| Revision as of 03:15, 23 January 2012 editWhoop whoop pull up (talk | contribs)Extended confirmed users35,195 editsmNo edit summary← Previous edit | Latest revision as of 14:11, 23 May 2024 edit undoEgidio24 (talk | contribs)Extended confirmed users10,444 editsm v2.05 - Fix errors for CW project (Reference before punctuation)Tag: WPCleaner | ||
| (44 intermediate revisions by 36 users not shown) | |||
| Line 1: | Line 1: | ||
| {{short description|Highly-strained hydrocarbon ring compound}} | |||
| {{correct title|reason=bracket|Propellane}} | {{correct title|reason=bracket|edit=omission|Propellane}} | ||
| {{Chembox | {{Chembox | ||
| |Verifiedfields = changed | |||
| | |
|verifiedrevid = 477208655 | ||
| | |
|Name=Propellane | ||
| | |
|ImageFileL1 = 1.1.1-propellane.svg | ||
| | ImageSizeL1 = 100px | |||
| | |
|ImageFileR1 = 1.1.1-propellane.png | ||
| | |
|ImageSizeR1 = 150px | ||
| | |
|PIN = Tricyclopentane | ||
| ⚫ | |Section1={{Chembox Identifiers | ||
| | OtherNames = | |||
| ⚫ | |CASNo = 35634-10-7 | ||
| ⚫ | | |
||
| |CASNo_Ref = {{cascite|changed|??}} | |||
| ⚫ | | |
||
| | |
|ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | |
|StdInChI_Ref = {{stdinchicite|correct|chemspider}} | ||
| ⚫ | |StdInChI = 1S/C5H6/c1-4-2-5(1,4)3-4/h1-3H2 | ||
| | |
|StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | ||
| ⚫ | | |
||
| ⚫ | |StdInChIKey = ZTXSPLGEGCABFL-UHFFFAOYSA-N | ||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| ⚫ | |ChemSpiderID = 125285 | ||
| ⚫ | | |
||
| ⚫ | |PubChem = 142022 | ||
| ⚫ | | |
||
| ⚫ | |SMILES = C1(C2)(C3)C23C1}} | ||
| ⚫ | | |
||
| ⚫ | |Section2={{Chembox Properties | ||
| ⚫ | | |
||
| ⚫ | |C=5 | H=6}} | ||
| ⚫ | | |
||
| ⚫ | | |
||
| | Appearance = | |||
| | Density = | |||
| | MeltingPt = | |||
| | BoilingPt = | |||
| | Solubility = }} | |||
| | Section3 = {{Chembox Hazards | |||
| | MainHazards = | |||
| | FlashPt = | |||
| | Autoignition = }} | |||
| }} | }} | ||
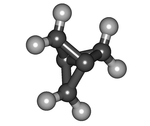
| '''Propellane''' is an ], the simplest member of the ] family. |
'''Propellane''' is an ], the simplest member of the ] family. It is a ] with formula {{chem2|C5H6}} or {{chem2|C2(CH2)3}}. The molecular structure consists of three rings of three ] atoms each, sharing one ]. | ||
| Propellane is a highly strained molecule. The bonds of the two central carbon atoms have an ], and the ] is 160 |
Propellane is a ] molecule. The bonds of the two central carbon atoms have an ], and the ] is 160 pm.<!--SO? WHAT IS A "NORMAL" VALUE?--> The ] is disputed; estimates vary from 59–65 ]/] to no strength at all. The energy of the ] state (with no central bond at all) is calculated to be 80 kcal/mol higher. At 114 °C it will spontaneously ] to ] ('''5''' below) with a ] of 5 minutes. Its ] is estimated to be 102 kcal/mol (427 ]/mol). Surprisingly, propellane is persistent at room temperature and is somewhat less susceptible to thermal decomposition than the less strained (90 kcal/mol) propellane system, which has an estimated half-life of only about 1 h at 25 °C.<ref>{{Cite web|url=https://www.thieme.de/shop/Houben-Weyl--SoS/de-Meijere-Butenschoen-Chow-Fitjer-Haufe-Houben-Weyl-Methods-of-Organic-Chemistry-9783131819840/p/000000009583004404|archive-url=https://web.archive.org/web/20171022033632/https://www.thieme.de/shop/Houben-Weyl--SoS/de-Meijere-Butenschoen-Chow-Fitjer-Haufe-Houben-Weyl-Methods-of-Organic-Chemistry-9783131819840/p/000000009583004404|url-status=dead|archive-date=October 22, 2017|title=Houben-Weyl Methods of Organic Chemistry Vol. E 17e, 4th Edition Supplement (E-Book PDF) - Thieme.de - Thieme Webshop - Armin de Meijere, Holger Butenschön, Hak-Fun Chow, Lutz Fitjer, Günter Haufe|website=Thieme Webshop|language=de|access-date=2017-10-21}}</ref> This unusual stability is attributed to delocalisation of electron density from the bond between the central carbon atoms onto the bridging carbon atoms.<ref>{{Cite journal|last1=Sterling|first1=Alistair J.|last2=Dürr|first2=Alexander|last3=Smith|first3=Russell|last4=Anderson|first4=Edward Alexander|last5=Duarte|first5=Fernanda|date=2020-04-13|title=Rationalizing the diverse reactivity of propellane through sigma-pi-delocalization|journal=Chemical Science|volume=11 |issue=19 |pages=4895–4903 |language=en|doi=10.1039/D0SC01386B |pmid=34122945 |pmc=8159217|issn=2041-6539|doi-access=free}}</ref> | ||
| The type of bonding in this molecule has been explained in terms of ]ing <ref> | The type of bonding in this molecule has been explained in terms of ]ing.<ref>{{cite journal|first1=Wei|last1=Wu|first2=Junjing|last2=Gu|first3=Jinshuai|last3=Song|first4=Sason|last4=Shaik|first5=Philippe C.|last5=Hiberty|date=2009|title=The Inverted Bond in Propellane is a Charge-Shift Bond|journal=]|volume=48|issue=8 |pages=1407–1410|doi=10.1002/anie.200804965|pmid=19072971|doi-access=free}}</ref> | ||
| Wei Wu, Junjing Gu , Jinshuai Song , Sason Shaik , Philippe C. Hiberty (2009), ''The Inverted Bond in Propellane is a Charge-Shift Bond''. ] volume 48, 1407–1410, {{DOI|10.1002/anie.200804965}} | |||
| </ref> | |||
| ==Synthesis== | ==Synthesis== | ||
| Propellane was first reported by ] and F. Walker in 1982. The synthesis commences with ] of ],<ref>{{cite journal|first1=K. B.|last1=Wiberg|first2=F. H.|last2=Walker|date=1982|title=Propellane|journal=]|volume=104|issue=19|pages=5239–5240|doi=10.1021/ja00383a046}}</ref> according to the following scheme: | |||
| Propellane was first synthesized by ] and ] in 1982,.<ref> | |||
| K. B. Wiberg, F H. Walker (1982), ''Propellane''. ] volume 104 issue 19, pp. 5239–5240; {{doi|10.1021/ja00383a046}} | |||
| </ref> according to the following schema: | |||
| :propellane]] | :propellane]] | ||
| Synthesis begins with conversion of the 1,3-di-] of pentane]] '''1''' in a ] to the corresponding di] '''2''' followed by a ] with ]. The final product '''3''' was isolated by ] at −30 °C. | |||
| However, a much simplified synthesis was published by.<ref> |
However, a much simplified synthesis was published by Szeimies.<ref>{{cite journal|first1=Johannes|last1=Belzner|first2=Uwe|last2=Bunz|first3=Klaus|last3=Semmler|first4=Günter|last4=Szeimies|first5=Klaus|last5=Opitz|first6=Arnulf-Dieter|last6=Schlüter|display-authors=etal|title=Concerning the synthesis of propellane|journal=]|year=1989 |volume=122|issue=2 |pages=397–398|doi=10.1002/cber.19891220233}}</ref> It starts with ] addition to the ] bond of 3-chloro-2-(chloromethyl)propene '''6''' followed by ] by ] and ]s in '''7'''.<ref>{{OrgSynth|title=Propellane|first1=Kathleen R.|last1=Mondanaro|first2=William P.|last2=Dailey|date=1998|collvol=10|collvolpage=658|volume=75|page=98|prep=v75p0098}}</ref> The product was not isolated but kept in solution at −196 °C. | ||
| (1998) ], Coll. Vol. 10, p. 658 (2004); Vol. 75, p.98 . | |||
| </ref> not isolated but kept in solution at −196 °C. | |||
| ==Reactions== | ==Reactions== | ||
| === Acetic acid addition === | === Acetic acid addition === | ||
| Propellane spontaneously reacts with ] to yield a ] ] ('''4''' above). | Propellane spontaneously reacts with ] to yield a ] ] ('''4''' above). | ||
| === Polymerization === | === Polymerization === | ||
| Propellane undergoes a ] reaction where the central C–C bond is split and connected to adjacent ] units, resulting in ]s.<ref>{{cite journal|first1=Piotr|last1=Kaszynski|first2=Josef|last2=Michl|date=1988|title=Staffanes: a molecular-size "Tinkertoy" construction set for nanotechnology. Preparation of end-functionalized telomers and a polymer of propellane|journal=]|volume=110|issue=15|pages=5225–5226|doi=10.1021/ja00223a070}}</ref> | |||
| Piotr Kaszynski and Josef Michl (1988), ''Staffanes: a molecular-size "Tinkertoy" construction set for nanotechnology. Preparation of end-functionalized telomers and a polymer of propellane'' ]; volume 110 issus 15, pp. 5225 - 5226; {{DOI|10.1021/ja00223a070}} | |||
| </ref> | |||
| :staffane |
:staffane]] | ||
| A ] initiated by ] and ] results in a distribution of ]s. An ] with ] results in a fully polymerized product. ] of the polymer shows that the connecting |
A ] initiated by ] and ] results in a distribution of ]s. An ] with ] results in a fully polymerized product. ] of the polymer shows that the connecting C–C bonds have ]s of only 1.48 Å, significantly shorter than the normal 1.54 Å. | ||
| The compound ], which can be viewed as a bridged |
The compound ], which can be viewed as a bridged propellane, also polymerizes in a similar way. | ||
| ==See also== | ==See also== | ||
| * |
*Propellane]] | ||
| *] which lacks a bond between the bridgehead carbons. | |||
| *-Propellane]] | |||
| ==References== | ==References== | ||
| Line 77: | Line 60: | ||
| {{DEFAULTSORT:Propellane, 1.1.1}} | {{DEFAULTSORT:Propellane, 1.1.1}} | ||
| ] | ] | ||
| ] | |||
Latest revision as of 14:11, 23 May 2024
Highly-strained hydrocarbon ring compound The correct title of this article is Propellane. The omission of any brackets is due to technical restrictions.
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name Tricyclopentane | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChemSpider | |||
| PubChem CID | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | C5H6 | ||
| Molar mass | 66.103 g·mol | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Propellane is an organic compound, the simplest member of the propellane family. It is a hydrocarbon with formula C5H6 or C2(CH2)3. The molecular structure consists of three rings of three carbon atoms each, sharing one C–C bond.
Propellane is a highly strained molecule. The bonds of the two central carbon atoms have an inverted tetrahedral geometry, and the length of the central bond is 160 pm. The strength of that bond is disputed; estimates vary from 59–65 kcal/mol to no strength at all. The energy of the biradical state (with no central bond at all) is calculated to be 80 kcal/mol higher. At 114 °C it will spontaneously isomerize to 3-methylidenecyclobutene (5 below) with a half-life of 5 minutes. Its strain energy is estimated to be 102 kcal/mol (427 kJ/mol). Surprisingly, propellane is persistent at room temperature and is somewhat less susceptible to thermal decomposition than the less strained (90 kcal/mol) propellane system, which has an estimated half-life of only about 1 h at 25 °C. This unusual stability is attributed to delocalisation of electron density from the bond between the central carbon atoms onto the bridging carbon atoms.
The type of bonding in this molecule has been explained in terms of charge-shift bonding.
Synthesis
Propellane was first reported by Kenneth B. Wiberg and F. Walker in 1982. The synthesis commences with cyclopropanation of 1,1-bis(chloromethyl)ethylene, according to the following scheme:
Synthesis begins with conversion of the 1,3-di-carboxylic acid of bicyclopentane 1 in a Hunsdiecker reaction to the corresponding dibromide 2 followed by a coupling reaction with n-butyllithium. The final product 3 was isolated by column chromatography at −30 °C.
However, a much simplified synthesis was published by Szeimies. It starts with dibromocarbene addition to the alkene bond of 3-chloro-2-(chloromethyl)propene 6 followed by deprotonation by methyllithium and nucleophilic displacements in 7. The product was not isolated but kept in solution at −196 °C.
Reactions
Acetic acid addition
Propellane spontaneously reacts with acetic acid to yield a methylidenecyclobutane ester (4 above).
Polymerization
Propellane undergoes a polymerization reaction where the central C–C bond is split and connected to adjacent monomer units, resulting in staffanes.
A radical polymerization initiated by methyl formate and benzoyl peroxide results in a distribution of oligomers. An anionic addition polymerization with n-butyllithium results in a fully polymerized product. X-ray diffraction of the polymer shows that the connecting C–C bonds have bond lengths of only 1.48 Å, significantly shorter than the normal 1.54 Å.
The compound 1,3-dehydroadamantane, which can be viewed as a bridged propellane, also polymerizes in a similar way.
See also
- Propellane
- Bicyclo(1.1.1)pentane which lacks a bond between the bridgehead carbons.
References
- "Houben-Weyl Methods of Organic Chemistry Vol. E 17e, 4th Edition Supplement (E-Book PDF) - Thieme.de - Thieme Webshop - Armin de Meijere, Holger Butenschön, Hak-Fun Chow, Lutz Fitjer, Günter Haufe". Thieme Webshop (in German). Archived from the original on October 22, 2017. Retrieved 2017-10-21.
- Sterling, Alistair J.; Dürr, Alexander; Smith, Russell; Anderson, Edward Alexander; Duarte, Fernanda (2020-04-13). "Rationalizing the diverse reactivity of [1.1.1]propellane through sigma-pi-delocalization". Chemical Science. 11 (19): 4895–4903. doi:10.1039/D0SC01386B. ISSN 2041-6539. PMC 8159217. PMID 34122945.
- Wu, Wei; Gu, Junjing; Song, Jinshuai; Shaik, Sason; Hiberty, Philippe C. (2009). "The Inverted Bond in [1.1.1]Propellane is a Charge-Shift Bond". Angew. Chem. Int. Ed. 48 (8): 1407–1410. doi:10.1002/anie.200804965. PMID 19072971.
- Wiberg, K. B.; Walker, F. H. (1982). "Propellane". J. Am. Chem. Soc. 104 (19): 5239–5240. doi:10.1021/ja00383a046.
- Belzner, Johannes; Bunz, Uwe; Semmler, Klaus; Szeimies, Günter; Opitz, Klaus; Schlüter, Arnulf-Dieter; et al. (1989). "Concerning the synthesis of propellane". Chem. Ber. 122 (2): 397–398. doi:10.1002/cber.19891220233.
- Mondanaro, Kathleen R.; Dailey, William P. "[1.1.1]Propellane". Organic Syntheses. 75: 98; Collected Volumes, vol. 10.
- Kaszynski, Piotr; Michl, Josef (1988). "Staffanes: a molecular-size "Tinkertoy" construction set for nanotechnology. Preparation of end-functionalized telomers and a polymer of propellane". J. Am. Chem. Soc. 110 (15): 5225–5226. doi:10.1021/ja00223a070.