| Revision as of 20:44, 5 February 2012 editLegacyOfValor (talk | contribs)713 edits updated thermochem properties & cite← Previous edit | Latest revision as of 01:47, 12 January 2025 edit undoVertexrocketry (talk | contribs)2 editsmNo edit summaryTag: Visual edit | ||
| (163 intermediate revisions by more than 100 users not shown) | |||
| Line 1: | Line 1: | ||
| {{chembox | {{chembox | ||
| | Watchedfields = changed | |||
| | verifiedrevid = 464211237 | |||
| | verifiedrevid = 477003444 | |||
| | Name = Potassium Chlorate | |||
| | |
| Name = Potassium chlorate | ||
| | |
| ImageFile = | ||
| | ImageFile1 = Potassium-chlorate-composition.png | |||
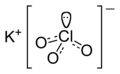
| | ImageName = The structure of the ions in potassium chlorate | |||
| | ImageSize1 = 120px | |||
| | ImageFile1 = Potassium-chlorate-crystal-3D-vdW.png | |||
| | ImageClass1 = skin-invert | |||
| | ImageSize1 = 200px | |||
| | |
| ImageName1 = The structure of the ions in potassium chlorate | ||
| | |
| ImageFile2 = Potassium chlorate-substance.jpg | ||
| | |
| ImageSize2 = 200px | ||
| | |
| ImageName2 = Potassium chlorate crystals | ||
| | |
| OtherNames = Potassium chlorate(V), Potcrate, Berthollet salt | ||
| | IUPACName = | |||
| | Section1 = {{Chembox Identifiers | |||
| | SystematicName = | |||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| | Section1 = {{Chembox Identifiers | |||
| | ChEMBL = 3188561 | |||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| | ChemSpiderID = 18512 | | ChemSpiderID = 18512 | ||
| | UNII_Ref = {{fdacite|correct|FDA}} | | UNII_Ref = {{fdacite|correct|FDA}} | ||
| Line 25: | Line 28: | ||
| | StdInChIKey = VKJKEPKFPUWCAS-UHFFFAOYSA-M | | StdInChIKey = VKJKEPKFPUWCAS-UHFFFAOYSA-M | ||
| | CASNo = 3811-04-9 | | CASNo = 3811-04-9 | ||
| | |
| CASNo_Ref = {{cascite|correct|CAS}} | ||
| | |
| PubChem = 6426889 | ||
| | |
| EINECS = 223-289-7 | ||
| | |
| RTECS = FO0350000 | ||
| | |
| UNNumber = 1485 | ||
| }} | }} | ||
| | Section2 = {{Chembox Properties | | Section2 = {{Chembox Properties | ||
| | |
| Formula = KClO<sub>3</sub> | ||
| | |
| MolarMass = 122.55 g mol<sup>−1</sup> | ||
| | |
| Appearance = white crystals or powder | ||
| | |
| Density = 2.32 g/cm<sup>3</sup> | ||
| | Solubility = 3.13 g/100 mL (0 °C)<br> 4.46 g/100 mL (10 °C)<br> 8.15 g/100 mL (25 °C)<br> 13.21 g/100 mL (40 °C)<br> 53.51 g/100 mL (100 °C)<br> 183 g/100 g (190 °C)<br> 2930 g/100 g (330 °C)<ref name=sioc>{{cite book|last1 = Seidell|first1 = Atherton|last2 = Linke|first2 = William F.|year = 1952|title = Solubilities of Inorganic and Organic Compounds|publisher = Van Nostrand|url = https://books.google.com/books?id=k2e5AAAAIAAJ |access-date = 2014-05-29}}</ref> | |||
| | Solubility = 7.19 g/100 ml (20 °C) <br> 57 g/100 mL (100 °C) | |||
| | |
| SolubleOther = soluble in ]<br> negligible in ] and liquid ]<ref name=chemister /> | ||
| | Solubility1 = 1 g/100 g (20 °C)<ref name=chemister /> | |||
| | MeltingPt = 356 °C | |||
| | Solvent1 = glycerol | |||
| | BoilingPt = 400 °C ''decomp.'' | |||
| | MeltingPtC = 356 | |||
| | RefractIndex = 1.40835 | |||
| | BoilingPtC = 400 | |||
| | BoilingPt_notes = decomposes<ref name=chemister /> | |||
| | RefractIndex = 1.40835 | |||
| | MagSus = −42.8·10<sup>−6</sup> cm<sup>3</sup>/mol | |||
| }} | }} | ||
| | Section3 = {{Chembox Structure | | Section3 = {{Chembox Structure | ||
| | |
| Coordination = | ||
| | |
| CrystalStruct = monoclinic | ||
| }} | }} | ||
| | Section4 = {{Chembox Thermochemistry | | Section4 = {{Chembox Thermochemistry | ||
| | |
| DeltaHf = −391.2 kJ/mol<ref name=b1>{{cite book| author = Zumdahl, Steven S.|title =Chemical Principles 6th Ed.| publisher = Houghton Mifflin Company| year = 2009| isbn = 978-0-618-94690-7|page=A22}}</ref><ref name=chemister>{{cite web|url=http://chemister.ru/Database/properties-en.php?dbid=1&id=331|title=potassium chlorate|access-date=9 July 2015}}</ref> | ||
| | |
| Entropy = 142.97 J/mol·K<ref name=b1 /><ref name=chemister /> | ||
| | DeltaGf = -289.9 kJ/mol<ref name=chemister /> | |||
| | HeatCapacity = 100.25 J/mol·K<ref name=chemister /> | |||
| }} | }} | ||
| | Section5 = | |||
| | Section7 = {{Chembox Hazards | |||
| | Section6 = | |||
| | ExternalMSDS = | |||
| | Section7 = {{Chembox Hazards | |||
| | EUClass = Oxidant ('''O''')<br/>Harmful ('''Xn''')<br/>Dangerous for the environment ('''N''') | |||
| | ExternalSDS = | |||
| | EUIndex = 017-004-00-3 | |||
| | GHSPictograms = {{GHS03}}{{GHS07}}{{GHS09}}<ref name="sigma">{{cite web|url=https://www.sigmaaldrich.com/US/en/product/sigald/255572|title=Potassium chlorate|access-date=14 February 2022}}</ref> | |||
| | RPhrases = {{R9}}, {{R22}}, {{R51/53}} | |||
| | GHSSignalWord = Danger | |||
| | SPhrases = {{S2}}, {{S13}}, {{S16}}, {{S27}}, {{S61}} | |||
| | HPhrases = {{H-phrases|271|302|332|411}}<ref name="sigma" /> | |||
| | NFPA-H = 2 | |||
| | PPhrases = {{P-phrases|220|273}}<ref name="sigma" /> | |||
| | NFPA-F = 0 | |||
| | |
| NFPA-H = 2 | ||
| | |
| NFPA-F = 0 | ||
| | |
| NFPA-R = 3 | ||
| | |
| NFPA-S = OX | ||
| | LD50 = 1870 mg/kg (oral, rat)<ref>{{cite web|url=https://chem.nlm.nih.gov/chemidplus/rn/3811-04-9|title=ChemIDplus - 3811-04-9 - VKJKEPKFPUWCAS-UHFFFAOYSA-M - Potassium chlorate - Similar structures search, synonyms, formulas, resource links, and other chemical information.|author=Michael Chambers|access-date=9 July 2015}}</ref> | |||
| }} | }} | ||
| | Section8 = {{Chembox Related | | Section8 = {{Chembox Related | ||
| | |
| OtherAnions = ]<br>]<br>] | ||
| | |
| OtherCations = ]<br>]<br>] | ||
| | |
| OtherCompounds = ]<br>]<br>]<br>] | ||
| }} | }} | ||
| }} | }} | ||
| '''Potassium chlorate''' is |
'''Potassium chlorate''' is the ] with the molecular formula KClO<sub>3</sub>. In its pure form, it is a white solid. After ], it is the second most common ] in industrial use. It is a strong ] and its most important application is in safety ]es.<ref name="ullmann_2000">{{cite book | ||
| | last1=Vogt | first1=Helmut | |||
| * as an ], | |||
| | last2=Balej | first2=Jan | |||
| * to prepare ], | |||
| | last3=Bennett | first3=John E. | |||
| * as a ], | |||
| | last4=Wintzer | first4=Peter | |||
| * in safety ]es, | |||
| | last5=Sheikh | first5=Saeed Akbar | |||
| * in ] and ], | |||
| | last6=Gallone | first6=Patrizio | |||
| * in ], forcing the blossoming stage of the ] tree, causing it to produce fruit in warmer climates.<ref>{{Cite journal | |||
| | date=June 15, 2000 | |||
| | last1 = Manochai | first1 = P. | |||
| | editor-last=Ullmann | |||
| | last2 = Sruamsiri | first2 = P. | |||
| | title=Ullmann's Encyclopedia of Industrial Chemistry | |||
| | last3 = Wiriya-alongkorn | first3 = W. | |||
| | publisher=Wiley‐VCH Verlag | |||
| | last4 = Naphrom | first4 = D. | |||
| | chapter=Chlorine Oxides and Chlorine Oxygen Acids | |||
| | last5 = Hegele | first5 = M. | |||
| | isbn=9783527303854 | |||
| | last6 = Bangerth | first6 = F. | |||
| | doi=10.1002/14356007.a06_483 | |||
| | title = Year around off season flower induction in longan (Dimocarpus longan, Lour.) trees by KClO3 applications: potentials and problems | |||
| }}</ref> In other applications it is mostly obsolete and has been replaced by safer alternatives in recent decades. It has been used | |||
| | journal = Scientia Horticulturae | |||
| * in ], ]s and ], | |||
| | volume = 104 | |||
| * to prepare ], both in the lab and in ]s, | |||
| | issue = 4 | |||
| * as a ], for example in ]s and medical ]es, | |||
| | pages = 379–390 | |||
| * in ] as an ]. | |||
| | location = Department of Horticulture, Maejo University, Chiang Mai, Thailand; Department of Horticulture, Chiang Mai University, Chiang Mai, Thailand; Institute of Special Crops and Crop Physiology, University of Hohenheim, 70593 Stuttgart, Germany | |||
| | date = February 12, 2005 | |||
| | url = http://www.actahort.org/books/863/863_48.htm | |||
| | accessdate = November 28, 2010}}</ref> | |||
| ==Production== | ==Production== | ||
| On the industrial scale, potassium chlorate is produced by the ] of ] and ]: | |||
| The compound is industrially produced by passing chlorine into the hot ], subsequently adding the ] (Liebig process).<ref>Реми, Г. Курс неорганической химиию, т. 1/Перевод с немецкого под ред. А. В. Новосёловой. Москва:Мир, 1972.- с. 770//(translated from:) Heinrich Remy. Lehrbuch der anorganischen Chemie. XI Auflage. Band 1. Leipzig:Geest & Portig K.-G., 1960.</ref> The ] of ] water solution is also used sometimes, then the chlorine formed at the ] reacts with KOH '']''. As KClO<sub>3</sub> ] in water decreases significantly when cooling, it can be simply isolated from the ]. | |||
| :{{chem2 | NaClO3 + KCl -> NaCl + KClO3 }} | |||
| The reaction is driven by the low solubility of potassium chlorate in water. The equilibrium of the reaction is shifted to the right hand side by the continuous precipitation of the product (]). The precursor sodium chlorate is produced industrially in very large quantities by ] of ], common table salt.<ref name="ullmann_2000"/> | |||
| The direct electrolysis of ] in aqueous solution is also used sometimes, in which elemental chlorine formed at the ] reacts with KOH '']''. The low ] of KClO<sub>3</sub> in water causes the salt to conveniently isolate itself from the ] by simply precipitating out of solution. | |||
| Can be produced in small amounts by ] a sodium hypochlorite solution followed by ] with potassium chloride<ref>http://chemistry.about.com/od/makechemicalsyourself/a/Potassium-Chlorate-From-Bleach-And-Salt-Substitute.htm</ref>: | |||
| :3 NaOCl → 2NaCl + NaClO3 | |||
| Potassium chlorate can be produced in small amounts by ] in a ] solution followed by ] with potassium chloride:<ref>{{cite web|url=http://chemistry.about.com/od/makechemicalsyourself/a/Potassium-Chlorate-From-Bleach-And-Salt-Substitute.htm|title=Potassium Chlorate Synthesis (Substitute) Formula|author=Anne Marie Helmenstine, Ph.D.|work=About.com Education|access-date=9 July 2015}}</ref> | |||
| :KCl + NaClO3 → NaCl + KClO3 | |||
| :{{chem2 | 3 NaOCl -> 2 NaCl + NaClO3 }} | |||
| Can also be produced by passing chlorine gas into a hot solution of caustic potash<ref>Pradyot Patnaik. ''Handbook of Inorganic Chemicals''. McGraw-Hill, 2002, ISBN 0-07-049439-8</ref>: | |||
| :{{chem2 | KCl + NaClO3 -> NaCl + KClO3 }} | |||
| :3Cl2(g) + 6KOH (aq) → KClO3 (aq) + 5KCl (aq) + 3H2O(l) | |||
| It can also be produced by passing chlorine gas into a hot solution of caustic potash:<ref name="Pradyot Patnaik 2002">Pradyot Patnaik. ''Handbook of Inorganic Chemicals''. McGraw-Hill, 2002, {{ISBN|0-07-049439-8}}</ref> | |||
| :{{chem2 | 3 Cl2 + 6 KOH -> KClO3 + 5 KCl + 3 H2O }} | |||
| :as seen in this | |||
| According to ], potassium chlorate is a dense salt-like structure consisting of chlorate and potassium ions in close association. | |||
| ] | |||
| ==Uses== | ==Uses== | ||
| Line 106: | Line 122: | ||
| Potassium chlorate was one key ingredient in early ] ]s (primers). It continues in that application, where not supplanted by ]. | Potassium chlorate was one key ingredient in early ] ]s (primers). It continues in that application, where not supplanted by ]. | ||
| Chlorate-based ]s are more efficient than traditional ] and are less susceptible to damage by water. However, they can be extremely unstable in the presence of ] or ] and are much more expensive. Chlorate propellants must be used only in equipment designed for them; failure to follow this precaution is a common source of accidents. |
Chlorate-based ]s are more efficient than traditional ] and are less susceptible to damage by water. However, they can be extremely unstable in the presence of ] or ] and are much more expensive. Chlorate propellants must be used only in equipment designed for them; failure to follow this precaution is a common source of accidents. Potassium chlorate, often in combination with ], is used in trick ] known as "crackers", "snappers", "pop-its", "caps" or "bang-snaps", a popular type of novelty ] | ||
| Another application of potassium chlorate is as the oxidizer in a ] such as that used in ]s. Since 2005, a cartridge with potassium chlorate mixed with ] and ] is used for generating the white smoke signaling the election of new pope by a ].<ref>{{cite news |title=New Round of Voting Fails to Name a Pope |author=Daniel J. Wakin and Alan Cowell |url=https://www.nytimes.com/2013/03/14/world/europe/vatican-pope-selection-conclave.html |newspaper=] |date=March 13, 2013 |access-date=March 13, 2013}}</ref> | |||
| When mixed with a suitable fuel, it may form an ], a so-called ]. The ] and slightly weaker ] is sometimes used as a safer and less expensive substitute for potassium chlorate. In ], mixes of potassium chlorate with ]s (such as ]) were the most common type of ] used, often filling grenades and other munitions. When used in explosives as an oxidizer, the explosive is ] meaning it burns rapidly rather than explodes. When mixed with a ], it may become ], requiring a ] (generally a commercial #8) to detonate properly. Potassium chlorate is rarely used in explosives now, as it is considered too sensitive for most uses. | |||
| High school and college laboratories often use potassium chlorate to generate oxygen gas. {{Citation needed|date=June 2009}} It is a far cheaper source than a pressurized or cryogenic oxygen tank. Potassium chlorate readily decomposes if heated while in contact with a ], typically ] (MnO<sub>2</sub>). Thus, it may be simply placed in a test tube and heated over a burner. If the test tube is equipped with a one-holed stopper and hose, warm oxygen can be drawn off. The reaction is as follows: | |||
| :{{chem2 | 2 KClO3(s) + MnO2(cat) -> 3 O2(g) + 2 KCl(s) }} | |||
| Heating it in the absence of a catalyst converts it into ]:<ref name="Pradyot Patnaik 2002"/> | |||
| : 2 KClO<sub>3</sub>(s) → 3 O<sub>2</sub>(g) + 2KCl(s) | |||
| :{{chem2 | 4 KClO3 -> 3 KClO4 + KCl }} | |||
| Heating it in the absence of a catalyst converts it into ]<ref>Pradyot Patnaik. ''Handbook of Inorganic Chemicals''. McGraw-Hill, 2002, ISBN 0-07-049439-8</ref>: | |||
| :4 KClO<sub>3</sub> → 3 KClO<sub>4</sub> + KCl | |||
| With further heating, potassium perchlorate decomposes to ] and oxygen: | With further heating, potassium perchlorate decomposes to ] and oxygen: | ||
| : |
:{{chem2 | KClO4 -> KCl + 2 O2 }} | ||
| The safe performance of this reaction requires very pure reagents and careful temperature control. Molten potassium chlorate is an extremely powerful oxidizer and |
The safe performance of this reaction requires very pure reagents and careful temperature control. Molten potassium chlorate is an extremely powerful oxidizer and spontaneously reacts with many common materials such as sugar. Explosions have resulted from liquid chlorates spattering into the latex or PVC tubes of oxygen generators and from contact between chlorates and hydrocarbon sealing greases. Impurities in potassium chlorate itself can also cause problems. When working with a new batch of potassium chlorate, it is advisable to take a small sample (~1 gram) and heat it strongly on an open glass plate. Contamination may cause this small quantity to explode, indicating that the chlorate should be discarded. | ||
| Potassium chlorate is used in ]s (also called chlorate candles or oxygen candles), employed as oxygen-supply systems of e.g. aircraft, space stations, and submarines, and has been responsible for at least one ]. A fire on the space station ] was |
Potassium chlorate is used in ]s (also called chlorate candles or oxygen candles), employed as oxygen-supply systems of e.g. aircraft, space stations, and submarines, and has been responsible for at least one ]. A fire on the space station ] was traced to oxygen generation candles that use a similar lithium perchlorate. The decomposition of potassium chlorate was also used to provide the oxygen supply for ]s. | ||
| Potassium chlorate is used also as a ]. In Finland it was sold under trade name Fegabit. | Potassium chlorate is used also as a ]. In Finland it was sold under trade name Fegabit. | ||
| Potassium chlorate can react with sulfuric acid to form a highly reactive solution of chloric acid and potassium sulfate: | Potassium chlorate can react with sulfuric acid to form a highly reactive solution of chloric acid and potassium sulfate: | ||
| :{{chem2 | 2 KClO3 + H2SO4 -> 2 HClO3 + K2SO4 }} | |||
| The solution so produced is sufficiently reactive that it spontaneously ignites if combustible material (sugar, paper, etc.) is present. | |||
| : 2 KClO<sub>3</sub> + H<sub>2</sub>SO<sub>4</sub> → 2 HClO<sub>3</sub> + K<sub>2</sub>SO<sub>4</sub> | |||
| ] | |||
| The solution so produced is sufficiently reactive that it will spontaneously ignite if combustible material (sugar, paper, etc.) is present. | |||
| In schools, molten potassium chlorate is used in ], ], ], and ] candy demonstration where the candy is dropped into the molten salt. | |||
| In chemical labs it is used to oxidize HCl and release small amounts of gaseous chlorine. | |||
| Militant groups in ] also use potassium chlorate extensively as a key component in the production of ] (IEDs). When significant effort was made to reduce the availability of ] fertilizer in Afghanistan, IED makers started using potassium chlorate as a cheap and effective alternative. In 2013, 60% of IEDs in Afghanistan used potassium chlorate, making it the most common ingredient used in IEDs.<ref>{{cite news|url=https://www.usatoday.com/story/news/world/2013/06/25/ammonium-nitrate-potassium-chlorate-ieds-afghanistan/2442191/ |title=Afghan bomb makers shifting to new explosives for IEDs |publisher=USAToday.com |date= June 25, 2013|access-date=2013-06-25}}</ref> | |||
| Potassium chlorate was also the main ingredient in the car bomb used in ] that killed 202 people. | |||
| Potassium chlorate is used to force the blossoming stage of the ] tree, causing it to produce fruit in warmer climates.<ref>{{Cite journal | |||
| | last1 = Manochai | first1 = P. | |||
| | last2 = Sruamsiri | first2 = P. | |||
| | last3 = Wiriya-alongkorn | first3 = W. | |||
| | last4 = Naphrom | first4 = D. | |||
| | last5 = Hegele | first5 = M. | |||
| | last6 = Bangerth | first6 = F. | |||
| | title = Year around off-season flower induction in longan (Dimocarpus longan, Lour.) trees by KClO3 applications: potentials and problems | |||
| | journal = Scientia Horticulturae | |||
| | volume = 104 | |||
| | issue = 4 | |||
| | pages = 379–390 | |||
| | location = Department of Horticulture, Maejo University, Chiang Mai, Thailand; Department of Horticulture, Chiang Mai University, Chiang Mai, Thailand; Institute of Special Crops and Crop Physiology, University of Hohenheim, 70593 Stuttgart, Germany | |||
| | date = February 12, 2005 | |||
| | url = http://www.actahort.org/books/863/863_48.htm | |||
| | access-date = November 28, 2010 | doi=10.1016/j.scienta.2005.01.004}}</ref> | |||
| ==Safety== | ==Safety== | ||
| Potassium chlorate should be handled with care. It reacts vigorously, and in some cases spontaneously ignites or explodes, when mixed with many ] materials. It |
Potassium chlorate should be handled with care. It reacts vigorously, and in some cases spontaneously ignites or explodes, when mixed with many ] materials. It burns vigorously in combination with virtually any combustible material, even those normally only slightly flammable (including ordinary dust and lint). Mixtures of potassium chlorate and a fuel can ignite by contact with sulfuric acid, so it should be kept away from this reagent. | ||
| ] should be avoided in pyrotechnic compositions containing potassium chlorate, as these mixtures are prone to spontaneous ]. Most sulfur contains trace quantities of sulfur-containing acids, and these can cause spontaneous ignition - "Flowers of sulfur" or "sublimed sulfur", despite the overall high purity, contains significant amounts of sulfur acids. Also, mixtures of potassium chlorate with any compound with ignition promoting properties |
] should be avoided in pyrotechnic compositions containing potassium chlorate, as these mixtures are prone to spontaneous ]. Most sulfur contains trace quantities of sulfur-containing acids, and these can cause spontaneous ignition - "Flowers of sulfur" or "sublimed sulfur", despite the overall high purity, contains significant amounts of sulfur acids. Also, mixtures of potassium chlorate with any compound with ignition promoting properties, such as ], are very dangerous to prepare, as they are extremely shock sensitive. | ||
| ==See also== | ==See also== | ||
| Line 140: | Line 179: | ||
| ==References== | ==References== | ||
| {{Reflist}} | |||
| <references/> | |||
| *"Chlorate de potassium. Chlorate de sodium", ''Fiche toxicol. n° 217'', Paris:Institut national de recherche et de sécurité, 2000. 4pp. | *"Chlorate de potassium. Chlorate de sodium", ''Fiche toxicol. n° 217'', Paris:Institut national de recherche et de sécurité, 2000. 4pp. | ||
| * | * | ||
| ==External links== | ==External links== | ||
| {{Commons category|Potassium chlorate}} | |||
| * | |||
| * | |||
| * | |||
| {{Potassium compounds}} | {{Potassium compounds}} | ||
| {{Chlorates}} | |||
| {{DEFAULTSORT:Potassium Chlorate}} | |||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 01:47, 12 January 2025
 | |
 | |
| Names | |
|---|---|
| Other names Potassium chlorate(V), Potcrate, Berthollet salt | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.021.173 |
| EC Number |
|
| PubChem CID | |
| RTECS number |
|
| UNII | |
| UN number | 1485 |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | KClO3 |
| Molar mass | 122.55 g mol |
| Appearance | white crystals or powder |
| Density | 2.32 g/cm |
| Melting point | 356 °C (673 °F; 629 K) |
| Boiling point | 400 °C (752 °F; 673 K) decomposes |
| Solubility in water | 3.13 g/100 mL (0 °C) 4.46 g/100 mL (10 °C) 8.15 g/100 mL (25 °C) 13.21 g/100 mL (40 °C) 53.51 g/100 mL (100 °C) 183 g/100 g (190 °C) 2930 g/100 g (330 °C) |
| Solubility | soluble in glycerol negligible in acetone and liquid ammonia |
| Solubility in glycerol | 1 g/100 g (20 °C) |
| Magnetic susceptibility (χ) | −42.8·10 cm/mol |
| Refractive index (nD) | 1.40835 |
| Structure | |
| Crystal structure | monoclinic |
| Thermochemistry | |
| Heat capacity (C) | 100.25 J/mol·K |
| Std molar entropy (S298) |
142.97 J/mol·K |
| Std enthalpy of formation (ΔfH298) |
−391.2 kJ/mol |
| Gibbs free energy (ΔfG) | -289.9 kJ/mol |
| Hazards | |
| GHS labelling: | |
| Pictograms |   
|
| Signal word | Danger |
| Hazard statements | H271, H302, H332, H411 |
| Precautionary statements | P220, P273 |
| NFPA 704 (fire diamond) |
 |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) | 1870 mg/kg (oral, rat) |
| Safety data sheet (SDS) | ICSC 0548 |
| Related compounds | |
| Other anions | Potassium bromate Potassium iodate Potassium nitrate |
| Other cations | Ammonium chlorate Sodium chlorate Barium chlorate |
| Related compounds | Potassium chloride Potassium hypochlorite Potassium chlorite Potassium perchlorate |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Potassium chlorate is the inorganic compound with the molecular formula KClO3. In its pure form, it is a white solid. After sodium chlorate, it is the second most common chlorate in industrial use. It is a strong oxidizing agent and its most important application is in safety matches. In other applications it is mostly obsolete and has been replaced by safer alternatives in recent decades. It has been used
- in fireworks, propellants and explosives,
- to prepare oxygen, both in the lab and in chemical oxygen generators,
- as a disinfectant, for example in dentifrices and medical mouthwashes,
- in agriculture as an herbicide.
Production
On the industrial scale, potassium chlorate is produced by the salt metathesis reaction of sodium chlorate and potassium chloride:
- NaClO3 + KCl → NaCl + KClO3
The reaction is driven by the low solubility of potassium chlorate in water. The equilibrium of the reaction is shifted to the right hand side by the continuous precipitation of the product (Le Chatelier's Principle). The precursor sodium chlorate is produced industrially in very large quantities by electrolysis of sodium chloride, common table salt.
The direct electrolysis of KCl in aqueous solution is also used sometimes, in which elemental chlorine formed at the anode reacts with KOH in situ. The low solubility of KClO3 in water causes the salt to conveniently isolate itself from the reaction mixture by simply precipitating out of solution.
Potassium chlorate can be produced in small amounts by disproportionation in a sodium hypochlorite solution followed by metathesis reaction with potassium chloride:
- 3 NaOCl → 2 NaCl + NaClO3
- KCl + NaClO3 → NaCl + KClO3
It can also be produced by passing chlorine gas into a hot solution of caustic potash:
- 3 Cl2 + 6 KOH → KClO3 + 5 KCl + 3 H2O
- as seen in this video
According to X-ray crystallography, potassium chlorate is a dense salt-like structure consisting of chlorate and potassium ions in close association.

Uses

Potassium chlorate was one key ingredient in early firearms percussion caps (primers). It continues in that application, where not supplanted by potassium perchlorate.
Chlorate-based propellants are more efficient than traditional gunpowder and are less susceptible to damage by water. However, they can be extremely unstable in the presence of sulfur or phosphorus and are much more expensive. Chlorate propellants must be used only in equipment designed for them; failure to follow this precaution is a common source of accidents. Potassium chlorate, often in combination with silver fulminate, is used in trick noise-makers known as "crackers", "snappers", "pop-its", "caps" or "bang-snaps", a popular type of novelty firework.
Another application of potassium chlorate is as the oxidizer in a smoke composition such as that used in smoke grenades. Since 2005, a cartridge with potassium chlorate mixed with lactose and rosin is used for generating the white smoke signaling the election of new pope by a papal conclave.
High school and college laboratories often use potassium chlorate to generate oxygen gas. It is a far cheaper source than a pressurized or cryogenic oxygen tank. Potassium chlorate readily decomposes if heated while in contact with a catalyst, typically manganese(IV) dioxide (MnO2). Thus, it may be simply placed in a test tube and heated over a burner. If the test tube is equipped with a one-holed stopper and hose, warm oxygen can be drawn off. The reaction is as follows:
- 2 KClO3(s) + MnO2(cat) → 3 O2(g) + 2 KCl(s)
Heating it in the absence of a catalyst converts it into potassium perchlorate:
- 4 KClO3 → 3 KClO4 + KCl
With further heating, potassium perchlorate decomposes to potassium chloride and oxygen:
- KClO4 → KCl + 2 O2
The safe performance of this reaction requires very pure reagents and careful temperature control. Molten potassium chlorate is an extremely powerful oxidizer and spontaneously reacts with many common materials such as sugar. Explosions have resulted from liquid chlorates spattering into the latex or PVC tubes of oxygen generators and from contact between chlorates and hydrocarbon sealing greases. Impurities in potassium chlorate itself can also cause problems. When working with a new batch of potassium chlorate, it is advisable to take a small sample (~1 gram) and heat it strongly on an open glass plate. Contamination may cause this small quantity to explode, indicating that the chlorate should be discarded.
Potassium chlorate is used in chemical oxygen generators (also called chlorate candles or oxygen candles), employed as oxygen-supply systems of e.g. aircraft, space stations, and submarines, and has been responsible for at least one plane crash. A fire on the space station Mir was traced to oxygen generation candles that use a similar lithium perchlorate. The decomposition of potassium chlorate was also used to provide the oxygen supply for limelights.
Potassium chlorate is used also as a pesticide. In Finland it was sold under trade name Fegabit.
Potassium chlorate can react with sulfuric acid to form a highly reactive solution of chloric acid and potassium sulfate:
- 2 KClO3 + H2SO4 → 2 HClO3 + K2SO4
The solution so produced is sufficiently reactive that it spontaneously ignites if combustible material (sugar, paper, etc.) is present.
In schools, molten potassium chlorate is used in screaming jelly babies, Gummy bear, Haribo, and Trolli candy demonstration where the candy is dropped into the molten salt.
In chemical labs it is used to oxidize HCl and release small amounts of gaseous chlorine.
Militant groups in Afghanistan also use potassium chlorate extensively as a key component in the production of improvised explosive devices (IEDs). When significant effort was made to reduce the availability of ammonium nitrate fertilizer in Afghanistan, IED makers started using potassium chlorate as a cheap and effective alternative. In 2013, 60% of IEDs in Afghanistan used potassium chlorate, making it the most common ingredient used in IEDs. Potassium chlorate was also the main ingredient in the car bomb used in 2002 Bali bombings that killed 202 people.
Potassium chlorate is used to force the blossoming stage of the longan tree, causing it to produce fruit in warmer climates.
Safety
Potassium chlorate should be handled with care. It reacts vigorously, and in some cases spontaneously ignites or explodes, when mixed with many combustible materials. It burns vigorously in combination with virtually any combustible material, even those normally only slightly flammable (including ordinary dust and lint). Mixtures of potassium chlorate and a fuel can ignite by contact with sulfuric acid, so it should be kept away from this reagent. Sulfur should be avoided in pyrotechnic compositions containing potassium chlorate, as these mixtures are prone to spontaneous deflagration. Most sulfur contains trace quantities of sulfur-containing acids, and these can cause spontaneous ignition - "Flowers of sulfur" or "sublimed sulfur", despite the overall high purity, contains significant amounts of sulfur acids. Also, mixtures of potassium chlorate with any compound with ignition promoting properties, such as antimony(III) sulfide, are very dangerous to prepare, as they are extremely shock sensitive.
See also
References
- ^ "potassium chlorate". Retrieved 9 July 2015.
- Seidell, Atherton; Linke, William F. (1952). Solubilities of Inorganic and Organic Compounds. Van Nostrand. Retrieved 2014-05-29.
- ^ Zumdahl, Steven S. (2009). Chemical Principles 6th Ed. Houghton Mifflin Company. p. A22. ISBN 978-0-618-94690-7.
- ^ "Potassium chlorate". Retrieved 14 February 2022.
- Michael Chambers. "ChemIDplus - 3811-04-9 - VKJKEPKFPUWCAS-UHFFFAOYSA-M - Potassium chlorate - Similar structures search, synonyms, formulas, resource links, and other chemical information". Retrieved 9 July 2015.
- ^ Vogt, Helmut; Balej, Jan; Bennett, John E.; Wintzer, Peter; Sheikh, Saeed Akbar; Gallone, Patrizio (June 15, 2000). "Chlorine Oxides and Chlorine Oxygen Acids". In Ullmann (ed.). Ullmann's Encyclopedia of Industrial Chemistry. Wiley‐VCH Verlag. doi:10.1002/14356007.a06_483. ISBN 9783527303854.
- Anne Marie Helmenstine, Ph.D. "Potassium Chlorate Synthesis (Substitute) Formula". About.com Education. Retrieved 9 July 2015.
- ^ Pradyot Patnaik. Handbook of Inorganic Chemicals. McGraw-Hill, 2002, ISBN 0-07-049439-8
- Daniel J. Wakin and Alan Cowell (March 13, 2013). "New Round of Voting Fails to Name a Pope". The New York Times. Retrieved March 13, 2013.
- "Afghan bomb makers shifting to new explosives for IEDs". USAToday.com. June 25, 2013. Retrieved 2013-06-25.
- Manochai, P.; Sruamsiri, P.; Wiriya-alongkorn, W.; Naphrom, D.; Hegele, M.; Bangerth, F. (February 12, 2005). "Year around off-season flower induction in longan (Dimocarpus longan, Lour.) trees by KClO3 applications: potentials and problems". Scientia Horticulturae. 104 (4). Department of Horticulture, Maejo University, Chiang Mai, Thailand; Department of Horticulture, Chiang Mai University, Chiang Mai, Thailand; Institute of Special Crops and Crop Physiology, University of Hohenheim, 70593 Stuttgart, Germany: 379–390. doi:10.1016/j.scienta.2005.01.004. Retrieved November 28, 2010.
{{cite journal}}: CS1 maint: location (link)
- "Chlorate de potassium. Chlorate de sodium", Fiche toxicol. n° 217, Paris:Institut national de recherche et de sécurité, 2000. 4pp.
- Continuous process for the manufacture of potassium chlorate by coupling with a sodium chlorate production plant
External links
| Potassium compounds | |
|---|---|
| H, (pseudo)halogens | |
| chalcogens | |
| pnictogens | |
| B, C group | |
| transition metals | |
| organic | |
| Salts and covalent derivatives of the chlorate ion | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||