| Revision as of 11:55, 9 July 2011 editEdgar181 (talk | contribs)Extended confirmed users196,325 edits minor cleanup; SMILES added; added Category:Pyridines using HotCat← Previous edit | Latest revision as of 17:54, 23 December 2024 edit undoInnerstream (talk | contribs)Autopatrolled, Extended confirmed users4,150 editsNo edit summary | ||
| (29 intermediate revisions by 12 users not shown) | |||
| Line 1: | Line 1: | ||
| {{Chembox | {{Chembox | ||
| | Watchedfields = changed | |||
| | verifiedrevid = |
| verifiedrevid = 438566750 | ||
| | ImageFile = 2,6-Pyridinedicarbothioicacid.svg | | ImageFile = 2,6-Pyridinedicarbothioicacid.svg | ||
| | |
| ImageSize = | ||
| | |
| ImageAlt = Skeletal formula of 2,6-pyridinedicarbothioic acid | ||
| | |
| ImageFile1 = 2,6-Pyridinedicarbothioic acid 3D ball.png | ||
| | ImageSize1 = 220 | |||
| | ImageAlt1 = Ball-and-stick model of the 2,6-pyridinedicarbothioic acid molecule | |||
| | IUPACName = 2,6-Pyridinedicarbothioic acid | |||
| | PIN = Pyridine-2,6-bis(carbothioic ''S''-acid) | |||
| | OtherNames = PDTC, dithiopyridinedicarbothioic acid | | OtherNames = PDTC, dithiopyridinedicarbothioic acid | ||
| | |
|Section1={{Chembox Identifiers | ||
| | CASNo_Ref = {{cascite|correct|CAS}} | |||
| | |
| CASNo = 69945-42-2 | ||
| ⚫ | | PubChem =25202662 | ||
| | UNII_Ref = {{fdacite|correct|FDA}} | |||
| ⚫ | | |
||
| | |
| UNII = KJ7K75Z4JS | ||
| ⚫ | <!--| PubChem =25202662 twice?--> | ||
| ⚫ | | |
||
| ⚫ | | SMILES = C1=CC(=NC(=C1)C(=O)S)C(=O)S | ||
| | PubChem = 10214455 | |||
| ⚫ | | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | ChemSpiderID = 8389947 | | ChemSpiderID = 8389947 | ||
| | |
| SMILES1 = O=C(S)c1nc(C(=O)S)ccc1 | ||
| | |
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChI=1S/C7H5NO2S2/c9-6(11)4-2-1-3-5(8-4)7(10)12/h1-3H,(H,9,11)(H,10,12) | | StdInChI=1S/C7H5NO2S2/c9-6(11)4-2-1-3-5(8-4)7(10)12/h1-3H,(H,9,11)(H,10,12) | ||
| | |
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChIKey = SSRIAMRLMUFTNV-UHFFFAOYSA-N | | StdInChIKey = SSRIAMRLMUFTNV-UHFFFAOYSA-N | ||
| }} | }} | ||
| | |
|Section2={{Chembox Properties | ||
| | |
| C=7 | H=5 | O=2 | S=2 | ||
| | |
| Appearance =White crystalline solid | ||
| | |
| Density = 1.415 g/cm<sup>3</sup> | ||
| | |
| MeltingPtC = 97 to 99 | ||
| | |
| BoilingPtC = 404.4 | ||
| ⚫ | | Solubility = 1000 g/L (5.02 mol/L)}} | ||
| | BoilingPtC = 404.4 | |||
| ⚫ | |Section3={{Chembox Hazards | ||
| ⚫ | | |
||
| ⚫ | |MainHazards = acidic | ||
| ⚫ | | |
||
| |FlashPtC = 198.4}} | |||
| ⚫ | | |
||
| | FlashPt = {{convert|198.4|C|F}} | |||
| | Autoignition = }} | |||
| }} | }} | ||
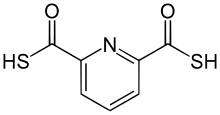
| '''2,6-Pyridinedicarbothioic acid''' (PDTC) is an ] compound that is produced by some bacteria. It functions as a |
'''2,6-Pyridinedicarbothioic acid''' (PDTC) is an ] compound that is produced by some bacteria. It functions as a , a low molecular weight compound that ]. Siderophores solubilize compounds by forming strong complexes. PDTC is secreted by the ] ] '']'' and '']''.<ref>{{cite book |doi=10.1007/978-3-211-99661-4_1 |year=2010 |last=Budzikiewicz |first=Herbert |title=Fortschritte der Chemie organischer Naturstoffe / Progress in the Chemistry of Organic Natural Products, Vol. 92 |editor-first=A. Douglas |editor-last=Kinghorn |editor-first2=Heinz |editor-last2=Falk |editor-first3=Junichi |editor-last3=Kobayashi |volume=92 |trans-title=Progress in the Chemistry of Organic Natural Products |chapter=Microbial Siderophores |pages=1–75 |pmid=20198464 |isbn=978-3-211-99660-7}}</ref> | ||
| ==Synthesis and biosynthesis== | ==Synthesis and biosynthesis== | ||
| PDTC can be synthesized by treating the pyridine-2,6-dicarboxylic |
PDTC can be synthesized in the laboratory by treating the diacid dichloride of ] with H<sub>2</sub>S in ]: | ||
| :{{chem2|NC5H3(COCl)2 + 2 H2S + 2 C5H5N → + Cl}} | |||
| :NC<sub>5</sub>H<sub>3</sub>(COOH)<sub>2</sub> + 2 H<sub>2</sub>S → NC<sub>5</sub>H<sub>3</sub>(COSH)<sub>2</sub> + 2 H<sub>2</sub>O | |||
| This produces |
This route produces the ] salt of pyridinium-2,6-dicarbothioate. Treatment of this orange-colored salt with ] gives colorless PDTC, which can then be extracted with ].<ref>{{cite journal |doi=10.1080/03086648308080490 |title=Zur Struktur Eines 1:1-Adduktes von Pyridin-2,6-Dicarbothiosäure und Pyridin |date=1983 |last1=Hildebrand |first1=U. |last2=Ockels |first2=W. |last3=Lex |first3=J. |last4=Budzikiewicz |first4=H. |journal=Phosphorus and Sulfur and the Related Elements |volume=16 |issue=3 |pages=361–364 }}</ref> | ||
| The biosynthesis of PDTC remains unclear although some insights can be deduced from the genetics.<ref>{{cite journal | doi = 10.1186/1471-2148-2-8 | year = 2002 | last1 = Cortese | first1 = Marc S | last2 = Caplan | first2 = Allan B | last3 = Crawford | first3 = Ronald L | journal = BMC Evolutionary Biology | volume = 2 | pages = 8 | pmid = 11972321 | title = Structural, functional, and evolutionary analysis of moeZ, a gene encoding an enzyme required for the synthesis of the Pseudomonas metabolite, pyridine-2,6-bis(thiocarboxylic acid) | pmc = 115864}}</ref> It is suggested that ''Pseudomonas stutzeri'' may have acquired at least one of the genes by lateral transfer from ].<ref name = cortese>{{cite journal | doi = 10.1023/A:1015241925322 | year = 2002 | last1 = Cortese | first1 = Marc S. | last2 = Paszczynski | first2 = Andrzej | last3 = Lewis | first3 = Thomas A. | last4 = Sebat | first4 = Jonathan L. | last5 = Borek | first5 = Vladimir | last6 = Crawford | first6 = Ronald L. | journal = BioMetals | volume = 15 | issue = 2 | pages = 103–120 | pmid = 12046919 | title = Metal chelating properties of pyridine-2,6-bis(thiocarboxylic acid) produced by Pseudomonas spp. And the biological activities of the formed complexes}}</ref> In a proposed biosynthetic sequence pyridine-2,6-dicarboxylic acid, a known bacterial metabolite,<ref name = cortese/> is activated as its bis-adenosine monophosphate (AMP) derivative. The sulfur donor and its activation remain uncertain.<ref name = budzi>{{cite journal | doi = 10.1023/A:1024012015127 | year = 2003 | last1 = Budzikiewicz | first1 = H. | journal = Biodegradation | volume = 14 | issue = 2 | pages = 65–72 | pmid = 12877462 | title = Heteroaromatic monothiocarboxylic acids from Pseudomonas spp}}</ref> | The biosynthesis of PDTC remains unclear although some insights can be deduced from the genetics.<ref>{{cite journal | doi = 10.1186/1471-2148-2-8 | year = 2002 | last1 = Cortese | first1 = Marc S | last2 = Caplan | first2 = Allan B | last3 = Crawford | first3 = Ronald L | journal = BMC Evolutionary Biology | volume = 2 | pages = 8 | pmid = 11972321 | title = Structural, functional, and evolutionary analysis of moeZ, a gene encoding an enzyme required for the synthesis of the Pseudomonas metabolite, pyridine-2,6-bis(thiocarboxylic acid) | pmc = 115864 | doi-access = free }}</ref> It is suggested that ''Pseudomonas stutzeri'' may have acquired at least one of the genes by lateral transfer from ].<ref name = cortese>{{cite journal | doi = 10.1023/A:1015241925322 | year = 2002 | last1 = Cortese | first1 = Marc S. | last2 = Paszczynski | first2 = Andrzej | last3 = Lewis | first3 = Thomas A. | last4 = Sebat | first4 = Jonathan L. | last5 = Borek | first5 = Vladimir | last6 = Crawford | first6 = Ronald L. | journal = BioMetals | volume = 15 | issue = 2 | pages = 103–120 | pmid = 12046919 | title = Metal chelating properties of pyridine-2,6-bis(thiocarboxylic acid) produced by ''Pseudomonas'' spp. And the biological activities of the formed complexes| s2cid = 5545637 }}</ref> In a proposed biosynthetic sequence pyridine-2,6-dicarboxylic acid, a known bacterial metabolite,<ref name = cortese/> is activated as its bis-adenosine monophosphate (AMP) derivative. The sulfur donor and its activation remain uncertain.<ref name = budzi>{{cite journal | doi = 10.1023/A:1024012015127 | year = 2003 | last1 = Budzikiewicz | first1 = H. | journal = Biodegradation | volume = 14 | issue = 2 | pages = 65–72 | pmid = 12877462 | title = Heteroaromatic monothiocarboxylic acids from ''Pseudomonas'' spp| s2cid = 29898226 }}</ref> | ||
| ==Coordination chemistry== | ==Coordination chemistry== | ||
| ] | |||
| PDTC binds to both Fe<sup>2+</sup> and Fe<sup>3+</sup>. The ferric complex is brown, whereas the ferrous complex is blue. In the presence of air, the ferrous complex oxidizes to the ferric compound.{{ |
PDTC binds to both Fe<sup>2+</sup> and Fe<sup>3+</sup>. The ferric complex is brown, whereas the ferrous complex is blue. In the presence of air, the ferrous complex oxidizes to the ferric compound.<ref>Ockels, W., Roemer, A., Budzikiewicz, H., Korth, H., Pulverer, G., "Bacterial constituents. II. An iron(II) complex of pyridine-2,6-di-(monothiocarboxylic acid) - a novel bacterial metabolic product", Tetrahedron Lett. 1978, 3341. {{doi|10.1016/S0040-4039(01)85634-3}}</ref> It is iron selective<ref name = cortese/> as only the Fe complex is soluble in water. PDTC is produced mainly during the exponential phase of bacterial growth. The conditions at which ''Pseudomonas'' produces PDTC is 25 °C, pH=8 and sufficient aeration.<ref name = budzi/> | ||
| == See also == | == See also == | ||
| Line 53: | Line 60: | ||
| {{DEFAULTSORT:Pyridinedicarbothioic acid, 2,6-}} | {{DEFAULTSORT:Pyridinedicarbothioic acid, 2,6-}} | ||
| ] | ] | ||
| ] | ] | ||
Latest revision as of 17:54, 23 December 2024
 | |
 | |
| Names | |
|---|---|
| IUPAC name 2,6-Pyridinedicarbothioic acid | |
| Preferred IUPAC name Pyridine-2,6-bis(carbothioic S-acid) | |
| Other names PDTC, dithiopyridinedicarbothioic acid | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C7H5O2S2 |
| Molar mass | 185.24 g·mol |
| Appearance | White crystalline solid |
| Density | 1.415 g/cm |
| Melting point | 97 to 99 °C (207 to 210 °F; 370 to 372 K) |
| Boiling point | 404.4 °C (759.9 °F; 677.5 K) |
| Solubility in water | 1000 g/L (5.02 mol/L) |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | acidic |
| Flash point | 198.4 °C (389.1 °F; 471.5 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
2,6-Pyridinedicarbothioic acid (PDTC) is an organosulfur compound that is produced by some bacteria. It functions as a , a low molecular weight compound that scavenges iron. Siderophores solubilize compounds by forming strong complexes. PDTC is secreted by the soil bacteria Pseudomonas stutzeri and Pseudomonas putida.
Synthesis and biosynthesis
PDTC can be synthesized in the laboratory by treating the diacid dichloride of pyridine-2,6-dicarboxylic with H2S in pyridine:
- NC5H3(COCl)2 + 2 H2S + 2 C5H5N → [C5H5NH][HNC5H3(COS)−2] + [C5H5NH]Cl
This route produces the pyridinium salt of pyridinium-2,6-dicarbothioate. Treatment of this orange-colored salt with sulfuric acid gives colorless PDTC, which can then be extracted with dichloromethane.
The biosynthesis of PDTC remains unclear although some insights can be deduced from the genetics. It is suggested that Pseudomonas stutzeri may have acquired at least one of the genes by lateral transfer from mycobacteria. In a proposed biosynthetic sequence pyridine-2,6-dicarboxylic acid, a known bacterial metabolite, is activated as its bis-adenosine monophosphate (AMP) derivative. The sulfur donor and its activation remain uncertain.
Coordination chemistry

PDTC binds to both Fe and Fe. The ferric complex is brown, whereas the ferrous complex is blue. In the presence of air, the ferrous complex oxidizes to the ferric compound. It is iron selective as only the Fe complex is soluble in water. PDTC is produced mainly during the exponential phase of bacterial growth. The conditions at which Pseudomonas produces PDTC is 25 °C, pH=8 and sufficient aeration.
See also
References
- Budzikiewicz, Herbert (2010). "Microbial Siderophores". In Kinghorn, A. Douglas; Falk, Heinz; Kobayashi, Junichi (eds.). Fortschritte der Chemie organischer Naturstoffe / Progress in the Chemistry of Organic Natural Products, Vol. 92 [Progress in the Chemistry of Organic Natural Products]. Vol. 92. pp. 1–75. doi:10.1007/978-3-211-99661-4_1. ISBN 978-3-211-99660-7. PMID 20198464.
- Hildebrand, U.; Ockels, W.; Lex, J.; Budzikiewicz, H. (1983). "Zur Struktur Eines 1:1-Adduktes von Pyridin-2,6-Dicarbothiosäure und Pyridin". Phosphorus and Sulfur and the Related Elements. 16 (3): 361–364. doi:10.1080/03086648308080490.
- Cortese, Marc S; Caplan, Allan B; Crawford, Ronald L (2002). "Structural, functional, and evolutionary analysis of moeZ, a gene encoding an enzyme required for the synthesis of the Pseudomonas metabolite, pyridine-2,6-bis(thiocarboxylic acid)". BMC Evolutionary Biology. 2: 8. doi:10.1186/1471-2148-2-8. PMC 115864. PMID 11972321.
- ^ Cortese, Marc S.; Paszczynski, Andrzej; Lewis, Thomas A.; Sebat, Jonathan L.; Borek, Vladimir; Crawford, Ronald L. (2002). "Metal chelating properties of pyridine-2,6-bis(thiocarboxylic acid) produced by Pseudomonas spp. And the biological activities of the formed complexes". BioMetals. 15 (2): 103–120. doi:10.1023/A:1015241925322. PMID 12046919. S2CID 5545637.
- ^ Budzikiewicz, H. (2003). "Heteroaromatic monothiocarboxylic acids from Pseudomonas spp". Biodegradation. 14 (2): 65–72. doi:10.1023/A:1024012015127. PMID 12877462. S2CID 29898226.
- Hildebrand, U.; Lex, J.; Taraz, K.; Winkler, S.; Ockels, W.; Budzikiewicz, H. (1984). "Untersuchungen zum Redox-System Bis-(pyridin-2,6-dicarbothioato)-Ferrat(II) /-Ferrat(III) [1]". Zeitschrift für Naturforschung B. 39 (11): 1607–1613. doi:10.1515/znb-1984-1123. S2CID 94908888.
- Ockels, W., Roemer, A., Budzikiewicz, H., Korth, H., Pulverer, G., "Bacterial constituents. II. An iron(II) complex of pyridine-2,6-di-(monothiocarboxylic acid) - a novel bacterial metabolic product", Tetrahedron Lett. 1978, 3341. doi:10.1016/S0040-4039(01)85634-3