| Revision as of 02:03, 27 September 2023 editCitation bot (talk | contribs)Bots5,459,523 edits Removed parameters. | Use this bot. Report bugs. | #UCB_CommandLine← Previous edit | Latest revision as of 23:07, 4 November 2023 edit undoNeko-chan (talk | contribs)Extended confirmed users, New page reviewers, Rollbackers20,610 edits →top: editing chem and a citeTags: Mobile edit Mobile app edit Android app edit | ||
| Line 40: | Line 40: | ||
| }} | }} | ||
| |Section2={{Chembox Properties | |Section2={{Chembox Properties | ||
| | Formula = {{chem2|C19H23N7O6}} | |||
| | Formula = C<sub>19</sub>H<sub>23</sub>N<sub>7</sub>O<sub>6</sub> | |||
| | MolarMass = 445.43 g/mol | | MolarMass = 445.43 g/mol | ||
| | Appearance = | | Appearance = | ||
| Line 73: | Line 73: | ||
| ==Functions== | ==Functions== | ||
| Tetrahydrofolic acid is a ] in many reactions, especially in the synthesis (or anabolism) of ]s and ]s. In addition, it serves as a carrier molecule for single-carbon moieties, that is, groups containing one carbon atom e.g. ], ], ], ], or formimino. When combined with one such single-carbon moiety as in ], it acts as a donor of a group with one carbon atom. Tetrahydrofolate gets this extra carbon atom by sequestering ] produced in other processes. These single-carbon moieties are important in the formation of precursors for DNA synthesis. A shortage in tetrahydrofolic acid (FH4) can cause ].<ref>{{Cite web |
Tetrahydrofolic acid is a ] in many reactions, especially in the synthesis (or anabolism) of ]s and ]s. In addition, it serves as a carrier molecule for single-carbon moieties, that is, groups containing one carbon atom e.g. ], ], ], ], or formimino. When combined with one such single-carbon moiety as in ], it acts as a donor of a group with one carbon atom. Tetrahydrofolate gets this extra carbon atom by sequestering ] produced in other processes. These single-carbon moieties are important in the formation of precursors for DNA synthesis. A shortage in tetrahydrofolic acid (FH4) can cause ].<ref>{{Cite web|date=2008-02-23|title=Biochemistry: The One-Carbon Pool: Folate and B12 Metabolism |url=https://liveonearth.livejournal.com/260487.html|archive-url=|archive-date=|access-date=2020-12-15|website=liveonearth.livejournal.com}}</ref><ref>{{Cite journal |last1=Yadav |first1=Manish K. |last2=Manoli |first2=Nandini M. |last3=Madhunapantula |first3=SubbaRao V. |date=2016-10-25 |editor-last=Roemer |editor-first=Klaus |title=Comparative Assessment of Vitamin-B12, Folic Acid and Homocysteine Levels in Relation to p53 Expression in Megaloblastic Anemia |journal=PLOS ONE |language=en |volume=11 |issue=10 |pages=e0164559 |doi=10.1371/journal.pone.0164559 |issn=1932-6203 |pmc=5079580 |pmid=27780269|bibcode=2016PLoSO..1164559Y |doi-access=free }}</ref><ref>{{Cite journal |last1=Aslinia |first1=F. |last2=Mazza |first2=J. J. |last3=Yale |first3=S. H. |date=2006-09-01 |title=Megaloblastic Anemia and Other Causes of Macrocytosis |journal=Clinical Medicine & Research |language=en |volume=4 |issue=3 |pages=236–241 |doi=10.3121/cmr.4.3.236 |issn=1539-4182 |pmc=1570488 |pmid=16988104}}</ref> | ||
| Methotrexate acts on dihydrofolate reductase, like ] or ], as an inhibitor and thus reduces the amount of tetrahydrofolate made. This may result in megaloblastic anemia. | Methotrexate acts on dihydrofolate reductase, like ] or ], as an inhibitor and thus reduces the amount of tetrahydrofolate made. This may result in megaloblastic anemia. | ||
| Tetrahydrofolic acid is involved in the conversion of ] to ]; this may reduce the amount of ] available for decarboxylation and protein synthesis, and hence the urinary histamine and formiminoglutamic acid may be decreased.<ref>{{cite journal |vauthors=Dawson W, Maudsley DV, West GB |title=Histamine formation in guinea-pigs |journal=J. Physiol. |volume=181 |issue=4 |pages=801–9 |date=December 1965 |pmid=5881255 |pmc=1357684 |doi=10.1113/jphysiol.1965.sp007798 |
Tetrahydrofolic acid is involved in the conversion of ] to ]; this may reduce the amount of ] available for decarboxylation and protein synthesis, and hence the urinary histamine and formiminoglutamic acid may be decreased.<ref>{{cite journal |vauthors=Dawson W, Maudsley DV, West GB |title=Histamine formation in guinea-pigs |journal=J. Physiol. |volume=181 |issue=4 |pages=801–9 |date=December 1965 |pmid=5881255 |pmc=1357684 |doi=10.1113/jphysiol.1965.sp007798 }}</ref> | ||
| <gallery> | <gallery> | ||
Latest revision as of 23:07, 4 November 2023
Not to be confused with Tetrahydrofuran. | |
 | |
| Names | |
|---|---|
| IUPAC name N-methyl}amino)benzoyl]-L-glutamic acid | |
| Systematic IUPAC name (2S)-2-methyl}amino)benzamido]pentanedioic acid | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| Beilstein Reference | 101189 |
| ChEBI | |
| ChemSpider | |
| DrugBank | |
| IUPHAR/BPS | |
| KEGG | |
| MeSH | 5,6,7,8-tetrahydrofolic+acid |
| PubChem CID | |
| UNII | |
InChI
| |
SMILES
| |
| Properties | |
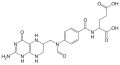
| Chemical formula | C19H23N7O6 |
| Molar mass | 445.43 g/mol |
| Melting point | 250 °C (482 °F; 523 K) |
| Solubility in water | 0.27 g/L |
| Acidity (pKa) | 3.51 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Tetrahydrofolic acid (THFA), or tetrahydrofolate, is a folic acid derivative.
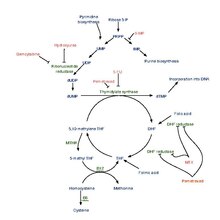
Metabolism
Human synthesis
Tetrahydrofolic acid is produced from dihydrofolic acid by dihydrofolate reductase. This reaction is inhibited by methotrexate.
It is converted into 5,10-methylenetetrahydrofolate by serine hydroxymethyltransferase.
Bacterial synthesis
Many bacteria use dihydropteroate synthetase to produce dihydropteroate, a molecule without function in humans. This makes it a useful target for sulfonamide antibiotics, which compete with the PABA precursor.
Functions
Tetrahydrofolic acid is a cofactor in many reactions, especially in the synthesis (or anabolism) of amino acids and nucleic acids. In addition, it serves as a carrier molecule for single-carbon moieties, that is, groups containing one carbon atom e.g. methyl, methylene, methenyl, formyl, or formimino. When combined with one such single-carbon moiety as in 10-formyltetrahydrofolate, it acts as a donor of a group with one carbon atom. Tetrahydrofolate gets this extra carbon atom by sequestering formaldehyde produced in other processes. These single-carbon moieties are important in the formation of precursors for DNA synthesis. A shortage in tetrahydrofolic acid (FH4) can cause megaloblastic anemia.
Methotrexate acts on dihydrofolate reductase, like pyrimethamine or trimethoprim, as an inhibitor and thus reduces the amount of tetrahydrofolate made. This may result in megaloblastic anemia.
Tetrahydrofolic acid is involved in the conversion of formiminoglutamic acid to glutamic acid; this may reduce the amount of histidine available for decarboxylation and protein synthesis, and hence the urinary histamine and formiminoglutamic acid may be decreased.
References
- Rajagopalan, P. T. Ravi; Zhang, Zhiquan; McCourt, Lynn; Dwyer, Mary; Benkovic, Stephen J.; Hammes, Gordon G. (2002-10-15). "Interaction of dihydrofolate reductase with methotrexate: Ensemble and single-molecule kinetics". Proceedings of the National Academy of Sciences. 99 (21): 13481–13486. Bibcode:2002PNAS...9913481R. doi:10.1073/pnas.172501499. ISSN 0027-8424. PMC 129699. PMID 12359872.
- "Biochemistry: The One-Carbon Pool: Folate and B12 Metabolism". liveonearth.livejournal.com. 2008-02-23. Retrieved 2020-12-15.
- Yadav, Manish K.; Manoli, Nandini M.; Madhunapantula, SubbaRao V. (2016-10-25). Roemer, Klaus (ed.). "Comparative Assessment of Vitamin-B12, Folic Acid and Homocysteine Levels in Relation to p53 Expression in Megaloblastic Anemia". PLOS ONE. 11 (10): e0164559. Bibcode:2016PLoSO..1164559Y. doi:10.1371/journal.pone.0164559. ISSN 1932-6203. PMC 5079580. PMID 27780269.
- Aslinia, F.; Mazza, J. J.; Yale, S. H. (2006-09-01). "Megaloblastic Anemia and Other Causes of Macrocytosis". Clinical Medicine & Research. 4 (3): 236–241. doi:10.3121/cmr.4.3.236. ISSN 1539-4182. PMC 1570488. PMID 16988104.
- Dawson W, Maudsley DV, West GB (December 1965). "Histamine formation in guinea-pigs". J. Physiol. 181 (4): 801–9. doi:10.1113/jphysiol.1965.sp007798. PMC 1357684. PMID 5881255.
External links
| Enzyme cofactors | |||||||
|---|---|---|---|---|---|---|---|
| Active forms |
| ||||||
| Base forms | |||||||