| Revision as of 22:21, 6 August 2011 editBeetstra (talk | contribs)Edit filter managers, Administrators172,128 edits Script assisted update of identifiers for the Chem/Drugbox validation project (updated: 'ChEBI').← Previous edit | Latest revision as of 04:51, 23 October 2024 edit undoCitation bot (talk | contribs)Bots5,461,325 edits Added publisher. | Use this bot. Report bugs. | Suggested by Dominic3203 | Category:Diketones | #UCB_Category 205/206 | ||
| (77 intermediate revisions by 55 users not shown) | |||
| Line 1: | Line 1: | ||
| {{distinguish|benzyl}} | {{distinguish|benzyl}} | ||
| {{ |
{{Chembox | ||
| |Watchedfields = changed | |||
| | verifiedrevid = 410983115 | |||
| |verifiedrevid = 443415454 | |||
| | Name = Benzil | |||
| |Name = Benzil | |||
| | ImageFile_Ref = {{chemboximage|correct|??}} | |||
| |ImageFile_Ref = {{chemboximage|correct|??}} | |||
| | ImageFile = Benzil-2D-skeletal.png | |||
| | |
|ImageFile = Benzil.svg | ||
| | |
|ImageName = Benzil | ||
| |ImageFile1 = Benzil-from-LT-monoclinic-xtal-CM-3D-balls.png | |||
| | |
|ImageFile2 = Benzil-from-LT-monoclinic-xtal-CM-3D-SF.png | ||
| |PIN = Diphenylethanedione <!-- Nomenclature of Organic Chemistry – IUPAC Recommendations and Preferred Names 2013 (Blue Book) --> | |||
| | IUPACName = 1,2-diphenylethane-1,2-dione | |||
| |SystematicName = 1,2-Diphenylethane-1,2-dione | |||
| | OtherNames = dibenzoyl<br />bibenzoyl<br />diphenylglyoxal | |||
| |OtherNames = Diphenylethane-1,2-dione<br />Benzil<br />Dibenzoyl<br />Bibenzoyl<br />Diphenylglyoxal | |||
| | Section1 = {{Chembox Identifiers | |||
| |Section1={{Chembox Identifiers | |||
| | ChEBI = 51507 | |||
| |ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| | SMILES = O=C(C(=O)c1ccccc1)c2ccccc2 | |||
| |ChEBI = 51507 | |||
| | InChI = 1/C14H10O2/c15-13(11-7-3-1-4-8-11)14(16)12-9-5-2-6-10-12/h1-10H | |||
| |SMILES = O=C(C(=O)c1ccccc1)c2ccccc2 | |||
| | InChIKey = WURBFLDFSFBTLW-UHFFFAOYAZ | |||
| |InChI = 1/C14H10O2/c15-13(11-7-3-1-4-8-11)14(16)12-9-5-2-6-10-12/h1-10H | |||
| | SMILES1 = c1ccc(cc1)C(=O)C(=O)c2ccccc2 | |||
| |InChIKey = WURBFLDFSFBTLW-UHFFFAOYAZ | |||
| | ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| |SMILES1 = c1ccccc1C(=O)C(=O)c2ccccc2 | |||
| | ChEMBL = 189886 | |||
| | |
|ChEMBL_Ref = {{ebicite|correct|EBI}} | ||
| |ChEMBL = 189886 | |||
| | StdInChI = 1S/C14H10O2/c15-13(11-7-3-1-4-8-11)14(16)12-9-5-2-6-10-12/h1-10H | |||
| | |
|StdInChI_Ref = {{stdinchicite|correct|chemspider}} | ||
| |StdInChI = 1S/C14H10O2/c15-13(11-7-3-1-4-8-11)14(16)12-9-5-2-6-10-12/h1-10H | |||
| | StdInChIKey = WURBFLDFSFBTLW-UHFFFAOYSA-N | |||
| | |
|StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | ||
| |StdInChIKey = WURBFLDFSFBTLW-UHFFFAOYSA-N | |||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| |ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| | ChemSpiderID=8329 | |||
| |ChemSpiderID = 8329 | |||
| | CASNo = 134-81-6 | |||
| |CASNo = 134-81-6 | |||
| | PubChem = 8651 | |||
| |CASNo_Ref = {{cascite|correct|CAS}} | |||
| | RTECS = | |||
| |UNII_Ref = {{fdacite|correct|FDA}} | |||
| }} | |||
| |UNII = S85X61172J | |||
| | Section2 = {{Chembox Properties | |||
| |PubChem = 8651 | |||
| |C=14|H=10|O=2 | |||
| |EC_number = 205-157-0 | |||
| | Appearance = yellow crystals or powder | |||
| |RTECS = DD1925000 | |||
| | Density = 1.23 g/cm<sup>3</sup>, solid (1.255 g/cm<sup>3</sup>, x-ray) | |||
| |Beilstein = 608047 | |||
| | Solubility = insoluble | |||
| }} | |||
| | Solvent = ] | |||
| |Section2={{Chembox Properties | |||
| | SolubleOther = soluble | |||
| |C=14 | H=10 | O=2 | |||
| | Solvent = ] | |||
| |Appearance = yellow crystalline powder | |||
| | SolubleOther = soluble | |||
| |Density = 1.23 g/cm<sup>3</sup>, solid (1.255 g/cm<sup>3</sup>, x-ray) | |||
| | Solvent = ] | |||
| |Solubility = insoluble | |||
| | SolubleOther = soluble | |||
| |Solvent1 = ethanol | |||
| | MeltingPt = 94.43-95.08 °C, 201.97-203.14 °F, 367-368 K | |||
| |Solubility1 = soluble | |||
| | BoilingPt = 346- 348 °C, 654.8-658.4 °F, 619-621 K | |||
| |Solvent2 = diethyl ether | |||
| }} | |||
| |Solubility2 = soluble | |||
| | Section3 = {{Chembox Structure | |||
| |Solvent3 = benzene | |||
| | CrystalStruct = P3<sub>1,2</sub>21<ref>''Acta Cryst.'' B43 398 (1987)</ref> | |||
| |Solubility3 = soluble | |||
| | Dipole = 3.8 ]<ref>''Spectrochim. Acta'' A60 (8-9) 1805 (2004)</ref> | |||
| |MeltingPtF = 201.2 to 204.8 | |||
| }} | |||
| |BoilingPtF = 654.8 to 658.4 | |||
| | Section7 = {{Chembox Hazards | |||
| |MagSus = -118.6·10<sup>−6</sup> cm<sup>3</sup>/mol | |||
| | ExternalMSDS = | |||
| }} | |||
| | MainHazards = ] | |||
| |Section3={{Chembox Structure | |||
| | FlashPt = | |||
| |CrystalStruct = P3<sub>1,2</sub>21<ref>''Acta Crystallogr.'' B43 398 (1987)</ref> | |||
| | RPhrases = | |||
| |Dipole = 3.8 ]<ref>''Spectrochim. Acta'' A60 (8-9) 1805 (2004)</ref> | |||
| | SPhrases = | |||
| }} | |||
| | NFPA-H = 2 | |||
| |Section7={{Chembox Hazards | |||
| | NFPA-F = 1 | |||
| |MainHazards = ] | |||
| | NFPA-R = 0 | |||
| | |
|NFPA-H = 2 | ||
| |NFPA-F = 1 | |||
| }} | |||
| |NFPA-R = 0 | |||
| | Section8 = {{Chembox Related | |||
| |GHSPictograms = {{GHS07}} | |||
| | Function = ]s | |||
| |GHSSignalWord = Warning | |||
| | OtherFunctn = ] | |||
| |HPhrases = {{H-phrases|315|319|335}} | |||
| | OtherCpds = ]<br />]<br />benzil | |||
| |PPhrases = {{P-phrases|261|264|271|280|302+352|304+340|305+351+338|312|321|332+313|337+313|362|403+233|405|501}} | |||
| }} | |||
| |LD50 = >3 g/kg (mouse, oral)<ref>{{cite web |title=Benzil |url=https://pubchem.ncbi.nlm.nih.gov/compound/Benzil#section=Toxicity |language=en}}</ref> | |||
| }} | |||
| |Section8={{Chembox Related | |||
| |OtherFunction_label = ]s | |||
| |OtherFunction = ] | |||
| |OtherCompounds = ]<br />]<br />] | |||
| }} | |||
| }} | }} | ||
| '''Benzil''' (systematically known as 1,2-diphenylethane-1,2-dione) is the ] with the formula (]])<sub>2</sub>, generally abbreviated (]CO)<sub>2</sub>. This yellow solid is one of the most common ]s. Its main use is as a ] in ].<ref name=Ullmann>Hardo Siegel, Manfred Eggersdorfer "Ketones" in Ullmann's Encyclopedia of Industrial Chemistry Wiley-VCH, 2002 by Wiley-VCH, Wienheim. {{DOI|10.1002/14356007.a15_077}}</ref> | |||
| '''Benzil''' (i.e. '''Bz<sub>2</sub>''', systematically known as 1,2-diphenylethane-1,2-dione) is the ] with the formula (]])<sub>2</sub>, generally abbreviated (]CO)<sub>2</sub>. This yellow solid is one of the most common ]s. Its main use is as a ] in ].<ref name=Ullmann>Hardo Siegel, Manfred Eggersdorfer "Ketones" in Ullmann's Encyclopedia of Industrial Chemistry Wiley-VCH, 2002 by Wiley-VCH, Weinheim. {{doi|10.1002/14356007.a15_077}}</ref> | |||
| ==Structure== | |||
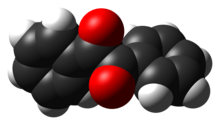
| The compound's most noteworthy structural feature is the long ] of 1.54 ], which indicates the absence of pi-bonding between the two carbonyl centers. The PhCO centers are planar, but the pair of benzoyl groups are twisted with respect to the other with a ] of 117°.<ref>Quang. Shen, Kolbjoern. Hagen "Gas-phase molecular structure and conformation of benzil as determined by electron diffraction" J. Phys. Chem., 1987, 91 (6), pp 1357–1360. {{DOI|10.1021/j100290a017}}.</ref> In less ] analogues (], ], ] derivatives), the (RCO)<sub>2</sub> groups adopts a planar, anti-conformation. | |||
| == |
== Structure == | ||
| The compound's most noteworthy structural feature is the long ] of 1.54 ], which indicates the absence of pi-bonding between the two carbonyl centers. The PhCO centers are planar, but the pair of benzoyl groups are twisted with respect to the other with a ] of 117°.<ref>Quang. Shen, Kolbjoern. Hagen "Gas-phase molecular structure and conformation of benzil as determined by electron diffraction" J. Phys. Chem., 1987, 91 (6), pp 1357–1360. {{doi|10.1021/j100290a017}}.</ref> In less ] analogues (], ], ] derivatives), the (RCO)<sub>2</sub> group adopts a planar, anti-conformation. | |||
| Most benzil is consumed for use in the free-radical ] of ] networks. ] radiation decomposes benzil, generating free-radical species within the material, promoting the formation of ]s. | |||
| == |
== Applications == | ||
| Most benzil can be used as a photoinitiator in the free-radical ] of ] networks. It absorbs ] radiation at a wavelength of 260 nm, leading to decomposition with formation of free-radical species and formation of ]s within the material. However, it is a relatively poor photoinitiator, and is seldom used. It undergoes ], which allows the curing light to reach deeper layers of the material on longer exposure.<ref name="Green2010">{{Cite book | url=https://books.google.com/books?id=b1jRBQAAQBAJ&dq=benzil+photoinitiator&pg=PA31 |page= 31 |title = Industrial Photoinitiators: A Technical Guide|isbn = 9781439827468|last1 = Green|first1 = W. Arthur |date = 2010-04-22 |publisher= CRC Press |access-date=2022-05-21}}</ref> ] derivatives, such as ], have better properties for this application.<ref name="Green2010"/> | |||
| Benzil is a standard building block in ]. It condenses with amines to give ]s ligands. A classic ] of benzil is the ], in which base catalyses the conversion of benzil to benzilic acid. This reactivity is exploited in the preparation of the drug ]. Benzil also reacts with ] in an ] to give ]. | |||
| Benzil is a potent ] of human ]s, ]s involved in the hydrolysis of carboxylesters and many clinically used drugs.<ref>Wadkins. R. M. et al "Identification and characterization of novel benzil (diphenylethane-1,2-dione) analogues as inhibitors of mammalian carboxylesterases. J. Med. Chem., 2005 48 pp 2906–15.</ref> | |||
| ==Preparation== | |||
| Benzil is prepared from ], which in turn is easily obtained via the ] from ].<ref>{{OrgSynth | author = Clarke, H. T.; Dreger.E. E. | title = Benzil | collvol = 1 | collvolpages = 87 | year = 1941 | prep = cv1p0087}}</ref> | |||
| :PhC(O)CH(OH)Ph + 2 Cu<sup>2+</sup> → PhC(O)C(O)Ph + 2 H<sup>+</sup> + 2 Cu<sup>+</sup> | |||
| == |
=== Reactions === | ||
| Benzil is a standard building block in ]. It condenses with amines to give ] ligands. A classic ] of benzil is the ], in which base catalyses the conversion of benzil to ]. This reactivity is exploited in the preparation of the drug ]. Benzil also reacts with ] in an ] to give ]. | |||
| <references/> | |||
| == Preparation == | |||
| ] | |||
| Benzil is prepared from ], for example with ]:<ref>{{cite journal|doi=10.1021/ed065p553|title=Synthesis of benzil from benzoin with copper(II) acetate|journal=Journal of Chemical Education|volume=65|issue=6|pages=553|year=1988|last1=Depreux|first1=P.|last2=Bethegnies|first2=G.|last3=Marcincal-Lefebvre|first3=A.|bibcode=1988JChEd..65..553D}}</ref> | |||
| :PhC(O)CH(OH)Ph + 2 Cu<sup>2+</sup> → PhC(O)C(O)Ph + 2 H<sup>+</sup> + 2 Cu<sup>+</sup> | |||
| Other suitable oxidizing agents such as ] (HNO<sub>3</sub>) are used routinely. | |||
| ] | |||
| ] | |||
| ] (FeCl<sub>3</sub>) can be used as an inexpensive catalyst for this chemical conversion.<ref>{{cite journal|doi=10.4067/S0717-97072011000200008|title=One-Pot Synthesis Benzils from Aldehydes Via Nhc-Catalyzed Benzoin Dimerization Under Metal-Free Conditions in Water|journal=Journal of the Chilean Chemical Society|volume=56|issue=2|pages=663|year=2011|last1=Bi|first1=Xiaoxin|last2=Wu|first2=Lintao|last3=Yan|first3=Chaoguo|last4=Jing|first4=Xiaobi|last5=Zhu|first5=Hongxiang|doi-access=free}}</ref> | |||
| ] | |||
| ] | |||
| == References == | |||
| ] | |||
| <references /> | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 04:51, 23 October 2024
Not to be confused with benzyl.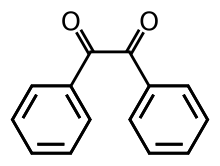 | |
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name Diphenylethanedione | |
| Systematic IUPAC name 1,2-Diphenylethane-1,2-dione | |
| Other names
Diphenylethane-1,2-dione Benzil Dibenzoyl Bibenzoyl Diphenylglyoxal | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| Beilstein Reference | 608047 |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.004.689 |
| EC Number |
|
| PubChem CID | |
| RTECS number |
|
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C14H10O2 |
| Molar mass | 210.232 g·mol |
| Appearance | yellow crystalline powder |
| Density | 1.23 g/cm, solid (1.255 g/cm, x-ray) |
| Melting point | 94.0 to 96.0 °C; 201.2 to 204.8 °F; 367.1 to 369.2 K |
| Boiling point | 346.0 to 348.0 °C; 654.8 to 658.4 °F; 619.1 to 621.1 K |
| Solubility in water | insoluble |
| Solubility in ethanol | soluble |
| Solubility in diethyl ether | soluble |
| Solubility in benzene | soluble |
| Magnetic susceptibility (χ) | -118.6·10 cm/mol |
| Structure | |
| Crystal structure | P31,221 |
| Dipole moment | 3.8 D |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | Irritant |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 |
| NFPA 704 (fire diamond) |
 |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) | >3 g/kg (mouse, oral) |
| Related compounds | |
| Related diketones | diacetyl |
| Related compounds | benzophenone glyoxal bibenzil |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Benzil (i.e. Bz2, systematically known as 1,2-diphenylethane-1,2-dione) is the organic compound with the formula (C6H5CO)2, generally abbreviated (PhCO)2. This yellow solid is one of the most common diketones. Its main use is as a photoinitiator in polymer chemistry.
Structure
The compound's most noteworthy structural feature is the long carbon-carbon bond of 1.54 Å, which indicates the absence of pi-bonding between the two carbonyl centers. The PhCO centers are planar, but the pair of benzoyl groups are twisted with respect to the other with a dihedral angle of 117°. In less hindered analogues (glyoxal, biacetyl, oxalic acid derivatives), the (RCO)2 group adopts a planar, anti-conformation.
Applications
Most benzil can be used as a photoinitiator in the free-radical curing of polymer networks. It absorbs ultraviolet radiation at a wavelength of 260 nm, leading to decomposition with formation of free-radical species and formation of cross-links within the material. However, it is a relatively poor photoinitiator, and is seldom used. It undergoes photobleaching, which allows the curing light to reach deeper layers of the material on longer exposure. Acetal derivatives, such as 2,2-dimethoxy-2-phenylacetophenone, have better properties for this application.
Benzil is a potent inhibitor of human carboxylesterases, enzymes involved in the hydrolysis of carboxylesters and many clinically used drugs.
Reactions
Benzil is a standard building block in organic synthesis. It condenses with amines to give diketimine ligands. A classic organic reaction of benzil is the benzilic acid rearrangement, in which base catalyses the conversion of benzil to benzilic acid. This reactivity is exploited in the preparation of the drug phenytoin. Benzil also reacts with 1,3-diphenylacetone in an aldol condensation to give tetraphenylcyclopentadienone.
Preparation
Benzil is prepared from benzoin, for example with copper(II) acetate:
- PhC(O)CH(OH)Ph + 2 Cu → PhC(O)C(O)Ph + 2 H + 2 Cu
Other suitable oxidizing agents such as nitric acid (HNO3) are used routinely.
Iron(III) chloride (FeCl3) can be used as an inexpensive catalyst for this chemical conversion.
References
- Acta Crystallogr. B43 398 (1987)
- Spectrochim. Acta A60 (8-9) 1805 (2004)
- "Benzil".
- Hardo Siegel, Manfred Eggersdorfer "Ketones" in Ullmann's Encyclopedia of Industrial Chemistry Wiley-VCH, 2002 by Wiley-VCH, Weinheim. doi:10.1002/14356007.a15_077
- Quang. Shen, Kolbjoern. Hagen "Gas-phase molecular structure and conformation of benzil as determined by electron diffraction" J. Phys. Chem., 1987, 91 (6), pp 1357–1360. doi:10.1021/j100290a017.
- ^ Green, W. Arthur (2010-04-22). Industrial Photoinitiators: A Technical Guide. CRC Press. p. 31. ISBN 9781439827468. Retrieved 2022-05-21.
- Wadkins. R. M. et al "Identification and characterization of novel benzil (diphenylethane-1,2-dione) analogues as inhibitors of mammalian carboxylesterases. J. Med. Chem., 2005 48 pp 2906–15.
- Depreux, P.; Bethegnies, G.; Marcincal-Lefebvre, A. (1988). "Synthesis of benzil from benzoin with copper(II) acetate". Journal of Chemical Education. 65 (6): 553. Bibcode:1988JChEd..65..553D. doi:10.1021/ed065p553.
- Bi, Xiaoxin; Wu, Lintao; Yan, Chaoguo; Jing, Xiaobi; Zhu, Hongxiang (2011). "One-Pot Synthesis Benzils from Aldehydes Via Nhc-Catalyzed Benzoin Dimerization Under Metal-Free Conditions in Water". Journal of the Chilean Chemical Society. 56 (2): 663. doi:10.4067/S0717-97072011000200008.