| Revision as of 05:24, 24 February 2011 editCheMoBot (talk | contribs)Bots141,565 edits Updating {{chembox}} (no changed fields - added verified revid - updated 'UNII_Ref', 'ChemSpiderID_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref', 'ChEMBL_Ref', 'KEGG_Ref') per Chem/Drugbox validation (← Previous edit |
Latest revision as of 13:23, 25 August 2022 edit undoFswitzer4 (talk | contribs)Extended confirmed users10,998 editsm Added UNII |
| (22 intermediate revisions by 19 users not shown) |
| Line 1: |
Line 1: |
|
{{chembox |
|
{{chembox |
|
|
| Verifiedfields = changed |
| ⚫ |
| verifiedrevid = 403156369 |
|
|
|
| Watchedfields = changed |
|
⚫ |
| verifiedrevid = 415641747 |
|
| ImageFileL1 = Oxalyl fluoride.svg |
|
| ImageFileL1 = Oxalyl fluoride.svg |
|
| ImageSizeL1 = 110px |
|
| ImageSizeL1 = 110px |
| Line 7: |
Line 9: |
|
| ImageSizeR1 = 120px |
|
| ImageSizeR1 = 120px |
|
| ImageNameR1 = Ball-and-stick model of oxalyl fluoride |
|
| ImageNameR1 = Ball-and-stick model of oxalyl fluoride |
|
| IUPACName = Oxalyl difluoride |
|
| PIN = Oxalyl difluoride |
|
| OtherNames = Oxalyl fluoride, ethanedioyl difluoride |
|
| OtherNames = TL-108 |
|
| Section1 = {{Chembox Identifiers |
|
|Section1={{Chembox Identifiers |
|
|
| CASNo_Ref = {{cascite|correct|??}} |
|
| CASNo = 359-40-0 |
|
|
| PubChem = 9668 |
|
| CASNo = 359-40-0 |
|
|
| UNII_Ref = {{fdacite|correct|FDA}} |
| ⚫ |
| EINECS = 206-630-4 |
|
|
|
| UNII = 2L7RR7QFL9 |
| ⚫ |
| SMILES = C(=O)(C(=O)F)F |
|
|
|
| PubChem = 9668 |
| ⚫ |
| InChI = 1/C2F2O2/c3-1(5)2(4)6 |
|
|
⚫ |
| EINECS = 206-630-4 |
|
⚫ |
| SMILES = C(=O)(C(=O)F)F |
|
⚫ |
| InChI = 1/C2F2O2/c3-1(5)2(4)6 |
|
|
| ChemSpiderID_Ref = {{chemspidercite|changed|chemspider}} |
|
|
| ChemSpiderID = 9287 |
|
}} |
|
}} |
|
| Section2 = {{Chembox Properties |
|
|Section2={{Chembox Properties |
|
| Formula = C<sub>2</sub>F<sub>2</sub>O<sub>2</sub> |
|
| Formula = C<sub>2</sub>F<sub>2</sub>O<sub>2</sub> |
|
| MolarMass = 94.017 g/mol |
|
| MolarMass = 94.017 g/mol |
|
| Appearance = |
|
| Appearance = |
|
| Density = |
|
| Density = |
|
| MeltingPt = |
|
| MeltingPtC = -3 |
|
| BoilingPt = |
|
| BoilingPtC = 26.6 |
|
| Solubility = |
|
| Solubility = |
| ⚫ |
}} |
|
| ⚫ |
| Section3 = {{Chembox Hazards |
|
| ⚫ |
| MainHazards = |
|
| ⚫ |
| FlashPt = |
|
|
| Autoignition = |
|
|
}} |
|
}} |
|
⚫ |
|Section3={{Chembox Hazards |
|
⚫ |
| MainHazards = |
|
⚫ |
| FlashPt = |
|
|
| AutoignitionPt = |
|
⚫ |
}} |
|
}} |
|
}} |
|
|
|
|
|
|
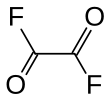
'''Oxalyl fluoride''' is the ] with the formula (COF)<sub>2</sub>. It is a ] derivative of ]. This colorless liquid is prepared by reaction of ] with ].<ref>{{cite journal|doi=10.1021/jo01081a050|title=Synthesis of Fluorides by Metathesis with Sodium Fluoride|author=C. W. Tullock, D. D. Coffman|journal=J. Org. Chem.|year=1960|volume=25|issue=11|page=2016–2019}}</ref> |
| ⚫ |
'''Oxalyl fluoride''' is a ] derivative of ]. It is being investigated for use in etching as a replacement for compounds which have the liability of high ].<ref>, US Patent 6635185.</ref><ref>{{cite journal|title=Evaluation of Oxalyl Fluoride for a Dielectric Etch Application in an Inductively Coupled Plasma Etch Tool|journal=J. Electrochem. Soc.|volume= 148|issue= 3|pages= G141–G149|year=2001|author=Simon Karecki, Ritwik Chatterjee, Laura Pruette, Rafael Reif, Terry Sparks, Laurie Beu, Victor Vartanian, and Konstantin Novoselovc|doi=10.1149/1.1348263}}</ref> |
|
|
|
|
|
⚫ |
Oxalyl fluoride is being investigated for use in etching as a replacement for compounds which have the liability of high ].<ref>, US Patent 6635185.</ref><ref>{{cite journal|title=Evaluation of Oxalyl Fluoride for a Dielectric Etch Application in an Inductively Coupled Plasma Etch Tool|journal=J. Electrochem. Soc.|volume= 148|issue= 3|pages= G141–G149|year=2001|author1=Simon Karecki |author2=Ritwik Chatterjee |author3=Laura Pruette |author4=Rafael Reif |author5=Terry Sparks |author6=Laurie Beu |author7=Victor Vartanian |author8=Konstantin Novoselovc |name-list-style=amp |doi=10.1149/1.1348263|bibcode=2001JElS..148G.141K}}</ref> |
|
|
|
|
|
==See also== |
|
==See also== |
|
* ] |
|
* ] |
|
|
* ] |
|
|
* ] |
|
|
* ] |
|
|
|
|
|
==References== |
|
==References== |
| Line 42: |
Line 54: |
|
] |
|
] |
|
] |
|
] |
|
|
] |
|
|
|
|
|
{{Organohalide-stub}} |
|
{{Organohalide-stub}} |
Oxalyl fluoride is being investigated for use in etching as a replacement for compounds which have the liability of high global warming potential.