| Revision as of 17:59, 25 November 2011 editCheMoBot (talk | contribs)Bots141,565 edits Updating {{drugbox}} (changes to verified fields - added verified revid - updated '') per Chem/Drugbox validation (report errors or bugs)← Previous edit | Latest revision as of 03:53, 12 January 2025 edit undoArthurfragoso (talk | contribs)Extended confirmed users, Template editors4,591 edits dark mode fix | ||
| (95 intermediate revisions by 61 users not shown) | |||
| Line 1: | Line 1: | ||
| {{short description|First of the synthetic quinolone antibiotics}} | |||
| {{Drugbox | |||
| {{Infobox drug | |||
| | |
| Watchedfields = changed | ||
| | verifiedrevid = 462258277 | | verifiedrevid = 462258277 | ||
| | IUPAC_name = 1- |
| IUPAC_name = 1-Ethyl-7-methyl-4-oxo-naphthyridine-3-carboxylic acid | ||
| | image = Nalidixic |
| image = Nalidixic Acid Structure.svg | ||
| | image_class = skin-invert-image | |||
| <!--Clinical data--> | <!--Clinical data--> | ||
| | tradename = | | tradename = NegGram, Wintomylon, others | ||
| | Drugs.com = {{drugs.com|CDI|nalidixic_acid}} | | Drugs.com = {{drugs.com|CDI|nalidixic_acid}} | ||
| | pregnancy_US = B | |||
| | pregnancy_category = B <small>]</small> | |||
| | legal_US = Not FDA approved | |||
| | legal_status = | |||
| | routes_of_administration = Oral | | routes_of_administration = Oral | ||
| <!--Pharmacokinetic data--> | <!--Pharmacokinetic data--> | ||
| | bioavailability = | | bioavailability = | ||
| Line 17: | Line 17: | ||
| | metabolism = Partially Hepatic | | metabolism = Partially Hepatic | ||
| | elimination_half-life = 6-7 hours, significantly longer in ] impairment | | elimination_half-life = 6-7 hours, significantly longer in ] impairment | ||
| <!--Identifiers--> | <!--Identifiers--> | ||
| | CASNo_Ref = {{cascite|correct|CAS}} | |||
| | CAS_number_Ref = {{cascite|correct|??}} | | CAS_number_Ref = {{cascite|correct|??}} | ||
| | CAS_number = 389-08-2 | | CAS_number = 389-08-2 | ||
| Line 26: | Line 24: | ||
| | ATC_supplemental = | | ATC_supplemental = | ||
| | PubChem = 4421 | | PubChem = 4421 | ||
| | DrugBank_Ref = {{drugbankcite| |
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | ||
| | DrugBank = |
| DrugBank = DB00779 | ||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | ChemSpiderID = 4268 | | ChemSpiderID = 4268 | ||
| Line 38: | Line 36: | ||
| | ChEMBL_Ref = {{ebicite|correct|EBI}} | | ChEMBL_Ref = {{ebicite|correct|EBI}} | ||
| | ChEMBL = 5 | | ChEMBL = 5 | ||
| <!--Chemical data--> | <!--Chemical data--> | ||
| | C=12 | H=12 | N=2 | O=3 |
| C=12 | H=12 | N=2 | O=3 | ||
| | molecular_weight = 232.235 g/mol | |||
| | smiles = O=C\2c1c(nc(cc1)C)N(/C=C/2C(=O)O)CC | | smiles = O=C\2c1c(nc(cc1)C)N(/C=C/2C(=O)O)CC | ||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | ||
| Line 48: | Line 44: | ||
| | StdInChIKey = MHWLWQUZZRMNGJ-UHFFFAOYSA-N | | StdInChIKey = MHWLWQUZZRMNGJ-UHFFFAOYSA-N | ||
| }} | }} | ||
| '''Nalidixic acid''' (tradenames '''Nevigramon''', '''Neggram''', '''Wintomylon''' and '''WIN 18,320''') is the first of the synthetic ] ]s. In the technical sense, it is a naphthyridone, not a quinolone: its ring structure is a 1,8-naphthyridines nucleus that contains two nitrogen atoms, unlike ], which has a single nitrogen atom.<ref name=jac2003>{{Cite doi|10.1093/jac/dkg208}}</ref> | |||
| '''Nalidixic acid''' (tradenames '''Nevigramon''', '''NegGram''', '''Wintomylon''' and '''WIN 18,320''') is the first of the synthetic ]s. | |||
| Synthetic quinolone antibiotics were discovered by George Lesher and coworkers as a byproduct of ] manufacture in the 1960s.<ref name=jac2003 /> | |||
| In a technical sense, it is a naphthyridone, not a quinolone: its ring structure is a ] nucleus that contains two nitrogen atoms, unlike quinoline, which has a single nitrogen atom.<ref name=jac2003>{{cite journal | vauthors = Emmerson AM, Jones AM | title = The quinolones: decades of development and use | journal = The Journal of Antimicrobial Chemotherapy | volume = 51 | issue = Suppl 1 | pages = 13–20 | date = May 2003 | pmid = 12702699 | doi = 10.1093/jac/dkg208 | doi-access = free }}</ref> | |||
| ⚫ | Nalidixic acid is effective against |
||
| Synthetic quinolone antibiotics were discovered by George Lesher and coworkers as a byproduct of ] manufacture in the 1960s;<ref>{{cite journal | vauthors = Lesher GY, Froelich EJ, Gruett MD, Bailey JH, Brundage RP | title = 1,8-Naphthyridine Derivatives. A New Class of Chemotherapeutic Agents | journal = Journal of Medicinal and Pharmaceutical Chemistry | volume = 5 | issue = 5 | pages = 1063–1065 | date = September 1962 | pmid = 14056431 | doi = 10.1021/jm01240a021 }}</ref> nalidixic acid itself was used clinically, starting in 1967. | |||
| It is especially used in treating ], caused, for example, by '']'', '']'', '']'', '']'', and '']''.. | |||
| ⚫ | It is also a tool in studies as a regulation of bacterial division. It selectively and reversibly blocks DNA replication in susceptible bacteria. Nalidixic acid and related antibiotics inhibit a subunit of ] and induce formation of |
||
| It is the only FDA approved quinolone for treating UTI infections in children (3). | |||
| ⚫ | Nalidixic acid is effective primarily against ], with minor anti-] activity. In lower concentrations, it acts in a ] manner; that is, it inhibits growth and reproduction. In higher concentrations, it is bactericidal, meaning that it kills bacteria instead of merely inhibiting their growth. | ||
| ⚫ | ==Adverse effects== | ||
| ] and ]<ref>{{cite journal |author=Fraser AG, Harrower AD |title=Convulsions and hyperglycaemia associated with nalidixic acid |journal=Br Med J |volume=2 |issue=6101 |pages=1518 |year=1977 |month=December |pmid=589309 |pmc=1632822 |doi= 10.1136/bmj.2.6101.1518 }}</ref> | |||
| It has historically been used for treating ], caused, for example, by '']'', '']'', '']'', '']'', and '']''. It is no longer clinically used for this indication in the US as less toxic and more effective agents are available. The marketing authorization for nalidixic acid has been suspended throughout the EU.<ref>{{Cite web|url=https://www.ema.europa.eu/en/medicines/human/referrals/quinolone-fluoroquinolone-containing-medicinal-products|title=Disabling and potentially permanent side effects lead to suspension or restrictions of quinolone and fluoroquinolone antibiotics|date=11 March 2019|website=European Medicines Agency}}</ref> | |||
| ⚫ | ==See also== | ||
| *] | |||
| *] | |||
| *] | |||
| ⚫ | It is also a tool in studies as a regulation of bacterial division. It selectively and reversibly blocks DNA replication in susceptible bacteria. Nalidixic acid and related antibiotics inhibit a subunit of ] and ] and induce formation of cleavage complexes.<ref>{{cite journal | vauthors = Pommier Y, Leo E, Zhang H, Marchand C | title = DNA topoisomerases and their poisoning by anticancer and antibacterial drugs | journal = Chemistry & Biology | volume = 17 | issue = 5 | pages = 421–433 | date = May 2010 | pmid = 20534341 | pmc = 7316379 | doi = 10.1016/j.chembiol.2010.04.012 | doi-access = free }}</ref> It also inhibits the nicking-closing activity on the subunit of DNA gyrase that releases the positive binding stress on the supercoiled DNA. | ||
| ⚫ | ==References== | ||
| ⚫ | {{reflist| |
||
| 3 Barkley, Nghiem, (Sept. 2011) "AAP reviews | |||
| use of systemic and topical Quinolones" | |||
| Medscape educational briefs | |||
| ⚫ | == Adverse effects == | ||
| ⚫ | ==External links== | ||
| ⚫ | * {{MedlinePlusDrugInfo|medmaster|a682042}} | ||
| Hives, rash, intense itching, or fainting soon after a dose may be a sign of ]. Common adverse effects include rash, itchy skin, blurred or double vision, halos around lights, changes in color vision, nausea, vomiting, and diarrhea. Nalidixic acid may also cause ]s and ],<ref>{{cite journal | vauthors = Fraser AG, Harrower AD | title = Convulsions and hyperglycaemia associated with nalidixic acid | journal = British Medical Journal | volume = 2 | issue = 6101 | pages = 1518 | date = December 1977 | pmid = 589309 | pmc = 1632822 | doi = 10.1136/bmj.2.6101.1518 }}</ref> photosensitivity reactions,<ref name="pmid4733958">{{cite journal | vauthors = Ramsay CA | title = Photosensitivity from nalidixic acid | journal = Proceedings of the Royal Society of Medicine | volume = 66 | issue = 8 | pages = 747 | date = August 1973 | pmid = 4733958 | pmc = 1645105 | doi = 10.1177/003591577306600805 }}</ref> and sometimes hemolytic anemia,<ref name="pmid4653901">{{cite journal | vauthors = Gilbertson C, Jones DR | title = Haemolytic anaemia with nalidixic acid | journal = British Medical Journal | volume = 4 | issue = 5838 | pages = 493 | date = November 1972 | pmid = 4653901 | pmc = 1786728 | doi = 10.1136/bmj.4.5838.493-a }}</ref><ref name="pmid6811074">{{cite journal | vauthors = Tafani O, Mazzoli M, Landini G, Alterini B | title = Fatal acute immune haemolytic anaemia caused by nalidixic acid | journal = British Medical Journal | volume = 285 | issue = 6346 | pages = 936–937 | date = October 1982 | pmid = 6811074 | pmc = 1499997 | doi = 10.1136/bmj.285.6346.936-a }}</ref> thrombocytopenia<ref name="pmid6435742">{{cite journal | vauthors = Meyboom RH | title = Thrombocytopenia induced by nalidixic acid | journal = British Medical Journal | volume = 289 | issue = 6450 | pages = 962 | date = October 1984 | pmid = 6435742 | pmc = 1443179 | doi = 10.1136/bmj.289.6450.962 }}</ref> or leukopenia. Particularly in ] and young children, has been reported occasionally increased ].<ref name="pmid6025983">{{cite journal | vauthors = Boréus LO, Sundström B | title = Intracranial hypertension in a child during treatment with nalidixic acid | journal = British Medical Journal | volume = 2 | issue = 5554 | pages = 744–745 | date = June 1967 | pmid = 6025983 | pmc = 1841777 | doi = 10.1136/bmj.2.5554.744 }}</ref><ref name="pmid6055749">{{cite journal | vauthors = Kremer L, Walton M, Wardle EN | title = Nalidixic acid and intracranial hypertension | journal = British Medical Journal | volume = 4 | issue = 5577 | pages = 488 | date = November 1967 | pmid = 6055749 | pmc = 1748506 | doi = 10.1136/bmj.4.5577.488-a }}</ref><ref name="pmid4419059">{{cite journal | vauthors = Deonna T, Guignard JP | title = Acute intracranial hypertension after nalidixic acid administration | journal = Archives of Disease in Childhood | volume = 49 | issue = 9 | pages = 743 | date = September 1974 | pmid = 4419059 | pmc = 1649016 | doi = 10.1136/adc.49.9.743 }}</ref> | |||
| * | |||
| == Overdose == | |||
| In case of overdose the patient experiences ], visual disturbances, balance disorders, ], metabolic acidosis and ].<ref name="pmid17175866">{{cite journal | vauthors = Eizadi-Mood N | title = Nalidixic acid overdose and metabolic acidosis | journal = CJEM | volume = 8 | issue = 2 | pages = 78 | date = March 2006 | pmid = 17175866 | doi = 10.1017/s148180350001349x | doi-access = free }}</ref> | |||
| == Spectrum of bacterial susceptibility and resistance == | |||
| '']'', '']'' and '']'' are generally susceptible to nalidixic acid, while other bacteria such as '']'', '']'', '']'' and '']'' are resistant.<ref>{{ cite web | title = Nalidixic acid spectrum of bacterial susceptibility and Resistance | url = http://www.toku-e.com/Upload/Products/PDS/20120522005430.pdf | publisher = Toku-E | date = 2011-09-14 | access-date = 2012-05-14 | archive-url = https://web.archive.org/web/20160110130057/http://www.toku-e.com/Upload/Products/PDS/20120522005430.pdf | archive-date = 2016-01-10 | url-status = dead }}</ref> ''Salmonella enterica'' serovar Typhimurium strain ATCC14028 acquires nalidixic acid resistance when ''gyrB'' gene is mutated (strain IR715).<ref name="pmid7868611">{{cite journal | vauthors = Stojiljkovic I, Bäumler AJ, Heffron F | title = Ethanolamine utilization in Salmonella typhimurium: nucleotide sequence, protein expression, and mutational analysis of the cchA cchB eutE eutJ eutG eutH gene cluster | journal = Journal of Bacteriology | volume = 177 | issue = 5 | pages = 1357–66 | date = March 1995 | pmid = 7868611 | pmc = 176743 | doi = 10.1128/jb.177.5.1357-1366.1995 }}</ref> | |||
| ⚫ | == See also == | ||
| *] | |||
| *] | |||
| ⚫ | == References == | ||
| ⚫ | {{reflist|33em}} | ||
| ⚫ | == External links == | ||
| ⚫ | * {{MedlinePlusDrugInfo|medmaster|a682042}}{{dead link|date=June 2012}} | ||
| * {{ cite web | url = http://www.healthdigest.org/topics/category/1464-nalidixic-acid-dosage-interactions-side-effects-how-to-use | publisher = HealthDigest.org | title = Nalidixic acid }} | |||
| {{QuinoloneAntiBiotics}} | {{QuinoloneAntiBiotics}} | ||
| {{Authority control}} | |||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 03:53, 12 January 2025
First of the synthetic quinolone antibiotics Pharmaceutical compound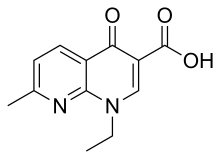 | |
| Clinical data | |
|---|---|
| Trade names | NegGram, Wintomylon, others |
| AHFS/Drugs.com | Consumer Drug Information |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 90% |
| Metabolism | Partially Hepatic |
| Elimination half-life | 6-7 hours, significantly longer in renal impairment |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.006.241 |
| Chemical and physical data | |
| Formula | C12H12N2O3 |
| Molar mass | 232.239 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Nalidixic acid (tradenames Nevigramon, NegGram, Wintomylon and WIN 18,320) is the first of the synthetic quinolone antibiotics.
In a technical sense, it is a naphthyridone, not a quinolone: its ring structure is a 1,8-naphthyridine nucleus that contains two nitrogen atoms, unlike quinoline, which has a single nitrogen atom.
Synthetic quinolone antibiotics were discovered by George Lesher and coworkers as a byproduct of chloroquine manufacture in the 1960s; nalidixic acid itself was used clinically, starting in 1967.
Nalidixic acid is effective primarily against Gram-negative bacteria, with minor anti-Gram-positive activity. In lower concentrations, it acts in a bacteriostatic manner; that is, it inhibits growth and reproduction. In higher concentrations, it is bactericidal, meaning that it kills bacteria instead of merely inhibiting their growth.
It has historically been used for treating urinary tract infections, caused, for example, by Escherichia coli, Proteus, Shigella, Enterobacter, and Klebsiella. It is no longer clinically used for this indication in the US as less toxic and more effective agents are available. The marketing authorization for nalidixic acid has been suspended throughout the EU.
It is also a tool in studies as a regulation of bacterial division. It selectively and reversibly blocks DNA replication in susceptible bacteria. Nalidixic acid and related antibiotics inhibit a subunit of DNA gyrase and topoisomerase IV and induce formation of cleavage complexes. It also inhibits the nicking-closing activity on the subunit of DNA gyrase that releases the positive binding stress on the supercoiled DNA.
Adverse effects
Hives, rash, intense itching, or fainting soon after a dose may be a sign of anaphylaxis. Common adverse effects include rash, itchy skin, blurred or double vision, halos around lights, changes in color vision, nausea, vomiting, and diarrhea. Nalidixic acid may also cause convulsions and hyperglycemia, photosensitivity reactions, and sometimes hemolytic anemia, thrombocytopenia or leukopenia. Particularly in infants and young children, has been reported occasionally increased intracranial pressure.
Overdose
In case of overdose the patient experiences headache, visual disturbances, balance disorders, mental confusion, metabolic acidosis and seizures.
Spectrum of bacterial susceptibility and resistance
Aeromonas hydrophila, Clostridium and Haemophilus are generally susceptible to nalidixic acid, while other bacteria such as Bifidobacteria, Lactobacillus, Pseudomonas and Staphylococcus are resistant. Salmonella enterica serovar Typhimurium strain ATCC14028 acquires nalidixic acid resistance when gyrB gene is mutated (strain IR715).
See also
References
- Emmerson AM, Jones AM (May 2003). "The quinolones: decades of development and use". The Journal of Antimicrobial Chemotherapy. 51 (Suppl 1): 13–20. doi:10.1093/jac/dkg208. PMID 12702699.
- Lesher GY, Froelich EJ, Gruett MD, Bailey JH, Brundage RP (September 1962). "1,8-Naphthyridine Derivatives. A New Class of Chemotherapeutic Agents". Journal of Medicinal and Pharmaceutical Chemistry. 5 (5): 1063–1065. doi:10.1021/jm01240a021. PMID 14056431.
- "Disabling and potentially permanent side effects lead to suspension or restrictions of quinolone and fluoroquinolone antibiotics". European Medicines Agency. 11 March 2019.
- Pommier Y, Leo E, Zhang H, Marchand C (May 2010). "DNA topoisomerases and their poisoning by anticancer and antibacterial drugs". Chemistry & Biology. 17 (5): 421–433. doi:10.1016/j.chembiol.2010.04.012. PMC 7316379. PMID 20534341.
- Fraser AG, Harrower AD (December 1977). "Convulsions and hyperglycaemia associated with nalidixic acid". British Medical Journal. 2 (6101): 1518. doi:10.1136/bmj.2.6101.1518. PMC 1632822. PMID 589309.
- Ramsay CA (August 1973). "Photosensitivity from nalidixic acid". Proceedings of the Royal Society of Medicine. 66 (8): 747. doi:10.1177/003591577306600805. PMC 1645105. PMID 4733958.
- Gilbertson C, Jones DR (November 1972). "Haemolytic anaemia with nalidixic acid". British Medical Journal. 4 (5838): 493. doi:10.1136/bmj.4.5838.493-a. PMC 1786728. PMID 4653901.
- Tafani O, Mazzoli M, Landini G, Alterini B (October 1982). "Fatal acute immune haemolytic anaemia caused by nalidixic acid". British Medical Journal. 285 (6346): 936–937. doi:10.1136/bmj.285.6346.936-a. PMC 1499997. PMID 6811074.
- Meyboom RH (October 1984). "Thrombocytopenia induced by nalidixic acid". British Medical Journal. 289 (6450): 962. doi:10.1136/bmj.289.6450.962. PMC 1443179. PMID 6435742.
- Boréus LO, Sundström B (June 1967). "Intracranial hypertension in a child during treatment with nalidixic acid". British Medical Journal. 2 (5554): 744–745. doi:10.1136/bmj.2.5554.744. PMC 1841777. PMID 6025983.
- Kremer L, Walton M, Wardle EN (November 1967). "Nalidixic acid and intracranial hypertension". British Medical Journal. 4 (5577): 488. doi:10.1136/bmj.4.5577.488-a. PMC 1748506. PMID 6055749.
- Deonna T, Guignard JP (September 1974). "Acute intracranial hypertension after nalidixic acid administration". Archives of Disease in Childhood. 49 (9): 743. doi:10.1136/adc.49.9.743. PMC 1649016. PMID 4419059.
- Eizadi-Mood N (March 2006). "Nalidixic acid overdose and metabolic acidosis". CJEM. 8 (2): 78. doi:10.1017/s148180350001349x. PMID 17175866.
- "Nalidixic acid spectrum of bacterial susceptibility and Resistance" (PDF). Toku-E. 2011-09-14. Archived from the original (PDF) on 2016-01-10. Retrieved 2012-05-14.
- Stojiljkovic I, Bäumler AJ, Heffron F (March 1995). "Ethanolamine utilization in Salmonella typhimurium: nucleotide sequence, protein expression, and mutational analysis of the cchA cchB eutE eutJ eutG eutH gene cluster". Journal of Bacteriology. 177 (5): 1357–66. doi:10.1128/jb.177.5.1357-1366.1995. PMC 176743. PMID 7868611.
External links
- MedlinePlus DrugInfo medmaster-a682042
- "Nalidixic acid". HealthDigest.org.
| Antibacterials that inhibit nucleic acid (J01E, J01M) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antifolates (inhibit bacterial purine metabolism, thereby inhibiting DNA and RNA synthesis) |
| ||||||||||||||||
| Quinolones (inhibit bacterial topoisomerase and/or DNA gyrase, thereby inhibiting DNA replication) |
| ||||||||||||||||
| Anaerobic DNA inhibitors |
| ||||||||||||||||
| RNA synthesis |
| ||||||||||||||||
| |||||||||||||||||