| Revision as of 14:20, 18 July 2011 editNono64 (talk | contribs)Autopatrolled, Pending changes reviewers, Rollbackers96,246 editsm Category:Phenolic compounds in wine← Previous edit | Latest revision as of 10:27, 25 November 2024 edit undoGraeme Bartlett (talk | contribs)Administrators250,307 edits chemspider | ||
| (18 intermediate revisions by 14 users not shown) | |||
| Line 1: | Line 1: | ||
| {{chembox | {{chembox | ||
| | Watchedfields = changed | |||
| | verifiedrevid = 442297034 | |||
| | Name = Castavinol C3 | | Name = Castavinol C3 | ||
| | ImageFile = Castavinol. |
| ImageFile = Castavinol 3.svg | ||
| | ImageSize = 200px | | ImageSize = 200px | ||
| | ImageName = Chemical structure of castavinol C3 | | ImageName = Chemical structure of castavinol C3 | ||
| Line 7: | Line 9: | ||
| | IUPACName = | | IUPACName = | ||
| | OtherNames = <!-- <br> --> | | OtherNames = <!-- <br> --> | ||
| |Section1= |
|Section1={{Chembox Identifiers | ||
| | CASNo = | | CASNo = 183607-17-2 | ||
| | CASNo_Ref = | | CASNo_Ref = {{cascite|correct|??}}= | ||
| | ChemSpiderID = 59694876 | |||
| | CASOther = | |||
| | UNII_Ref = {{fdacite|correct|FDA}} | |||
| ⚫ | | PubChem = | ||
| | UNII = ZHZ5SCW9G9 | |||
| ⚫ | | PubChem = 57515151 | ||
| | SMILES = OC2C(O)C(O)C(OC2CO)OC4C3C(C)(C(C)=O)OC4(c(cc1OC)cc(O)c1O)Oc5c3c(O)cc(O)c5 | | SMILES = OC2C(O)C(O)C(OC2CO)OC4C3C(C)(C(C)=O)OC4(c(cc1OC)cc(O)c1O)Oc5c3c(O)cc(O)c5 | ||
| | SMILES1 = COc1cc(23Oc4cc(O)ccc4(2O2O(CO)(O)(O)2O)C(C)(C(C)=O)O3)cc(O)c1O | |||
| | InChI = | |||
| | InChI=1S/C26H30O14/c1-9(28)25(2)18-17-12(30)6-11(29)7-14(17)39-26(40-25,10-4-13(31)19(32)15(5-10)36-3)23(18)38-24-22(35)21(34)20(33)16(8-27)37-24/h4-7,16,18,20-24,27,29-35H,8H2,1-3H3 | |||
| | InChIKey = ADFRCNLBRJARNV-UHFFFAOYSA-N | |||
| | InChIKey1 =ALSDFAORYWHNNX-UFFWUXHJSA-N | |||
| | MeSHName = | | MeSHName = | ||
| }} | }} | ||
| |Section2= |
|Section2={{Chembox Properties | ||
| | |
| C=26 | H=30 | O=14 | ||
| | MolarMass = 566.50 g/mol | |||
| | ExactMass = 566.163555 u | |||
| | Appearance = | | Appearance = | ||
| | Density = | | Density = | ||
| | MeltingPt = |
| MeltingPt = | ||
| | BoilingPt = |
| BoilingPt = | ||
| | Solubility = | | Solubility = | ||
| }} | }} | ||
| | |
|Section3={{Chembox Hazards | ||
| | MainHazards = | | MainHazards = | ||
| | FlashPt = | | FlashPt = | ||
| | |
| AutoignitionPt = | ||
| | GHSPictograms = | |||
| | RPhrases = <!-- {{R10}}, {{R23}}, {{R34}}, {{R50}} etc. --> | |||
| | GHSSignalWord = | |||
| | SPhrases = <!-- {{S1/2}}, {{S9}}, {{S16}}, {{S26}}, {{S36/37/39}}, {{S45}}, {{S61}} etc. --> | |||
| | HPhrases = {{HPhrases|}} | |||
| | PPhrases = {{PPhrases|}} | |||
| | GHS_ref = <!-- no GHS data in pubchem Dec2021 --> | |||
| }} | }} | ||
| }} | }} | ||
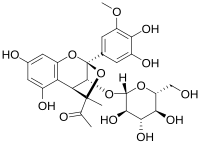
| ⚫ | '''Castavinol C3''' is a ], a natural phenolic compound found in ].<ref>Castavinol, a new series of polyphenols from Bordeaux red wines |
||
| ⚫ | '''Castavinol C3''' is a ], a natural phenolic compound found in ]s.<ref>{{cite journal | doi = 10.1016/0040-4039(96)01761-3| title = Castavinol, a new series of polyphenols from Bordeaux red wines| date = 1996| last1 = Castagnino| first1 = Chantal| last2 = Vercauteren| first2 = Joseph| journal = Tetrahedron Letters| volume = 37| issue = 43| pages = 7739–7742}}</ref> | ||
| ⚫ | ==See also== | ||
| ⚫ | == See also == | ||
| * ] | * ] | ||
| ==References== | == References == | ||
| {{reflist}} | {{reflist}} | ||
| ==External links== | == External links == | ||
| * (French) | * {{Webarchive|url=https://web.archive.org/web/20111004120343/http://www.socpharmbordeaux.asso.fr/pdf/pdf-136/136-019-036.pdf |date=2011-10-04 }} (French) | ||
| ] | ] | ||
| ] | |||
| {{Natural-phenol-stub}} | |||
| {{aromatic-stub}} | |||
Latest revision as of 10:27, 25 November 2024
 | |
| Identifiers | |
|---|---|
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C26H30O14 |
| Molar mass | 566.512 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Castavinol C3 is a castavinol, a natural phenolic compound found in red wines.
See also
References
- Castagnino, Chantal; Vercauteren, Joseph (1996). "Castavinol, a new series of polyphenols from Bordeaux red wines". Tetrahedron Letters. 37 (43): 7739–7742. doi:10.1016/0040-4039(96)01761-3.
External links
- 1996 : Les molécules des futurs millésimes Bordelais ? C. Castagnino, C. Chèze and J. Vercauteren, Bull. Soc. Pharm. Bordeaux, 1997, 136, pp. 19-36 Archived 2011-10-04 at the Wayback Machine (French)
This article about an aromatic compound is a stub. You can help Misplaced Pages by expanding it. |