| Revision as of 13:27, 24 April 2011 editWilliam Avery (talk | contribs)Autopatrolled, Extended confirmed users, Page movers, Pending changes reviewers, Rollbackers479,213 edits Disambiguated: polymorph → polymorphism (materials science) using Dab solver← Previous edit | Latest revision as of 05:33, 17 November 2024 edit undoGraeme Bartlett (talk | contribs)Administrators250,281 edits more ids | ||
| (30 intermediate revisions by 20 users not shown) | |||
| Line 1: | Line 1: | ||
| {{ |
{{Chembox | ||
| | Verifiedfields = changed | |||
| ⚫ | | |
||
| | Watchedfields = changed | |||
| | ImageFile = Sb2O4 structure.jpg | |||
| | verifiedrevid = 434226759 | |||
| ⚫ | | Name = Antimony tetroxide | ||
| | ImageFile1 = Alpha-Sb2O4-xtal-b-2x2x2-3D-bs-17.png | |||
| | ImageCaption1 = ''α''-{{chem2|Sb2O4}} | |||
| | ImageFile2 = Beta-Sb2O4-xtal-b-2x2x2-3D-bs-17.png | |||
| | ImageCaption2 = ''β''-{{chem2|Sb2O4}}<br><br>{{Color box|#bd80e3|border=darkgray}} ] {{Color box|#ee2010|border=darkgray}} ] | |||
| | IUPACName = antimony(III,V) oxide | | IUPACName = antimony(III,V) oxide | ||
| | |
| ImageName = | ||
| | |
| OtherNames = | ||
| | |
|Section1={{Chembox Identifiers | ||
| | |
| CASNo = 1332-81-6 | ||
| | CASNo_Ref = {{cascite|correct|CAS}} | |||
| | CASOther = 1332-81-6 | |||
| | ChemSpiderID = 66628 | |||
| ⚫ | |||
| | EINECS = 215-576-0 | |||
| ⚫ | | |
||
| | UNII_Ref = {{fdacite|correct|FDA}} | |||
| ⚫ | | |
||
| | UNII = XB7RVZ5DN5 | |||
| ⚫ | | |
||
| | PubChem = 74002 | |||
| ⚫ | | |
||
| ⚫ | }} | ||
| ⚫ | | |
||
| ⚫ | |Section2={{Chembox Properties | ||
| ⚫ | | |
||
| ⚫ | | Formula = SbO<sub>2</sub>; Sb<sub>2</sub>O<sub>4</sub> | ||
| ⚫ | | |
||
| ⚫ | | MolarMass = 153.7588; 307.5176 g/mol | ||
| | BoilingPt = dec. | |||
| ⚫ | | Appearance = white solid | ||
| ⚫ | | |
||
| ⚫ | | Density = 6.64 g/cm<sup>3</sup> (orthorhombic form) <ref name="Amador" /> | ||
| ⚫ | | Solubility = insoluble | ||
| ⚫ | | MeltingPt = > | ||
| | MeltingPtC = 930 | |||
| | MeltingPt_notes = (decomposes) | |||
| | BoilingPt_notes = decomposes | |||
| ⚫ | | pKa = | ||
| | RefractIndex = 2.0 | | RefractIndex = 2.0 | ||
| }} | |||
| | |
|Section3={{Chembox Structure | ||
| | |
| Coordination = | ||
| | |
| CrystalStruct = ] | ||
| | |
| Dipole = | ||
| }} | |||
| | |
|Section7={{Chembox Hazards | ||
| | |
| ExternalSDS = | ||
| ⚫ | | NFPA-H = 2 | ||
| | EUClass = | |||
| | |
| NFPA-F = 1 | ||
| | |
| NFPA-R = 0 | ||
| | REL = TWA 0.5 mg/m<sup>3</sup> (as Sb)<ref name=PGCH>{{PGCH|0036}}</ref> | |||
| | SPhrases = | |||
| | PEL = TWA 0.5 mg/m<sup>3</sup> (as Sb)<ref name=PGCH/> | |||
| ⚫ | | |
||
| ⚫ | }} | ||
| | NFPA-F = 1 | |||
| ⚫ | |Section8={{Chembox Related | ||
| | NFPA-R = 0 | |||
| ⚫ | | OtherAnions = | ||
| ⚫ | |||
| ⚫ | | OtherCations = | ||
| ⚫ | | |
||
| ⚫ | | OtherCompounds = ]<br />] | ||
| ⚫ | | |
||
| ⚫ | }} | ||
| ⚫ | | |
||
| ⚫ | | |
||
| ⚫ | |||
| }} | }} | ||
| '''Antimony tetroxide''' is an ] with the formula Sb<sub>2</sub>O<sub>4</sub>. This material, which exists as the mineral cervantite,<ref>{{cite web| url = http://webmineral.com/data/Cervantite.shtml| title = Cervantite| |
'''Antimony tetroxide''' is an ] with the formula Sb<sub>2</sub>O<sub>4</sub>. This material, which exists as the mineral cervantite,<ref>{{cite web| url = http://webmineral.com/data/Cervantite.shtml| title = Cervantite| access-date = 2009-06-06| publisher = Webminerals}}</ref> is white but reversibly yellows upon heating. The material, with empirical formula SbO<sub>2</sub>, is called antimony tetroxide to signify the presence of two kinds of Sb centers.<ref name="G&E">{{Greenwood&Earnshaw2nd|page=576}}</ref> | ||
| ==Formation and structure== | ==Formation and structure== | ||
| The material forms when ] is heated in air:<ref>Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN |
The material forms when ] is heated in air:<ref>Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. {{ISBN|0-12-352651-5}}.</ref> | ||
| :Sb<sub>2</sub>O<sub>3</sub> + 0.5 O<sub>2</sub> → Sb<sub>2</sub>O<sub>4</sub> ΔH = −187 kJ/mol | :Sb<sub>2</sub>O<sub>3</sub> + 0.5 O<sub>2</sub> → Sb<sub>2</sub>O<sub>4</sub> ΔH = −187 kJ/mol | ||
| At 800 °C, ] loses oxygen to give the same material: | At 800 °C, ] loses oxygen to give the same material: | ||
| :Sb<sub>2</sub>O<sub>5</sub> → Sb<sub>2</sub>O<sub>4</sub> + 0.5 O<sub>2</sub> ΔH = −64 kJ/mol | :Sb<sub>2</sub>O<sub>5</sub> → Sb<sub>2</sub>O<sub>4</sub> + 0.5 O<sub>2</sub> ΔH = −64 kJ/mol | ||
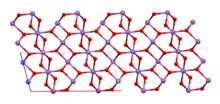
| The material is mixed valence, containing both Sb(V) and Sb(III) centers. Two ] are known, one orthorhombic (shown in the infobox) and one monoclinic.<ref name="Amador">J. |
The material is mixed valence, containing both Sb(V) and Sb(III) centers. Two ] are known, one orthorhombic (shown in the infobox) and one monoclinic.<ref name="Amador">{{cite journal | last1 = Amador | first1 = J. | last2 = Puebla | first2 = E. Gutierrez | last3 = Monge | first3 = M. A. | last4 = Rasines | first4 = I. | last5 = Valero | first5 = C. Ruiz | year = 1988 | title = Diantimony Tetraoxides Revisited | journal = Inorganic Chemistry | volume = 27 | issue = 8 | pages = 1367–1370 | doi = 10.1021/ic00281a011 }}</ref> Both forms feature octahedral Sb(V) centers arranged in sheets with distorted Sb(III) centers bound to four oxides. | ||
| ==References== | ==References== | ||
| Line 56: | Line 67: | ||
| {{Antimony compounds}} | {{Antimony compounds}} | ||
| {{Oxides}} | |||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ] | |||
| ] | |||
Latest revision as of 05:33, 17 November 2024
 | |
 Sb O | |
| Names | |
|---|---|
| IUPAC name antimony(III,V) oxide | |
| Identifiers | |
| CAS Number | |
| ChemSpider | |
| ECHA InfoCard | 100.014.161 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Properties | |
| Chemical formula | SbO2; Sb2O4 |
| Molar mass | 153.7588; 307.5176 g/mol |
| Appearance | white solid |
| Density | 6.64 g/cm (orthorhombic form) |
| Melting point | > 930 °C (1,710 °F; 1,200 K) (decomposes) |
| Boiling point | decomposes |
| Solubility in water | insoluble |
| Refractive index (nD) | 2.0 |
| Structure | |
| Crystal structure | orthorhombic |
| Hazards | |
| NFPA 704 (fire diamond) |
 |
| NIOSH (US health exposure limits): | |
| PEL (Permissible) | TWA 0.5 mg/m (as Sb) |
| REL (Recommended) | TWA 0.5 mg/m (as Sb) |
| Related compounds | |
| Related compounds | Antimony trioxide Antimony pentoxide |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Antimony tetroxide is an inorganic compound with the formula Sb2O4. This material, which exists as the mineral cervantite, is white but reversibly yellows upon heating. The material, with empirical formula SbO2, is called antimony tetroxide to signify the presence of two kinds of Sb centers.
Formation and structure
The material forms when Sb2O3 is heated in air:
- Sb2O3 + 0.5 O2 → Sb2O4 ΔH = −187 kJ/mol
At 800 °C, antimony(V) oxide loses oxygen to give the same material:
- Sb2O5 → Sb2O4 + 0.5 O2 ΔH = −64 kJ/mol
The material is mixed valence, containing both Sb(V) and Sb(III) centers. Two polymorphs are known, one orthorhombic (shown in the infobox) and one monoclinic. Both forms feature octahedral Sb(V) centers arranged in sheets with distorted Sb(III) centers bound to four oxides.
References
- ^ Amador, J.; Puebla, E. Gutierrez; Monge, M. A.; Rasines, I.; Valero, C. Ruiz (1988). "Diantimony Tetraoxides Revisited". Inorganic Chemistry. 27 (8): 1367–1370. doi:10.1021/ic00281a011.
- ^ NIOSH Pocket Guide to Chemical Hazards. "#0036". National Institute for Occupational Safety and Health (NIOSH).
- "Cervantite". Webminerals. Retrieved 2009-06-06.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 576. ISBN 978-0-08-037941-8.
- Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
| Antimony compounds | |||
|---|---|---|---|
| Antimonides | |||
| Sb(III) |
| ||
| Sb(III,V) | |||
| Sb(V) |
| ||