| Revision as of 07:35, 23 September 2011 editCheMoBot (talk | contribs)Bots141,565 edits Updating {{chembox}} (no changed fields - added verified revid - updated 'DrugBank_Ref', 'UNII_Ref', 'ChEMBL_Ref', 'ChEBI_Ref', 'KEGG_Ref') per Chem/Drugbox validation (report errors or [[user← Previous edit | Latest revision as of 18:00, 9 July 2024 edit undoSmokefoot (talk | contribs)Autopatrolled, Extended confirmed users, Pending changes reviewers, Rollbackers75,107 edits dithionate | ||
| (30 intermediate revisions by 21 users not shown) | |||
| Line 1: | Line 1: | ||
| {{Chembox | {{Chembox | ||
| | verifiedrevid = |
| verifiedrevid = 451984881 | ||
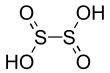
| | ImageFileL1 = Dithionous acid.svg | | ImageFileL1 = Dithionous acid.svg | ||
| | ImageSizeL1 = 120px | |||
| | ImageFileR1 = Dithionous-acid-3D-balls.png | | ImageFileR1 = Dithionous-acid-3D-balls.png | ||
| | ImageSizeR1 = 120px | |||
| | IUPACName = Dithionous acid | | IUPACName = Dithionous acid | ||
| | OtherNames = Hydrosulfurous acid; Hyposulfurous acid | | OtherNames = Hydrosulfurous acid; Hyposulfurous acid | ||
| | |
|Section1={{Chembox Identifiers | ||
| | |
| CASNo = 15959-26-9 | ||
| | |
| CASNo_Ref = {{cascite|correct|??}} | ||
| | |
| PubChem = 24490 | ||
| | |
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | ChemSpiderID = 22898 | | ChemSpiderID = 22898 | ||
| | |
| ChEBI_Ref = {{ebicite|correct|EBI}} | ||
| | ChEBI = 29253 | | ChEBI = 29253 | ||
| | SMILES = O=S(O)S(=O)O | | SMILES = O=S(O)S(=O)O | ||
| | |
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChI = 1S/H2O4S2/c1-5(2)6(3)4/h(H,1,2)(H,3,4) | | StdInChI = 1S/H2O4S2/c1-5(2)6(3)4/h(H,1,2)(H,3,4) | ||
| | |
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChIKey = GRWZHXKQBITJKP-UHFFFAOYSA-N | | StdInChIKey = GRWZHXKQBITJKP-UHFFFAOYSA-N | ||
| | |
| InChI = 1/H2O4S2/c1-5(2)6(3)4/h(H,1,2)(H,3,4) | ||
| | |
| InChIKey = GRWZHXKQBITJKP-UHFFFAOYAX | ||
| }} | }} | ||
| | |
|Section2={{Chembox Properties | ||
| | |
| Formula = H<sub>2</sub>S<sub>2</sub>O<sub>4</sub> | ||
| | |
| MolarMass = 130.144 g/mol | ||
| | |
| Appearance = | ||
| | |
| Density = | ||
| | |
| MeltingPt = | ||
| | |
| BoilingPt = | ||
| | |
| Solubility = | ||
| | ConjugateBase = ] | |||
| | pKa = 0.35, 2.45 <ref name="InorgChem">{{cite book | |||
| | title = Inorganic Chemistry, 3rd Edition | |||
| | chapter = Chapter 16: The group 16 elements | |||
| | author1 = Catherine E. Housecroft | |||
| | author2 = Alan G. Sharpe | |||
| | publisher = Pearson | |||
| | year = 2008 | |||
| | isbn = 978-0-13-175553-6 | |||
| | page = 520 | |||
| }}</ref> | |||
| }} | }} | ||
| | |
|Section3={{Chembox Hazards | ||
| | |
| MainHazards = | ||
| | |
| FlashPt = | ||
| | |
| AutoignitionPt = | ||
| }} | |||
| |Section4={{Chembox Related | |||
| | OtherCompounds = ]<br />]<br />] | |||
| }} | }} | ||
| }} | }} | ||
| '''Dithionous acid''' is a ] with the chemical formula H<sub>2</sub>S<sub>2</sub>O<sub>4</sub>. It |
'''Dithionous acid''' is a ] with the chemical formula H<sub>2</sub>S<sub>2</sub>O<sub>4</sub>. It has not been observed experimentally.<ref name="drozdova">{{cite journal | doi = 10.1021/jp972658d | title = Structures and Energies of Various Isomers of Dithionous Acid, H2S2O4, and of Its Anion HS2O4- 1 | year = 1998 | last1 = Drozdova | first1 = Yana | last2 = Steudel | first2 = Ralf | last3 = Hertwig | first3 = Roland H. | last4 = Koch | first4 = Wolfram | last5 = Steiger | first5 = Thomas | journal = The Journal of Physical Chemistry A | volume = 102 | issue = 6 | pages = 990–996 | bibcode = 1998JPCA..102..990D}}</ref> It is the ] of the ] dianion, S<sub>2</sub>O<sub>4</sub><sup>2-</sup>. Dithionite is a well-known reducing agent. | ||
| == |
==Related compounds== | ||
| *] (H<sub>2</sub>S<sub>2</sub>O<sub>6</sub>), another unstable protonated sulfur oxide, derived from dithionate. | |||
| * ] | |||
| * ] | |||
| ==References== | ==References== | ||
| {{reflist}} | {{reflist}} | ||
| ⚫ | ] | ||
| ⚫ | {{inorganic-compound-stub}} | ||
| ⚫ | ] | ||
| ] | ] | ||
| ] | |||
| ⚫ | {{inorganic-compound-stub}} | ||
Latest revision as of 18:00, 9 July 2024
| |||
| Names | |||
|---|---|---|---|
| IUPAC name Dithionous acid | |||
| Other names Hydrosulfurous acid; Hyposulfurous acid | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChEBI | |||
| ChemSpider | |||
| PubChem CID | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | H2S2O4 | ||
| Molar mass | 130.144 g/mol | ||
| Acidity (pKa) | 0.35, 2.45 | ||
| Conjugate base | Dithionite | ||
| Related compounds | |||
| Related compounds | Oxalic acid Sodium dithionite Potassium dithionite | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Dithionous acid is a sulfur oxoacid with the chemical formula H2S2O4. It has not been observed experimentally. It is the conjugate base of the dithionite dianion, S2O4. Dithionite is a well-known reducing agent.
Related compounds
- Dithionic acid (H2S2O6), another unstable protonated sulfur oxide, derived from dithionate.
References
- Catherine E. Housecroft; Alan G. Sharpe (2008). "Chapter 16: The group 16 elements". Inorganic Chemistry, 3rd Edition. Pearson. p. 520. ISBN 978-0-13-175553-6.
- Drozdova, Yana; Steudel, Ralf; Hertwig, Roland H.; Koch, Wolfram; Steiger, Thomas (1998). "Structures and Energies of Various Isomers of Dithionous Acid, H2S2O4, and of Its Anion HS2O4- 1". The Journal of Physical Chemistry A. 102 (6): 990–996. Bibcode:1998JPCA..102..990D. doi:10.1021/jp972658d.
This inorganic compound–related article is a stub. You can help Misplaced Pages by expanding it. |