| Revision as of 07:31, 25 January 2012 editCheMoBot (talk | contribs)Bots141,565 edits Updating {{chembox}} (no changed fields - added verified revid - updated 'DrugBank_Ref', 'UNII_Ref', 'ChEMBL_Ref', 'ChEBI_Ref', 'KEGG_Ref', 'CASNo_Ref') per Chem/Drugbox validation (report [[Wikipedia_talk:WikiProject_Chemicals|er...← Previous edit | Latest revision as of 07:40, 22 August 2024 edit undoLamro (talk | contribs)Autopatrolled, Extended confirmed users84,339 edits + | ||
| (22 intermediate revisions by 18 users not shown) | |||
| Line 1: | Line 1: | ||
| {{orphan|date=September 2010}} | |||
| {{Chembox | {{Chembox | ||
| | Verifiedfields = changed | |||
| ⚫ | | verifiedrevid = |
||
| | Watchedfields = changed | |||
| ⚫ | | ImageFile = | ||
| ⚫ | | verifiedrevid = 476995911 | ||
| ⚫ | | |
||
| ⚫ | | ImageFile = (NH4)3AlF6.png | ||
| ⚫ | | |
||
| ⚫ | | ImageSize = | ||
| ⚫ | | ImageAlt = | ||
| | IUPACName = | | IUPACName = | ||
| | PIN = | | PIN = | ||
| | OtherNames = Ammonium aluminium fluoride | | OtherNames = Ammonium aluminium fluoride | ||
| | |
|Section1={{Chembox Identifiers | ||
| | |
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | ChemSpiderID = 11253835 | | ChemSpiderID = 11253835 | ||
| | InChI = 1/Al.6FH.3H3N/h;6*1H;3*1H3/q+3;;;;;;;;;/p-3/rAlF6.3H3N/c2-1(3,4,5,6)7;;;/h;3*1H3/q-3;;;/p+3 | | InChI = 1/Al.6FH.3H3N/h;6*1H;3*1H3/q+3;;;;;;;;;/p-3/rAlF6.3H3N/c2-1(3,4,5,6)7;;;/h;3*1H3/q-3;;;/p+3 | ||
| Line 18: | Line 18: | ||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChIKey = OYHBNKHFKHBTRQ-UHFFFAOYSA-K | | StdInChIKey = OYHBNKHFKHBTRQ-UHFFFAOYSA-K | ||
| | CASNo_Ref = {{cascite|correct| |
| CASNo_Ref = {{cascite|correct|CAS}} | ||
| | CASNo = 7784-19-2 | | CASNo = 7784-19-2 | ||
| | UNII_Ref = {{fdacite|correct|FDA}} | |||
| ⚫ | | |
||
| | UNII = SW274S3321 | |||
| ⚫ | | |
||
| ⚫ | | PubChem = 24565 | ||
| | EC_number = 264-415-0 | |||
| ⚫ | | SMILES = ...F(F)(F)(F)(F)F | ||
| }} | }} | ||
| | |
|Section2={{Chembox Properties | ||
| | |
| Formula = (NH<sub>4</sub>)<sub>3</sub> | ||
| | |
| MolarMass = 195.09 g/mol | ||
| | |
| Appearance = White crystalline powder | ||
| | |
| Density = 1.78 g/cm<sup>3</sup> at 20 °C | ||
| | |
| MeltingPtC = 126.1 | ||
| | MeltingPt_notes = | |||
| | BoilingPt = 239.5°C | |||
| | |
| BoilingPtC = 239.5 | ||
| | BoilingPt_notes = | |||
| ⚫ | | |
||
| | Solubility = }} | |||
| ⚫ | | |
||
| ⚫ | |Section3={{Chembox Hazards | ||
| | FlashPt = | |||
| ⚫ | | MainHazards = Irritant ('''Xi''') | ||
| | Autoignition = | |||
| | |
| FlashPt = | ||
| | |
| AutoignitionPt = | ||
| | |
| NFPA-H = 2 | ||
| | |
| NFPA-F = 0 | ||
| | NFPA-R = 0 | |||
| | RPhrases = {{R23/24/25}} | |||
| | NFPA-S = | |||
| | SPhrases = {{S26}}, {{S28}}, {{S36/37/39}}, {{S45}} | |||
| | GHSPictograms = {{GHS06}} | |||
| | GHSSignalWord = Danger | |||
| | HPhrases = {{H-phrases|301|311|330|331}} | |||
| | PPhrases = {{P-phrases|260|261|264|270|271|280|284|301+310|302+352|304+340|310|311|312|320|321|322|330|361|363|403+233|405|501}} | |||
| }} | }} | ||
| }} | }} | ||
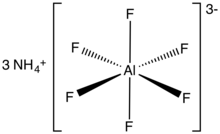
| '''Ammonium hexafluoroaluminate''' is an ] with the ] of (NH<sub>4</sub>)<sub>3</sub>. It is a white solid. Upon heating, it converts to ], a reaction that releases ].<ref>{{cite journal |doi=10.1021/cm991195g|title=Preparation and Characterization of Different Phases of Aluminum Trifluoride|year=2000|last1=Alonso|first1 =C.|last2=Morato|first2=A.|last3=Medina|first3=F.|last4=Guirado|first4=F. |last5=Cesteros|first5=Y.|last6=Salagre|first6=P.|last7=Sueiras|first7=J. E.|last8=Terrado|first8=R.|last9=Giralt|first9=A.|journal= Chemistry of Materials|volume=12|issue=4|pages=1148–1155}}</ref> It has also been used as a precursor to ]s.<ref>{{cite journal |doi=10.1016/j.molcata.2005.03.026|title=Post-synthesis alumination of mesoporous silica SBA-15 with high framework aluminum content using ammonium hexafluoroaluminate|year=2005|last1=Kao|first1=Hsien-Ming|last2=Ting|first2=Chun-Chiang|last3=Chao|first3=Shih-Wei|journal=Journal of Molecular Catalysis A: Chemical|volume=235|issue=1–2|pages=200–208}}</ref> | |||
| '''Ammonium hexafluoroaluminate''' is a ] which has the ] of (NH<sub>4</sub>)<sub>3</sub>AlF<sub>6</sub>. It is a white crystalline powder that is usually stable, but can release ] ] if it ]. | |||
| ==Preparation== | |||
| Ammonium hexafluoroaluminate can be obtained by the reaction of ] and ].<ref name="brauer">{{cite book | author=hrsg. von Georg Brauer. Unter Mitarb. von M. Baudler | title=Handbuch der präparativen anorganischen Chemie / 1. | publisher=Enke | publication-place=Stuttgart | date=1975 | isbn=3-432-02328-6 | oclc=310719485 | language=de|page=239}}</ref> | |||
| :<math>\mathrm{6 \ NH_4F + Al(OH)_3 \longrightarrow (NH_4)_3 + 3 \ NH_4OH}</math> | |||
| ==References== | |||
| <references /> | |||
| {{Ammonium salts}} | |||
| {{Fluorine compounds}} | |||
| ⚫ | ] | ||
| ⚫ | ] | ||
| ⚫ | ] | ||
| ] | |||
| {{inorganic-compound-stub}} | {{inorganic-compound-stub}} | ||
| ⚫ | ] | ||
| ⚫ | ] | ||
| ⚫ | ] | ||
| ] | |||
Latest revision as of 07:40, 22 August 2024
 | |
| Names | |
|---|---|
| Other names Ammonium aluminium fluoride | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.029.138 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | (NH4)3 |
| Molar mass | 195.09 g/mol |
| Appearance | White crystalline powder |
| Density | 1.78 g/cm at 20 °C |
| Melting point | 126.1 °C (259.0 °F; 399.2 K) |
| Boiling point | 239.5 °C (463.1 °F; 512.6 K) |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | Irritant (Xi) |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Danger |
| Hazard statements | H301, H311, H330, H331 |
| Precautionary statements | P260, P261, P264, P270, P271, P280, P284, P301+P310, P302+P352, P304+P340, P310, P311, P312, P320, P321, P322, P330, P361, P363, P403+P233, P405, P501 |
| NFPA 704 (fire diamond) |
 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Ammonium hexafluoroaluminate is an inorganic compound with the chemical formula of (NH4)3. It is a white solid. Upon heating, it converts to aluminium trifluoride, a reaction that releases hydrogen fluoride. It has also been used as a precursor to zeolites.
Preparation
Ammonium hexafluoroaluminate can be obtained by the reaction of ammonium fluoride and aluminium hydroxide.
References
- Alonso, C.; Morato, A.; Medina, F.; Guirado, F.; Cesteros, Y.; Salagre, P.; Sueiras, J. E.; Terrado, R.; Giralt, A. (2000). "Preparation and Characterization of Different Phases of Aluminum Trifluoride". Chemistry of Materials. 12 (4): 1148–1155. doi:10.1021/cm991195g.
- Kao, Hsien-Ming; Ting, Chun-Chiang; Chao, Shih-Wei (2005). "Post-synthesis alumination of mesoporous silica SBA-15 with high framework aluminum content using ammonium hexafluoroaluminate". Journal of Molecular Catalysis A: Chemical. 235 (1–2): 200–208. doi:10.1016/j.molcata.2005.03.026.
- hrsg. von Georg Brauer. Unter Mitarb. von M. Baudler (1975). Handbuch der präparativen anorganischen Chemie / 1 (in German). Stuttgart: Enke. p. 239. ISBN 3-432-02328-6. OCLC 310719485.
| Fluorine compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PF−6, AsF−6, SbF−6 compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AlF2−5, AlF3−6 compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| chlorides, bromides, iodides and pseudohalogenides | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SiF2−6, GeF2−6 compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxyfluorides | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Organofluorides | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| with transition metal, lanthanide, actinide, ammonium | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| nitric acids | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| bifluorides | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| thionyl, phosphoryl, and iodosyl | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical formulas | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
This inorganic compound–related article is a stub. You can help Misplaced Pages by expanding it. |
