| Revision as of 21:09, 18 September 2021 editKent G. Budge (talk | contribs)Autopatrolled, Extended confirmed users19,645 edits →Further reading: Already cited in article← Previous edit | Latest revision as of 16:15, 30 September 2024 edit undoDiscospinster (talk | contribs)Administrators466,213 editsm Reverted edit by 81.35.177.246 (talk) to last version by NamesnikTag: Rollback | ||
| (64 intermediate revisions by 40 users not shown) | |||
| Line 1: | Line 1: | ||
| {{Short description|Copper carbonate mineral}} | {{Short description|Copper carbonate mineral}} | ||
| {{About|the carbonate mineral|the FDPSO vessel|Azurite FDPSO|the green fluorescent protein derivative|Green fluorescent protein#GFP derivatives|the mountain|Azurite Peak}} | {{About|the carbonate mineral|the FDPSO vessel|Azurite FDPSO|the green fluorescent protein derivative|Green fluorescent protein#GFP derivatives|the mountain|Azurite Peak}} | ||
| {{Other uses|Azure spar}} | |||
| {{Infobox mineral | {{Infobox mineral | ||
| |boxbgcolor=# |
|boxbgcolor=#0066CC| name = Azurite | ||
| | boxtextcolor = #fff | |||
| | category = ] | | category = ] | ||
| | image = Azurite, |
| image = Azurite - New Nevada Lode, La Sal, Utah, USA.jpg | ||
| | imagesize = 260px | | imagesize = 260px | ||
| | alt = | | alt = | ||
| | caption = Azurite from |
| caption = Azurite from New Nevada lode, La Sal, Utah, USA | ||
| | formula = Cu<sub>3</sub>(CO<sub>3</sub>)<sub>2</sub>(OH)<sub>2</sub> | | formula = Cu<sub>3</sub>(CO<sub>3</sub>)<sub>2</sub>(OH)<sub>2</sub> | ||
| |IMAsymbol=Azu<ref>{{Cite journal|last=Warr|first=L.N.|date=2021|title=IMA–CNMNC approved mineral symbols|url=https://www.cambridge.org/core/journals/mineralogical-magazine/article/imacnmnc-approved-mineral-symbols/62311F45ED37831D78603C6E6B25EE0A|journal=Mineralogical Magazine|volume=85|issue=3|pages=291–320|doi=10.1180/mgm.2021.43|bibcode=2021MinM...85..291W|s2cid=235729616|doi-access=free}}</ref> | |||
| | molweight = 344.67 g/mol | | molweight = 344.67 g/mol | ||
| | strunz = 5.BA.05 | | strunz = 5.BA.05 | ||
| Line 39: | Line 42: | ||
| }} | }} | ||
| '''Azurite''' is a soft, deep-blue ] ] produced by weathering of copper ore deposits. During the early 19th century, it was also known as '''chessylite''', after the ] at ] near ], ].<ref name=Mindat/> The mineral, a basic ] with the chemical formula Cu<sub>3</sub>(CO<sub>3</sub>)<sub>2</sub>(OH)<sub>2</sub>, has been known since ancient times, and was mentioned in ]'s '']'' under the Greek name {{transl|el|kuanos}} (κυανός: "deep blue," root of English |
'''Azurite''' or '']''<ref name="kriv">''Krivovichev V. G.'' Mineralogical glossary. Scientific editor ]. — St.Petersburg: St.Petersburg Univ. Publ. House. 2009. — 556 p. — ISBN 978-5-288-04863-0. ''(in Russian)''</ref>{{rp|14}} is a soft, deep-blue ] ] produced by weathering of copper ore deposits. During the early 19th century, it was also known as '''chessylite''', after the ] at ] near ], ].<ref name=Mindat/> The mineral, a basic ] with the chemical formula Cu<sub>3</sub>(CO<sub>3</sub>)<sub>2</sub>(OH)<sub>2</sub>, has been known since ancient times, and was mentioned in ]'s '']'' under the Greek name {{transl|el|kuanos}} (κυανός: "deep blue," root of English ]) and the Latin name '']''.<ref> {{webarchive |url=https://web.archive.org/web/20051220155850/http://www.ancientlibrary.com/smith-dgra/0328.html |date=December 20, 2005 }}</ref> Copper (Cu<sup>2+</sup>) gives it its blue color.<ref>{{Cite web |title=Minerals Colored by Metal Ions |url=http://minerals.gps.caltech.edu/color_causes/Metal_Ion/index.html |access-date=2023-03-01 |website=minerals.gps.caltech.edu}}</ref> | ||
| ==Mineralogy== | ==Mineralogy== | ||
| ] | ] | ||
| Azurite has the formula Cu<sub>3</sub>(CO<sub>3</sub>)<sub>2</sub>(OH)<sub>2</sub>, with the copper(II) ]s linked to two different anions, ] and ]. It is one of two relatively common basic copper(II) ], the other being bright green ]. ] is a rare basic carbonate of copper and ].<ref name="HurlbutKlein">{{cite book |last1=Klein |first1=Cornelis |last2=Hurlbut |first2=Cornelius S. |
Azurite has the formula Cu<sub>3</sub>(CO<sub>3</sub>)<sub>2</sub>(OH)<sub>2</sub>, with the copper(II) ]s linked to two different anions, ] and ]. It is one of two relatively common basic copper(II) ], the other being bright green ]. ] is a rare basic carbonate of copper and ].<ref name="HurlbutKlein">{{cite book |last1=Klein |first1=Cornelis |last2=Hurlbut |first2=Cornelius S. Jr. |title=Manual of mineralogy : (after James D. Dana) |date=1993 |publisher=Wiley |location=New York |isbn=047157452X |edition=21st |pages=417–418}}</ref> Simple ] (CuCO<sub>3</sub>) is not known to exist in nature, due to the high affinity of the {{chem|Cu|2+}} ion for the ] anion {{chem|H|O|-}}.<ref>{{Cite book|title=Principles of Corrosion Engineering and Corrosion Control|last=Ahmad|first=Zaki|date=2006|publisher=Butterworth-Heinemann|isbn=9780750659246|location=Oxford|pages=120–270}}</ref> | ||
| Azurite crystallizes in the ].<ref>{{cite journal|title=Verfeinerung der Struktur von Azurit, Cu<sub>3</sub>(OH)<sub>2</sub>(CO<sub>3</sub>)<sub>2</sub>, durch Neutronenbeugung|author1=Zigan, F.|author2=Schuster, H.D.|journal=Zeitschrift für Kristallographie, Kristallgeometrie, Kristallphysik, Kristallchemie|year=1972|volume=135|issue=5–6|pages=416–436|doi=10.1524/zkri.1972.135.5-6.416|bibcode=1972ZK....135..416Z}}</ref> |
Azurite crystallizes in the ].<ref>{{cite journal|title=Verfeinerung der Struktur von Azurit, Cu<sub>3</sub>(OH)<sub>2</sub>(CO<sub>3</sub>)<sub>2</sub>, durch Neutronenbeugung|author1=Zigan, F.|author2=Schuster, H.D.|journal=Zeitschrift für Kristallographie, Kristallgeometrie, Kristallphysik, Kristallchemie|year=1972|volume=135|issue=5–6|pages=416–436|doi=10.1524/zkri.1972.135.5-6.416|bibcode=1972ZK....135..416Z|s2cid=95738208 }}</ref> Large crystals are dark blue, often prismatic.<ref name=Mindat/><ref name=Webmin/><ref name="HurlbutKlein"/> Azurite specimens can be massive to nodular or can occur as ] crystals lining a cavity.<ref name=Sinkankas>{{cite book |last1=Sinkankas |first1=John |title=Mineralogy for amateurs. |date=1964 |publisher=Van Nostrand |location=Princeton, N.J. |isbn=0442276249 |pages=379–381}}</ref> | ||
| Azurite |
Azurite has a ] of 3.5 to 4. The ] of azurite is 3.7 to 3.9. Characteristic of a carbonate, specimens effervesce upon treatment with hydrochloric acid. The combination of deep blue color and effervescence when moistened with hydrochloric acid are identifying characteristics of the mineral.<ref name="HurlbutKlein"/><ref name=Sinkankas/> | ||
| ===Color=== | ===Color=== | ||
| The optical properties (color, intensity) of minerals such as azurite and malachite are characteristic of copper(II). Many ] of copper(II) exhibit similar colors. According to ], the color results from low energy d-d transitions associated with the d<sup>9</sup> metal center.<ref>{{cite journal |last1=Nassau |first1=K. |year=1978 |title=The origins of color in minerals |journal=American |
The optical properties (color, intensity) of minerals such as azurite and malachite are characteristic of copper(II). Many ] of copper(II) exhibit similar colors. According to ], the color results from low energy d-d transitions associated with the d<sup>9</sup> metal center.<ref>{{cite journal |last1=Nassau |first1=K. |year=1978 |title=The origins of color in minerals |journal=American Mineralogist |volume=63 |number=3–4 |pages=219–229}}</ref>{{sfn|Klein|Hurlbut|1993|pp=260-263}} | ||
| ===Weathering=== | ===Weathering=== | ||
| Line 62: | Line 65: | ||
| ===Occurrences=== | ===Occurrences=== | ||
| ] | |||
| Azurite is found in the same geologic settings as its sister mineral, malachite, though it is usually less abundant. Both minerals occur widely as ] copper minerals, formed in the ] zone of copper ore deposits. Here they are associated with ], ], and various ] minerals.<ref name=HurlbutKlein/> | Azurite is found in the same geologic settings as its sister mineral, malachite, though it is usually less abundant. Both minerals occur widely as ] copper minerals, formed in the ] zone of copper ore deposits. Here they are associated with ], ], and various ] minerals.<ref name=HurlbutKlein/> | ||
| Fine specimens can be found at many locations. Among the best specimens are found at ], and nearby locations, and have included clusters of crystals several inches long and spherical aggregates and rosettes up to {{convert|2|in||sp=us}} in diameter. Similar rosettes are found at ], France. The best crystals, up to {{convert|10|in||sp=us}} in length, are found at ], ]. Other notable occurrences are in ]; ]; the ] and ]; ]; ]; ]; and ].<ref name=Sinkankas/> | Fine specimens can be found at many locations. Among the best specimens are found at ], and nearby locations, and have included clusters of crystals several inches long and spherical aggregates and rosettes up to {{convert|2|in||sp=us}} in diameter. Similar rosettes are found at ], France. The best crystals, up to {{convert|10|in||sp=us}} in length, are found at ], ]. Other notable occurrences are in ]; ]; the ] and ]; ]; ]; ]; Brazil and ].<ref name=Sinkankas/> | ||
| ==Uses== | ==Uses== | ||
| ===Pigments=== | ===Pigments=== | ||
| Azurite is unstable in air |
Azurite is unstable in air, however it was used as a ] in antiquity.<ref>Gettens, R.J. and Fitzhugh, E.W., Azurite and Blue Verditer, in Artists’ Pigments. A Handbook of Their History and Characteristics, Vol. 2: A. Roy (Ed.) Oxford University Press 1993, p. 23–24</ref> Azurite is naturally occurring in Sinai and the Eastern Desert of Egypt. It was reported by ] (1895) in the following examples; a shell used as a pallet in a ] (2613 to 2494 BCE) context in ], a cloth over the face of a ] (2494 to 2345 BCE) mummy also at ] and a number of ] (1543–1292 BCE) wall paintings.<ref>{{Cite book|title=Ancient Egyptian Materials and Technology|last1=Nicholson|first1=Paul|last2=Shaw|first2=Ian|publisher=Cambridge University Press|year=2000|isbn=978-0521452571}}</ref> Depending on the degree of fineness to which it was ground, and its basic content of copper carbonate, it gave a wide range of blues. It has been known as ''mountain blue'', ''Armenian stone'', and ''azurro della Magna'' (''blue from Germany'' in ]). When mixed with oil it turns slightly green. When mixed with ] it turns green-grey. It is also known by the names '']'' and ''blue verditer'', though '']'' usually refers to a pigment made by chemical process. Older examples of azurite pigment may show a more greenish tint due to weathering into malachite. Much azurite was mislabeled '']'', a term applied to many blue pigments. As chemical analysis of paintings from the ] improves, azurite is being recognized as a major source of the blues used by medieval painters. Lapis lazuli (the pigment ultramarine) was chiefly supplied from Afghanistan during the Middle Ages, whereas azurite was a common mineral in Europe at the time. Sizable deposits were found near Lyons, France. It was mined since the 12th century in ], in the silver mines located there.<ref>Andersen, Frank J. ''Riches of the Earth''. W.H. Smith Publishers, New York, 1981, {{ISBN|0-8317-7739-7}}</ref> | ||
| Heating can be used to distinguish azurite from purified natural ] blue, a more expensive but more stable blue pigment, as described by ]. Ultramarine withstands heat, whereas azurite converts to black copper oxide.<ref>{{cite journal |last1=Muller |first1=Norman E. |title=Three Methods of Modelling the Virgin's Mantle in Early Itallan Painting |journal=Journal of the American Institute for Conservation |date=January 1978 |volume=17 |issue=2 |pages=10–18 |doi=10.1179/019713678806029166}}</ref> However, gentle heating of azurite produces a deep blue pigment used in Japanese painting techniques.<ref>{{cite journal |last1=Nishio |first1=Yoshiyuki |title= |
Heating can be used to distinguish azurite from purified natural ] blue, a more expensive but more stable blue pigment, as described by ]. Ultramarine withstands heat, whereas azurite converts to black copper oxide.<ref>{{cite journal |last1=Muller |first1=Norman E. |title=Three Methods of Modelling the Virgin's Mantle in Early Itallan Painting |journal=Journal of the American Institute for Conservation |date=January 1978 |volume=17 |issue=2 |pages=10–18 |doi=10.1179/019713678806029166}}</ref> However, gentle heating of azurite produces a deep blue pigment used in Japanese painting techniques.<ref>{{cite journal |last1=Nishio |first1=Yoshiyuki |title=Pigments Used in Japanese Paintings |journal=The Paper Conservator |date=January 1987 |volume=11 |issue=1 |pages=39–45 |doi=10.1080/03094227.1987.9638544}}</ref> | ||
| Azurite pigment can be synthesized by precipitating ] from a solution of ] with ] (]) and treating the precipitate with a concentrated solution of ] and lime. This pigment is likely to contain traces of basic copper(II) chlorides.<ref>{{cite journal |last1=Orna |first1=Mary Virginia |last2=Low |first2=Manfred J. D. |last3=Baer |first3=Norbert S. |title=Synthetic Blue Pigments: Ninth to Sixteenth Centuries. I. Literature |journal=Studies in Conservation |date=May 1980 |volume=25 |issue=2 |pages=53 |doi=10.2307/1505860}}</ref> | Azurite pigment can be synthesized by precipitating ] from a solution of ] with ] (]) and treating the precipitate with a concentrated solution of ] and lime. This pigment is likely to contain traces of basic copper(II) chlorides.<ref>{{cite journal |last1=Orna |first1=Mary Virginia |last2=Low |first2=Manfred J. D. |last3=Baer |first3=Norbert S. |title=Synthetic Blue Pigments: Ninth to Sixteenth Centuries. I. Literature |journal=Studies in Conservation |date=May 1980 |volume=25 |issue=2 |pages=53 |doi=10.2307/1505860|jstor=1505860 }}</ref> | ||
| <gallery mode=packed heights= |
<gallery mode="packed" heights="160px"> | ||
| File:Azuritepigment.jpg|Ground azurite for use as a pigment | File:Azuritepigment.jpg|Ground azurite for use as a pigment | ||
| File:Lady with a Squirrel.jpg|The background of ''Lady with a Squirrel'' by ] was painted with |
File:Lady with a Squirrel.jpg|The background of ''Lady with a Squirrel'' by ] was painted with azurite | ||
| File:Madonna and Child Enthroned with Saints.jpg|The greenish tint of the Madonna's mantle in ]'s ''Madonna and Child Enthroned with Saints'' is due to azurite weathering to malachite | File:Madonna and Child Enthroned with Saints.jpg|The greenish tint of the Madonna's mantle in ]'s ''Madonna and Child Enthroned with Saints'' is due to azurite weathering to malachite | ||
| </gallery> | </gallery> | ||
| ===Jewelry=== | ===Jewelry=== | ||
| Azurite is used occasionally as beads and as ], and also as an ornamental stone.<ref>{{cite journal |last1=Mueller |first1=Wolfgang |title=Arizona Gemstones |journal=Rocks & Minerals |date=31 January 2012 |volume=87 |issue=1 |pages=64–70 |doi=10.1080/00357529.2012.636241 |language=en |issn=0035-7529}}</ref> However, its softness and tendency to lose its deep blue color as it weathers |
Azurite is used occasionally as beads and as ], and also as an ornamental stone.<ref>{{cite journal |last1=Mueller |first1=Wolfgang |title=Arizona Gemstones |journal=Rocks & Minerals |date=31 January 2012 |volume=87 |issue=1 |pages=64–70 |doi=10.1080/00357529.2012.636241 |s2cid=219714562 |language=en |issn=0035-7529}}</ref> However, its softness and tendency to lose its deep blue color as it weathers leaves it with fewer uses.<ref>{{cite book |last1=Schumann |first1=Walter |title=Gemstones of the world |date=2009 |publisher=Sterling |location=New York |isbn=9781402768293 |edition=4th, newly rev. & expanded |url=https://books.google.com/books?id=V9PqVxpxeiEC&q=azurite |access-date=18 September 2021}}</ref> Heating destroys azurite easily, so all mounting of azurite specimens must be done at room temperature. | ||
| ===Collecting=== | ===Collecting=== | ||
| Line 91: | Line 95: | ||
| ==History== | ==History== | ||
| Azurite was known in the pre-classical ]. It was used in ] as a pigment, obtained from mines in ]. ]n writers report the use of a special ] for grinding it. It was also used in ancient ], for example on the ] in Athens. It does not appear to have been used in ] wall paintings but Roman writers certainly knew about its use as a pigment.<ref>Robert James Forbes, ''Studies in Ancient Technology'', vol. 1, p. 216, Leiden: E. J. Brill, 1955 {{oclc|312267983}}.</ref> The ] of glass and azurite was developed in ancient Mesopotamia.<ref>Emmerich Paszthory, "Electricity generation or magic? The analysis of an unusual group of finds from Mesopotamia", p. 34 in, Stuart J. Fleming, Helen R. Schenck, ''History of Technology: The Role of Metals'', University of Pennsylvania Museum of Archaeology, 1989 {{ISBN|0924171952}}.</ref> | |||
| The use of azurite and malachite as copper ore indicators led indirectly to the name of the element ] in the English language. ], a principal ore of nickel that is also known as niccolite, weathers at the surface into a green mineral (]) that resembles malachite. This resemblance resulted in occasional attempts to ] nickeline in the belief that it was copper ore, but such attempts always ended in failure due to high smelting temperatures needed to ] nickel. In Germany this deceptive mineral came to be known as ], literally "copper ]." The ] ] Baron ] (who had been trained by ], the discoverer of the nickel-like metal ]) realized that there was probably a new metal hiding within the kupfernickel ore, and in 1751 he succeeded in smelting kupfernickel to produce a previously unknown (except in certain ]) silvery white, iron-like metal. Logically, Cronstedt named his new metal after the ''nickel'' part of ''kupfernickel''. | |||
| ==Gallery |
==Gallery== | ||
| <gallery widths=" |
<gallery widths="125" heights="125" mode="packed"> | ||
| Azurite from China.jpg|Azurite crystals from China | Azurite from China.jpg|Azurite crystals from China | ||
| File:Azurite from Arizona, collected by Dr John Hunter in the 18th century, Hunterian Museum, Glasgow.jpg|Azurite from Arizona, collected by Dr John Hunter in the 18th century, Hunterian Museum, Glasgow | File:Azurite from Arizona, collected by Dr John Hunter in the 18th century, Hunterian Museum, Glasgow.jpg|Azurite from Arizona, collected by Dr John Hunter in the 18th century, Hunterian Museum, Glasgow | ||
| Line 104: | Line 108: | ||
| File:Azurite-den07-03b.jpg|Azurite from ], Namibia | File:Azurite-den07-03b.jpg|Azurite from ], Namibia | ||
| File:Azurite Bisbee ROM.jpg|Azurite, cross-section through merged ]s, ] | File:Azurite Bisbee ROM.jpg|Azurite, cross-section through merged ]s, ] | ||
| File:Sparkly Azurite Crystal 01.jpg|Azurite crystal, from the minerals |
File:Batu Azurite.jpg|Azurite from ], Indonesia | ||
| File:Sparkly Azurite Crystal 01.jpg|Azurite crystal, from the minerals collection at the ]. | |||
| File:Azurite spheroids.jpg|Spheroidal azurite specimens from ] | File:Azurite spheroids.jpg|Spheroidal azurite specimens from ] | ||
| </gallery> | </gallery> | ||
| Line 112: | Line 117: | ||
| * ] | * ] | ||
| * ] | * ] | ||
| * ] | |||
| ==References== | ==References== | ||
| Line 126: | Line 132: | ||
| ] | ] | ||
| ] | ] | ||
| ] | |||
| ] | ] | ||
| ] | ] | ||
Latest revision as of 16:15, 30 September 2024
Copper carbonate mineral This article is about the carbonate mineral. For the FDPSO vessel, see Azurite FDPSO. For the green fluorescent protein derivative, see Green fluorescent protein § GFP derivatives. For the mountain, see Azurite Peak. For other uses, see Azure spar.| Azurite | |
|---|---|
 Azurite from New Nevada lode, La Sal, Utah, USA Azurite from New Nevada lode, La Sal, Utah, USA | |
| General | |
| Category | Carbonate mineral |
| Formula (repeating unit) | Cu3(CO3)2(OH)2 |
| IMA symbol | Azu |
| Strunz classification | 5.BA.05 |
| Crystal system | Monoclinic |
| Crystal class | Prismatic (2/m) (same H-M symbol) |
| Space group | P21/c |
| Unit cell | a = 5.01 Å, b = 5.85 Å c = 10.35 Å; β = 92.43°; Z = 2 |
| Identification | |
| Formula mass | 344.67 g/mol |
| Color | Azure-blue, dark to pale blue; pale blue in transmitted light |
| Crystal habit | Massive, prismatic, stalactitic, tabular |
| Twinning | Rare, twin planes {101}, {102} or {001} |
| Cleavage | Perfect on {011}, fair on {100}, poor on {110} |
| Fracture | Conchoidal |
| Tenacity | brittle |
| Mohs scale hardness | 3.5 to 4 |
| Luster | Vitreous |
| Streak | Light blue |
| Diaphaneity | Transparent to translucent |
| Specific gravity | 3.773 (measured), 3.78 (calculated) |
| Optical properties | Biaxial (+) |
| Refractive index | nα = 1.730 nβ = 1.758 nγ = 1.838 |
| Birefringence | δ = 0.108 |
| Pleochroism | Visible shades of blue |
| 2V angle | Measured: 68°, calculated: 64° |
| Dispersion | relatively weak |
| References | |
Azurite or Azure spar is a soft, deep-blue copper mineral produced by weathering of copper ore deposits. During the early 19th century, it was also known as chessylite, after the type locality at Chessy-les-Mines near Lyon, France. The mineral, a basic carbonate with the chemical formula Cu3(CO3)2(OH)2, has been known since ancient times, and was mentioned in Pliny the Elder's Natural History under the Greek name kuanos (κυανός: "deep blue," root of English cyan) and the Latin name caeruleum. Copper (Cu) gives it its blue color.
Mineralogy

Azurite has the formula Cu3(CO3)2(OH)2, with the copper(II) cations linked to two different anions, carbonate and hydroxide. It is one of two relatively common basic copper(II) carbonate minerals, the other being bright green malachite. Aurichalcite is a rare basic carbonate of copper and zinc. Simple copper carbonate (CuCO3) is not known to exist in nature, due to the high affinity of the Cu
ion for the hydroxide anion HO
.
Azurite crystallizes in the monoclinic system. Large crystals are dark blue, often prismatic. Azurite specimens can be massive to nodular or can occur as drusy crystals lining a cavity.
Azurite has a Mohs hardness of 3.5 to 4. The specific gravity of azurite is 3.7 to 3.9. Characteristic of a carbonate, specimens effervesce upon treatment with hydrochloric acid. The combination of deep blue color and effervescence when moistened with hydrochloric acid are identifying characteristics of the mineral.
Color
The optical properties (color, intensity) of minerals such as azurite and malachite are characteristic of copper(II). Many coordination complexes of copper(II) exhibit similar colors. According to crystal field theory, the color results from low energy d-d transitions associated with the d metal center.
Weathering
Azurite is unstable in open air compared to malachite, and often is pseudomorphically replaced by malachite. This weathering process involves the replacement of some of the carbon dioxide (CO2) units with water (H2O), changing the carbonate:hydroxide ratio of azurite from 1:1 to the 1:2 ratio of malachite:
- 2 Cu3(CO3)2(OH)2 + H2O → 3 Cu2(CO3)(OH)2 + CO2
From the above equation, the conversion of azurite into malachite is attributable to the low partial pressure of carbon dioxide in air.
Azurite is quite stable under ordinary storage conditions, so that specimens retain their deep blue color for long periods of time.
Occurrences

Azurite is found in the same geologic settings as its sister mineral, malachite, though it is usually less abundant. Both minerals occur widely as supergene copper minerals, formed in the oxidized zone of copper ore deposits. Here they are associated with cuprite, native copper, and various iron oxide minerals.
Fine specimens can be found at many locations. Among the best specimens are found at Bisbee, Arizona, and nearby locations, and have included clusters of crystals several inches long and spherical aggregates and rosettes up to 2 inches (51 mm) in diameter. Similar rosettes are found at Chessy, Rhône, France. The best crystals, up to 10 inches (250 mm) in length, are found at Tsumeb, Namibia. Other notable occurrences are in Utah; Mexico; the Ural and Altai Mountains; Sardinia; Laurion, Greece; Wallaroo, South Australia; Brazil and Broken Hill.
Uses
Pigments
Azurite is unstable in air, however it was used as a blue pigment in antiquity. Azurite is naturally occurring in Sinai and the Eastern Desert of Egypt. It was reported by F. C. J. Spurrell (1895) in the following examples; a shell used as a pallet in a Fourth Dynasty (2613 to 2494 BCE) context in Meidum, a cloth over the face of a Fifth Dynasty (2494 to 2345 BCE) mummy also at Meidum and a number of Eighteenth Dynasty (1543–1292 BCE) wall paintings. Depending on the degree of fineness to which it was ground, and its basic content of copper carbonate, it gave a wide range of blues. It has been known as mountain blue, Armenian stone, and azurro della Magna (blue from Germany in Italian). When mixed with oil it turns slightly green. When mixed with egg yolk it turns green-grey. It is also known by the names blue bice and blue verditer, though verditer usually refers to a pigment made by chemical process. Older examples of azurite pigment may show a more greenish tint due to weathering into malachite. Much azurite was mislabeled lapis lazuli, a term applied to many blue pigments. As chemical analysis of paintings from the Middle Ages improves, azurite is being recognized as a major source of the blues used by medieval painters. Lapis lazuli (the pigment ultramarine) was chiefly supplied from Afghanistan during the Middle Ages, whereas azurite was a common mineral in Europe at the time. Sizable deposits were found near Lyons, France. It was mined since the 12th century in Saxony, in the silver mines located there.
Heating can be used to distinguish azurite from purified natural ultramarine blue, a more expensive but more stable blue pigment, as described by Cennino D'Andrea Cennini. Ultramarine withstands heat, whereas azurite converts to black copper oxide. However, gentle heating of azurite produces a deep blue pigment used in Japanese painting techniques.
Azurite pigment can be synthesized by precipitating copper(II) hydroxide from a solution of copper(II) chloride with lime (calcium hydroxide) and treating the precipitate with a concentrated solution of potassium carbonate and lime. This pigment is likely to contain traces of basic copper(II) chlorides.
-
 Ground azurite for use as a pigment
Ground azurite for use as a pigment
-
 The background of Lady with a Squirrel by Hans Holbein the Younger was painted with azurite
The background of Lady with a Squirrel by Hans Holbein the Younger was painted with azurite
-
 The greenish tint of the Madonna's mantle in Raphael's Madonna and Child Enthroned with Saints is due to azurite weathering to malachite
The greenish tint of the Madonna's mantle in Raphael's Madonna and Child Enthroned with Saints is due to azurite weathering to malachite
Jewelry
Azurite is used occasionally as beads and as jewelry, and also as an ornamental stone. However, its softness and tendency to lose its deep blue color as it weathers leaves it with fewer uses. Heating destroys azurite easily, so all mounting of azurite specimens must be done at room temperature.
Collecting
The intense color of azurite makes it a popular collector's stone. The notion that specimens must be carefully protected from bright light, heat, and open air to retain their intensity of color over time may be an urban legend. Paul E. Desautels, former curator of gems and minerals at the Smithsonian Institution, has written that azurite is stable under ordinary storage conditions.
Prospecting
While not a major ore of copper itself, the presence of azurite is a good surface indicator of the presence of weathered copper sulfide ores. It is usually found in association with the chemically similar malachite, producing a striking color combination of deep blue and bright green that is strongly indicative of the presence of copper ores.
History
Azurite was known in the pre-classical ancient world. It was used in ancient Egypt as a pigment, obtained from mines in Sinai. Ancient Mesopotamian writers report the use of a special mortar and pestle for grinding it. It was also used in ancient Greece, for example on the Acropolis in Athens. It does not appear to have been used in ancient Roman wall paintings but Roman writers certainly knew about its use as a pigment. The fusing of glass and azurite was developed in ancient Mesopotamia.
Gallery
-
 Azurite crystals from China
Azurite crystals from China
-
 Azurite from Arizona, collected by Dr John Hunter in the 18th century, Hunterian Museum, Glasgow
Azurite from Arizona, collected by Dr John Hunter in the 18th century, Hunterian Museum, Glasgow
-
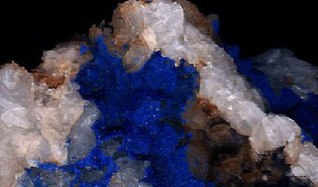 Fresh, unweathered azurite crystals showing the deep blue of unaltered azurite. From Špania Dolina, Slovakia
Fresh, unweathered azurite crystals showing the deep blue of unaltered azurite. From Špania Dolina, Slovakia
-
 Azurite with Malachite, Copper Queen mine, Bisbee, Arizona
Azurite with Malachite, Copper Queen mine, Bisbee, Arizona
-
 Azurite from Touissit, Morocco
Azurite from Touissit, Morocco
-
 Azurite, Morenci, Arizona
Azurite, Morenci, Arizona
-
 Azurite in siltstone, Malbunka mine, Northern Territory, Australia
Azurite in siltstone, Malbunka mine, Northern Territory, Australia
-
 Azurite from Tsumeb, Namibia
Azurite from Tsumeb, Namibia
-
 Azurite, cross-section through merged stalactites, Bisbee, Arizona
Azurite, cross-section through merged stalactites, Bisbee, Arizona
-
 Azurite from Bandung, Indonesia
Azurite from Bandung, Indonesia
-
 Azurite crystal, from the minerals collection at the Natural History Museum, London.
Azurite crystal, from the minerals collection at the Natural History Museum, London.
-
 Spheroidal azurite specimens from Utah
Spheroidal azurite specimens from Utah
See also
References
- Warr, L.N. (2021). "IMA–CNMNC approved mineral symbols". Mineralogical Magazine. 85 (3): 291–320. Bibcode:2021MinM...85..291W. doi:10.1180/mgm.2021.43. S2CID 235729616.
- Handbook of Mineralogy
- ^ Mindat.org
- ^ Webmineral.com Webmineral Data
- Krivovichev V. G. Mineralogical glossary. Scientific editor A. G. Bulakh. — St.Petersburg: St.Petersburg Univ. Publ. House. 2009. — 556 p. — ISBN 978-5-288-04863-0. (in Russian)
- The Ancient Library: Smith, Dictionary of Greek and Roman Antiquities, p.321, right col., under BLUE Archived December 20, 2005, at the Wayback Machine
- "Minerals Colored by Metal Ions". minerals.gps.caltech.edu. Retrieved 2023-03-01.
- ^ Klein, Cornelis; Hurlbut, Cornelius S. Jr. (1993). Manual of mineralogy : (after James D. Dana) (21st ed.). New York: Wiley. pp. 417–418. ISBN 047157452X.
- Ahmad, Zaki (2006). Principles of Corrosion Engineering and Corrosion Control. Oxford: Butterworth-Heinemann. pp. 120–270. ISBN 9780750659246.
- Zigan, F.; Schuster, H.D. (1972). "Verfeinerung der Struktur von Azurit, Cu3(OH)2(CO3)2, durch Neutronenbeugung". Zeitschrift für Kristallographie, Kristallgeometrie, Kristallphysik, Kristallchemie. 135 (5–6): 416–436. Bibcode:1972ZK....135..416Z. doi:10.1524/zkri.1972.135.5-6.416. S2CID 95738208.
- ^ Sinkankas, John (1964). Mineralogy for amateurs. Princeton, N.J.: Van Nostrand. pp. 379–381. ISBN 0442276249.
- Nassau, K. (1978). "The origins of color in minerals". American Mineralogist. 63 (3–4): 219–229.
- Klein & Hurlbut 1993, pp. 260–263.
- ^ Desautels, Paul E. (January 1991). "Some Thoughts about Azurite". Rocks & Minerals. 66 (1): 14–23. doi:10.1080/00357529.1991.11761595.
- Gettens, R.J. and Fitzhugh, E.W., Azurite and Blue Verditer, in Artists’ Pigments. A Handbook of Their History and Characteristics, Vol. 2: A. Roy (Ed.) Oxford University Press 1993, p. 23–24
- Nicholson, Paul; Shaw, Ian (2000). Ancient Egyptian Materials and Technology. Cambridge University Press. ISBN 978-0521452571.
- Andersen, Frank J. Riches of the Earth. W.H. Smith Publishers, New York, 1981, ISBN 0-8317-7739-7
- Muller, Norman E. (January 1978). "Three Methods of Modelling the Virgin's Mantle in Early Itallan Painting". Journal of the American Institute for Conservation. 17 (2): 10–18. doi:10.1179/019713678806029166.
- Nishio, Yoshiyuki (January 1987). "Pigments Used in Japanese Paintings". The Paper Conservator. 11 (1): 39–45. doi:10.1080/03094227.1987.9638544.
- Orna, Mary Virginia; Low, Manfred J. D.; Baer, Norbert S. (May 1980). "Synthetic Blue Pigments: Ninth to Sixteenth Centuries. I. Literature". Studies in Conservation. 25 (2): 53. doi:10.2307/1505860. JSTOR 1505860.
- Mueller, Wolfgang (31 January 2012). "Arizona Gemstones". Rocks & Minerals. 87 (1): 64–70. doi:10.1080/00357529.2012.636241. ISSN 0035-7529. S2CID 219714562.
- Schumann, Walter (2009). Gemstones of the world (4th, newly rev. & expanded ed.). New York: Sterling. ISBN 9781402768293. Retrieved 18 September 2021.
- Robert James Forbes, Studies in Ancient Technology, vol. 1, p. 216, Leiden: E. J. Brill, 1955 OCLC 312267983.
- Emmerich Paszthory, "Electricity generation or magic? The analysis of an unusual group of finds from Mesopotamia", p. 34 in, Stuart J. Fleming, Helen R. Schenck, History of Technology: The Role of Metals, University of Pennsylvania Museum of Archaeology, 1989 ISBN 0924171952.
External links
- Spencer, Leonard James (1911). "Azurite" . Encyclopædia Britannica. Vol. 3 (11th ed.). p. 86.
- Azurite, Colourlex