| Revision as of 21:25, 7 February 2012 editR. S. Shaw (talk | contribs)Extended confirmed users, Pending changes reviewers, Rollbackers11,694 edits →Side effects: usage:, replaced: adverse affects → adverse effects using AWB← Previous edit | Latest revision as of 05:29, 12 May 2024 edit undoBoghog (talk | contribs)Autopatrolled, Extended confirmed users, IP block exemptions, New page reviewers, Pending changes reviewers, Rollbackers, Template editors137,946 edits consistent citation formatting | ||
| (256 intermediate revisions by 99 users not shown) | |||
| Line 1: | Line 1: | ||
| {{Short description|Chemical compound}} | |||
| {{cs1 config|name-list-style=vanc|display-authors=6}} | |||
| {{Drugbox | {{Drugbox | ||
| | Verifiedfields = changed | | Verifiedfields = changed | ||
| | Watchedfields = changed | |||
| | verifiedrevid = 470630524 | |||
| | verifiedrevid = 470630524 | |||
| | IUPAC_name = <small>(3α,16α)-Eburnamenine-14-carboxylic acid ethyl ester</small> | |||
| | IUPAC_name = (3α,16α)-Eburnamenine-14-carboxylic acid ethyl ester | |||
| | image = Vinpocetine.svg | |||
| | image = Vinpocetine.svg | |||
| | width = 200px | |||
| | width = 200px | |||
| | image2 = Vinpocetine ball-and-stick.png | |||
| | width2 = 200px | |||
| <!--Clinical data--> | <!--Clinical data-->| tradename = | ||
| | Drugs.com = {{drugs.com|international|vinpocetine}} | |||
| | tradename = | |||
| | pregnancy_category = Not recommended<ref name=FDA2019Preg/> | |||
| | Drugs.com = {{drugs.com|international|vinpocetine}} | |||
| | legal_EU = Rx-only | |||
| | pregnancy_category = not recommended | |||
| | legal_EU_comment = {{citation needed|date=December 2016}} | |||
| | legal_status = OTC | |||
| | legal_US = Unapproved "New Drug" (as defined by 21 U.S. Code § 321(p)(1)). Use in ]s, ], or ] is unlawful; otherwise uncontrolled. <ref>{{Cite web |date=February 22, 2023 |title=Vinpocetine in Dietary Supplements |url=https://www.fda.gov/food/dietary-supplement-ingredient-directory/vinpocetine-dietary-supplements |access-date=June 9, 2023 |website=]}}</ref> | |||
| | routes_of_administration = oral, intravenous | |||
| | routes_of_administration = Oral, intravenous | |||
| <!--Pharmacokinetic data--> | <!--Pharmacokinetic data-->| bioavailability = 56.6 ± 8.9% | ||
| | metabolism = hepatic | |||
| | bioavailability = 56.6 +/- 8.9% | |||
| | elimination_half-life = 2.54 ± 0.48 hours | |||
| | metabolism = hepatic | |||
| | excretion = renal | |||
| | elimination_half-life = 2.54 +/- 0.48 hours | |||
| | excretion = renal | |||
| <!--Identifiers--> | <!--Identifiers-->| IUPHAR_ligand = 5285 | ||
| | CAS_number_Ref = {{cascite|changed|??}} | | CAS_number_Ref = {{cascite|changed|??}} | ||
| | CAS_number = 42971-09-5 | | CAS_number = 42971-09-5 | ||
| | ATC_prefix = N06 | | ATC_prefix = N06 | ||
| | ATC_suffix = BX18 | | ATC_suffix = BX18 | ||
| | ChEMBL_Ref = {{ebicite|correct|EBI}} | | ChEMBL_Ref = {{ebicite|correct|EBI}} | ||
| | ChEMBL = 71752 | | ChEMBL = 71752 | ||
| | PubChem = 443955 | | PubChem = 443955 | ||
| | DrugBank_Ref = {{drugbankcite|correct|drugbank}} | | DrugBank_Ref = {{drugbankcite|correct|drugbank}} | ||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | ChemSpiderID = 392007 | | ChemSpiderID = 392007 | ||
| | UNII_Ref = {{fdacite|correct|FDA}} | | UNII_Ref = {{fdacite|correct|FDA}} | ||
| | UNII = 543512OBTC | | UNII = 543512OBTC | ||
| | KEGG_Ref = {{keggcite|correct|kegg}} | | KEGG_Ref = {{keggcite|correct|kegg}} | ||
| | KEGG = D01371 | | KEGG = D01371 | ||
| <!--Chemical data--> | <!--Chemical data-->| C = 22 | ||
| | |
| H = 26 | ||
| | N = 2 | |||
| | molecular_weight = 350.454 g/mol | |||
| | O = 2 | |||
| | smiles = O=C(OCC)C=4n1c3c(c2ccccc12)CCN53(C=4)(CCC5)CC | |||
| | smiles = O=C(OCC)C=4n1c3c(c2ccccc12)CCN53(C=4)(CCC5)CC | |||
| | InChI = 1/C22H26N2O2/c1-3-22-11-7-12-23-13-10-16-15-8-5-6-9-17(15)24(19(16)20(22)23)18(14-22)21(25)26-4-2/h5-6,8-9,14,20H,3-4,7,10-13H2,1-2H3/t20-,22+/m1/s1 | |||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| | InChIKey = DDNCQMVWWZOMLN-IRLDBZIGBY | |||
| | StdInChI = 1S/C22H26N2O2/c1-3-22-11-7-12-23-13-10-16-15-8-5-6-9-17(15)24(19(16)20(22)23)18(14-22)21(25)26-4-2/h5-6,8-9,14,20H,3-4,7,10-13H2,1-2H3/t20-,22+/m1/s1 | |||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| | StdInChI = 1S/C22H26N2O2/c1-3-22-11-7-12-23-13-10-16-15-8-5-6-9-17(15)24(19(16)20(22)23)18(14-22)21(25)26-4-2/h5-6,8-9,14,20H,3-4,7,10-13H2,1-2H3/t20-,22+/m1/s1 | |||
| | StdInChIKey = DDNCQMVWWZOMLN-IRLDBZIGSA-N | |||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| | StdInChIKey = DDNCQMVWWZOMLN-IRLDBZIGSA-N | |||
| }} | }} | ||
| '''Vinpocetine''' (brand names: '''Cavinton''', '''Intelectol'''; chemical name: '''ethyl apovincaminate''') is a semisynthetic derivative alkaloid of ] (sometimes described as "a ] ] of ]"),<ref>{{Cite journal|author=Lörincz C, Szász K, Kisfaludy L |title=The synthesis of ethyl apovincaminate |journal=Arzneimittel-Forschung |volume=26 |issue=10a |page=1907 |year=1976 |pmid=1037211}}</ref> an extract from the ] plant. | |||
| '''Vinpocetine''' ('''ethyl apovincaminate''') is a synthetic ] of the ] ], differing by the removal of a ] group and by being the ethyl rather than the methyl ester of the underlying carboxylic acid. Vincamine is extracted from either the seeds of '']'' or the leaves of '']'' (lesser periwinkle). | |||
| Vinpocetine is reported to have cerebral blood-flow enhancing<ref>{{Cite journal|author=Szilágyi G, Nagy Z, Balkay L, ''et al.'' |title=Effects of vinpocetine on the redistribution of cerebral blood flow and glucose metabolism in chronic ischemic stroke patients: a PET study |journal=Journal of the Neurological Sciences |volume=229-230 |pages=275–84 |year=2005 |pmid=15760651 |doi=10.1016/j.jns.2004.11.053}}</ref> and neuroprotective effects,<ref>{{Cite journal|author=Dézsi L, Kis-Varga I, Nagy J, Komlódi Z, Kárpáti E |title= |language=Hungarian |journal=Acta Pharmaceutica Hungarica |volume=72 |issue=2 |pages=84–91 |year=2002 |pmid=12498034}}</ref> and is used as a drug in Eastern Europe for the treatment of ] and age-related memory impairment.<ref>{{Cite journal|title=Vinpocetine. Monograph |journal=Alternative Medicine Review |volume=7 |issue=3 |pages=240–3 |year=2002 |pmid=12126465 |url=http://www.thorne.com/altmedrev/.fulltext/7/3/240.pdf}}</ref> | |||
| ==Medical uses== | |||
| Vinpocetine is widely marketed as a supplement for ] and as a ] for the improvement of memory. In other words, Vinpocetine may help support brain functions such as concentration and memory by activating cerebral metabolism. A small subset of users report uncomfortable, adverse reactions to vinpocetine. A low initial dosage is ordinarily recommended. | |||
| Vinpocetine has been used in many Asian and European countries for treatment of cerebrovascular disorders such as stroke and dementia for over three decades.<ref name="pmid29183836">{{cite journal | vauthors = Zhang YS, Li JD, Yan C | title = An update on vinpocetine: New discoveries and clinical implications | journal = European Journal of Pharmacology | volume = 819 | issue = | pages = 30–34 | date = January 2018 | pmid = 29183836 | doi = 10.1016/j.ejphar.2017.11.041 | doi-access = free | pmc = 5766389 }}</ref> | |||
| The ] has tentatively ruled that vinpocetine, due to its synthetic nature and proposed therapeutic uses, is ineligible to be marketed as ] under the ].<ref name="Newsweek_011217">{{cite magazine| vauthors = Schmitt R |title=Marketers exploit the aged with unproven brain-health claims|url=http://www.newsweek.com/marketers-exploit-aged-unproven-brain-health-claims-541709?rx=us|access-date=January 18, 2016|magazine=]|date=January 12, 2017}}</ref><ref name="NI_090816">{{cite web| vauthors = Hank S |title=FDA rules vinpocetine not a legal dietary ingredient despite successful NDI filings |url=http://www.nutraingredients-usa.com/Regulation/FDA-rules-vinpocetine-not-a-legal-dietary-ingredient-despite-successful-NDI-filings|website=NutraIngredients|date=7 September 2016 |publisher=William Reed Business Media, England|access-date=September 8, 2016}}</ref><ref name="NW_011816">{{cite web|title=FDA Concludes Vinpocetine Ineligible as a Dietary Ingredient|url=http://www.nutraceuticalsworld.com/contents/view_online-exclusives/2016-09-20/fda-concludes-vinpocetine-ineligible-as-a-dietary-ingredient|website=Nutraceuticals World|publisher=Rodman Media|access-date=January 18, 2017|date=September 20, 2016}}</ref><ref name="NW_020317">{{cite magazine| vauthors = Schmitt R |title=Dubious doses|url=https://www.pressreader.com/usa/newsweek/20170203/282071981620035|access-date=September 24, 2017|magazine=]|date=February 3, 2017}}</ref> Despite this, vinpocetine remains widely available in dietary supplements often marketed as ].<ref name="DTA2015">{{cite journal | vauthors = Avula B, Chittiboyina AG, Sagi S, Wang YH, Wang M, Khan IA, Cohen PA | title = Identification and quantification of vinpocetine and picamilon in dietary supplements sold in the United States | journal = Drug Testing and Analysis | volume = 8 | issue = 3–4 | pages = 334–343 | date = March 2016 | pmid = 26426301 | doi = 10.1002/dta.1853 | doi-access = free }}</ref><ref name="Mayo2015">{{cite journal | vauthors = Cohen PA | title = Vinpocetine: An Unapproved Drug Sold as a Dietary Supplement | journal = Mayo Clinic Proceedings | volume = 90 | issue = 10 | pages = 1455 | date = October 2015 | pmid = 26434971 | doi = 10.1016/j.mayocp.2015.07.008 | doi-access = free }}</ref><ref name="FTC070815"/><ref name="Erickson"/> | |||
| Vinpocetine has been identified as a potent anti-inflammatory agent that might have a potential role in the treatment of ] and ].<ref name="Jeon">{{Cite journal|last1=Jeon|first1=KI|last2=Xu|first2=X|last3=Aizawa|first3=T|last4=Lim|first4=JH|last5=Jono|first5=H|last6=Kwon|first6=DS|last7=Abe|first7=J|last8=Berk|first8=BC|last9=Li|first9=JD|title=Vinpocetine inhibits NF-kappaB-dependent inflammation via an IKK-dependent but PDE-independent mechanism.|journal=Proceedings of the National Academy of Sciences of the United States of America|volume=107|issue=21|pages=9795–800|doi=10.1073/pnas.0914414107|year=2010|pmc=2906898|pmid=20448200}}</ref><ref name="Medina">{{Cite journal|last1=Medina|first1=AE|title=Vinpocetine as a potent antiinflammatory agent.|journal=Proceedings of the National Academy of Sciences of the United States of America|volume=107|issue=22|pages=9921–2|doi=10.1073/pnas.1005138107|year=2010|pmc=2890434|pmid=20495091}}</ref> | |||
| Vinpocetine does not fully support a benefit in either ] or ].<ref name="Szatmari2003">{{cite journal | vauthors = Szatmari SZ, Whitehouse PJ | title = Vinpocetine for cognitive impairment and dementia | journal = The Cochrane Database of Systematic Reviews | volume = 2003 | issue = 1 | pages = CD003119 | year = 2003 | pmid = 12535455 | pmc = 8406981 | doi = 10.1002/14651858.CD003119 }}</ref><ref name="Mayo2015" /><ref name="Bereczki2009" /> As of 2003, three controlled ]s had tested "older adults with memory problems".<ref name="McDaniel2003">{{cite journal | vauthors = McDaniel MA, Maier SF, Einstein GO | title = "Brain-specific" nutrients: a memory cure? | journal = Nutrition | volume = 19 | issue = 11–12 | pages = 957–975 | year = 2003 | pmid = 14624946 | doi = 10.1016/S0899-9007(03)00024-8 }}</ref> | |||
| ==Controlled clinical trials== | |||
| As of 2003 only three controlled ]s had tested "older adults with memory problems,"<ref name=McDaniel2003/> and these studies had promising results,<ref name=McDaniel2003>{{Cite journal|author=McDaniel MA, Maier SF, Einstein GO |title='Brain-specific' nutrients: a memory cure? |journal=Nutrition |volume=19 |issue=11-12 |pages=957–75 |year=2003 |pmid=14624946 |doi=10.1016/S0899-9007(03)00024-8}}</ref> in which a 2003 Cochrane review decided that the results were inconclusive.<ref name=Szatmari2003>{{Cite journal|author=Szatmari SZ, Whitehouse PJ|editor1-last=Szatmári|editor1-first=Szabolcs |title=Vinpocetine for cognitive impairment and dementia |journal=Cochrane Database of Systematic Reviews |issue=1 |pages=CD003119 |year=2003 |pmid=12535455 |doi=10.1002/14651858.CD003119}}</ref> | |||
| Vinpocetine has also been studied for the prevention and recovery of acquired hearing loss in a phase II, longitudinal and prospective open clinical study on humans.<ref>{{cite journal | vauthors = Gutiérrez-Farfán I, Reyes-Legorreta C, Solís-Olguín M, Alatorre-Miguel E, Verduzco-Mendoza A, Durand-Rivera A | title = Evaluation of vinpocetine as a therapy in patients with sensorineural hearing loss: A phase II, open-label, single-center study | journal = Journal of Pharmacological Sciences | volume = 145 | issue = 4 | pages = 313–318 | date = April 2021 | pmid = 33712282 | doi = 10.1016/j.jphs.2021.01.010 | doi-access = free }}</ref> | |||
| Prior to 2003, a different study from 1985<ref>{{Cite journal|author=Subhan Z, Hindmarch I |title=Psychopharmacological effects of vinpocetine in normal healthy volunteers |journal=European Journal of Clinical Pharmacology |volume=28 |issue=5 |pages=567–71 |year=1985 |pmid=3899677 |doi=10.1007/BF00544068}}</ref> had tested young, healthy adults, but this study had 12 subjects and used a short treatment period.<ref name=McDaniel2003/> | |||
| ==Side effects== | |||
| ==Use as a vasodilator== | |||
| Use during pregnancy may harm the baby or result in ].<ref name="FDA2019Preg">{{cite web | author = Office of the Commissioner |date=3 June 2019|title=Statement on warning for women of childbearing age about possible safety risks of dietary supplements containing vinpocetine|url=https://www.fda.gov/news-events/press-announcements/statement-warning-women-childbearing-age-about-possible-safety-risks-dietary-supplements-containing|access-date=5 June 2019|website=FDA|language=en}}</ref> | |||
| Vinpocetine is widely used in the body building community as a ]. Although no studies have been conducted on the effectiveness of vinpocetine on performance enhancement during exercise, in trial both beneficial and adverse effects have been reported on body building forums. | |||
| Adverse effects of vinpocetine include flushing, nausea, dizziness, dry mouth, transient hypo- and hyper-tension, headaches, heartburn, and decreased blood pressure.<ref name="DTA2015" /><ref name="NTP">{{cite web |author1=National Toxicology Program |author-link=National Toxicology Program |title=Chemical Information Review Document for Vinpocetine (CAS No. 42971-09-5) |url=https://ntp.niehs.nih.gov/ntp/htdocs/chem_background/exsumpdf/vinpocetine091613_508.pdf |publisher=] |access-date=December 28, 2018 |date=September 2013}}</ref> FDA issued a statement in 2019 warning that "vinpocetine may cause a miscarriage or harm fetal development".<ref>{{Cite web|url=https://www.fda.gov/news-events/press-announcements/statement-warning-women-childbearing-age-about-possible-safety-risks-dietary-supplements-containing|title=Statement on warning for women of childbearing age about possible safety risks of dietary supplements containing vinpocetine | author = Office of the Commissioner |date=2019-06-03|website=FDA|language=en|access-date=2019-06-04}}</ref> | |||
| ==Anti-inflammatory action== | |||
| Vinpocetine has been identified as a novel ] agent.<ref name="Jeon"/><ref name="Medina"/> Vinpocetine inhibits the up-regulation of ] by ] in various cell tests. ] also shows that it reduced the TNFα-induced expression of the ] of proinflammatory molecules such as ], ] (MCP-1), and ] (VCAM-1). In mice, vinpocetine reduced ] inoculation induced ] infiltration into the lung.<ref name="Jeon"/><ref name="Medina"/> Neuroinflammatory processes can result in neuronal death in ] (PD) and ] (AD). It has been suggested that "it would be interesting to test whether vinpocetine’s antiinflammatory properties would have a protective effect in models of neurodegenerative conditions such as AD and PD."<ref name="Medina"/> | |||
| ==Mechanism of action== | ==Mechanism of action== | ||
| Vinpocetine’s ] has been postulated to involve three potential effects: blockage of ], reduction of cellular calcium influx, and ] activity.<ref name="Bereczki2009">{{cite journal | vauthors = Bereczki D, Fekete I | title = Vinpocetine for acute ischaemic stroke | journal = The Cochrane Database of Systematic Reviews | volume = 2008 | issue = 1 | pages = CD000480 | date = January 2008 | pmid = 18253980 | pmc = 7034523 | doi = 10.1002/14651858.CD000480.pub2 }}</ref> Studies have also suggested that vinpocetine can inhibit ] in isolated rabbit aorta;<ref>{{cite journal | vauthors = Hagiwara M, Endo T, Hidaka H | title = Effects of vinpocetine on cyclic nucleotide metabolism in vascular smooth muscle | journal = Biochemical Pharmacology | volume = 33 | issue = 3 | pages = 453–457 | date = February 1984 | pmid = 6322804 | doi = 10.1016/0006-2952(84)90240-5 }}</ref> inhibit ] ], preventing ] degradation and the following translocation of ] to the cell nucleus;<ref name="Jeon">{{cite journal | vauthors = Jeon KI, Xu X, Aizawa T, Lim JH, Jono H, Kwon DS, Abe J, Berk BC, Li JD, Yan C | title = Vinpocetine inhibits NF-kappaB-dependent inflammation via an IKK-dependent but PDE-independent mechanism | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 107 | issue = 21 | pages = 9795–9800 | date = May 2010 | pmid = 20448200 | pmc = 2906898 | doi = 10.1073/pnas.0914414107 | doi-access = free }}</ref><ref name="Medina">{{cite journal | vauthors = Medina AE | title = Vinpocetine as a potent antiinflammatory agent | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 107 | issue = 22 | pages = 9921–9922 | date = June 2010 | pmid = 20495091 | pmc = 2890434 | doi = 10.1073/pnas.1005138107 | doi-access = free | bibcode = 2010PNAS..107.9921M }}</ref> and increase ], a metabolic breakdown product of ], in isolated striatal nerve endings of rats.<ref name=pmid11478921>{{cite journal | vauthors = Trejo F, Nekrassov V, Sitges M | title = Characterization of vinpocetine effects on DA and DOPAC release in striatal isolated nerve endings | journal = Brain Research | volume = 909 | issue = 1–2 | pages = 59–67 | date = August 2001 | pmid = 11478921 | doi = 10.1016/S0006-8993(01)02621-X | s2cid = 38990597 }}</ref> | |||
| Vinpocetine has been shown to selectively inhibit voltage-sensitive Na+ channels, resulting in a dose-dependent decrease in evoked extracellular Ca+ ions in striatal nerve endings.<ref>{{Cite journal|author=Sitges M, Galván E, Nekrassov V |title=Vinpocetine blockade of sodium channels inhibits the rise in sodium and calcium induced by 4-aminopyridine in synaptosomes |journal=Neurochemistry International |volume=46 |issue=7 |pages=533–40 |year=2005 |pmid=15843047 |doi=10.1016/j.neuint.2005.02.001}}</ref> The Na+ channel inhibiting properties of vinpocetine are thought to contribute to a general neuroprotective effect through blockade of excitotoxicity and attenuation of neuronal damage induced by cerebral ischemia/reperfusion.<ref>{{Cite journal|author=Adám-Vizi V |title= |language=Hungarian |journal=Orvosi Hetilap |volume=141 |issue=23 |pages=1279–86 |year=2000 |pmid=10905082}}</ref> | |||
| ==Dietary supplement== | |||
| Vinpocetine is also a ] (PDE) type-1 inhibitor,<ref>{{Cite journal|author=Hagiwara M, Endo T, Hidaka H |title=Effects of vinpocetine on cyclic nucleotide metabolism in vascular smooth muscle |journal=Biochemical Pharmacology |volume=33 |issue=3 |pages=453–7 |year=1984 |pmid=6322804 |doi=10.1016/0006-2952(84)90240-5}}</ref> (with an ] of approximately 10<sup>−5</sup> M.) leading to increases in intracellular levels of cyclic guanosine 3'5'-monophosphate (cGMP), an action that causes the vasorelaxant effects of vinpocetine on cerebral smooth muscle tissue.<ref>{{Cite journal|author=Truss MC, Uckert S, Stief CG, Forssmann WG, Jonas U |title=Cyclic nucleotide phosphodiesterase (PDE) isoenzymes in the human detrusor smooth muscle. II. Effect of various PDE inhibitors on smooth muscle tone and cyclic nucleotide levels in vitro |journal=Urological Research |volume=24 |issue=3 |pages=129–34 |year=1996 |pmid=8839479}}</ref><ref>{{Cite journal|author=Gurkovskaia AV, Gokina NI, Buryĭ VA, Shuba MF |title= |language=Russian |journal=Biulleten' Eksperimental'noĭ Biologii I Meditsiny |volume=103 |issue=1 |pages=68–71 |year=1987 |pmid=3801654}}</ref> | |||
| The inclusion of vinpocetine in ] in the U.S. has come under scrutiny due to the lack of defined dosage parameters, unproven short- and long-term benefits, and risks to human health.<ref name="French">{{cite journal | vauthors = French JM, King MD, McDougal OM | title = Quantitative Determination of Vinpocetine in Dietary Supplements | journal = Natural Product Communications | volume = 11 | issue = 5 | pages = 607–609 | date = May 2016 | doi = 10.1177/1934578X1601100512 | pmid = 27319129 | pmc = 5345962 }}</ref> In the U.S., vinpocetine supplements are marketed as sports supplements, brain enhancers, and weight loss supplements.<ref name="Mayo2015"/> | |||
| A 2015 analysis of 23 brands of vinpocetine dietary supplements sold at ] and ] retail stores reported widespread labeling errors.<ref name="Newsweek_011217"/> Only 6 of the 23 supplement labels (26%) provided consumers with accurate dosages of vinpocetine (ranging from 0.3 to 32 mg per recommended daily serving), while 6 of 23 (26%) contained no vinpocetine at all, despite their labels claiming that the ingredient was in them.<ref name="Mayo2015"/><ref name="DTA2015"/> In total, 9 of the 23 products tested were mislabeled, and 17 of 23 (74%) did not provide any information on the quantity of vinpocetine.<ref name="DTA2015"/> | |||
| Independent of vinpocetine's action on PDE, vinpocetine inhibits ] preventing ] degradation and the following translocation of ] to the cell nucleus.<ref name="Jeon"/><ref name="Medina"/> | |||
| In response to the study, then-senator ], while at the time serving as the top ] on the ], urged the FDA to suspend sales of vinpocetine supplements and asked 10 retailers to voluntarily stop selling vinpocetine products. McCaskill stated: "The way we regulate these supplements isn’t working—and it’s putting the lives and well-being of consumers at risk. We’ve seen products with false labels, tainted ingredients, wildly illegal claims, and, now, products containing synthesized ingredients that are classified as prescription drugs in other countries."<ref name="Erickson">{{cite journal | vauthors = Erickson BE |title=Vinpocetine: drug or dietary supplement? |journal=Chemical & Engineering News |date=October 31, 2016 |volume=94 |issue=43 |pages=16–17 |url=https://cen.acs.org/content/cen/articles/94/i43/Vinpocetine-drug-dietary-supplement.html |access-date=December 28, 2018 |issn=0009-2347}}</ref> | |||
| Increases in neuronal levels of DOPAC, a metabolic breakdown product of ], have been shown to occur in striatal isolated nerve endings as a result of exposure to vinpocetine.<ref name=pmid11478921>{{Cite journal|author=Trejo F, Nekrassov V, Sitges M |title=Characterization of vinpocetine effects on DA and DOPAC release in striatal isolated nerve endings |journal=Brain Research |volume=909 |issue=1-2 |pages=59–67 |year=2001 |pmid=11478921 |doi=10.1016/S0006-8993(01)02621-X}}</ref> Such an effect is consistent with the biogenic pharmacology of ], a structural relative of vinpocetine, which depletes catecholamine levels and causes ] as a side effect of the cardiovascular and anti-psychotic effects.<ref name=pmid11478921/> However, this effect tends to be reversible upon cessation of Vinpocetine administration, with full remission typically occurring within 3–4 weeks. | |||
| == |
===Lawsuits=== | ||
| Procera AVH is a dietary supplement containing undisclosed amounts of vinpocetine in combination with ] and ].<ref name="TBT070915">{{cite news | vauthors = McGrory K |title=Tampa diet supplement firm pays $1.4 million settlement over 'brain power' pill claims |url=https://www.tampabay.com/news/health/tampa-distributor-related-companies-face-14-million-penalty-for-brain/2236830 |access-date=January 1, 2019 |newspaper=] |date=July 9, 2015}}</ref><ref name="SBM091812">{{cite web | vauthors = Hall H |title=Procera AVH: A Pill to Restore Memory |url=https://sciencebasedmedicine.org/procera-avh-a-pill-to-restore-memory/ |website=] |access-date=January 1, 2019 |date=September 18, 2012}}</ref> In 2012, manufacturer Brain Research Labs (BRL) agreed to pay $500,000 to settle a class action lawsuit which alleged that the company had falsely marketed Procera AVH as capable of improving brain function, in violation of the Consumer Fraud Act.<ref name="OCR070615">{{cite news | vauthors = Almada B |title=False claims for brain supplement draw $152 million penalty from FTC |url=https://www.ocregister.com/2015/07/06/false-claims-for-brain-supplement-draw-152-million-penalty-from-ftc/ |access-date=January 1, 2019 |newspaper=] |date=July 6, 2015}}</ref> | |||
| Vinpocetine is generally tolerated well and without many cases of adverse reaction reported.<ref>{{Cite web|url=http://thyroid.about.com/cs/alternativehelp/a/vinpocetine.htm |title=Is Vinpocetine the Answer to Brain Fog, Cognitive and Memory Problems? |publisher=about.com |accessdate=2011-06-30}}</ref> No serious side effects have been reported in any clinical trials,<ref>{{Cite web|url=http://www.foundhealth.com/vinpocetine/side-effects-and-warnings |title=Vinpocetine Side Effects and Warnings |publisher=foundhealth |accessdate=2011-07-02}}</ref> although none of these trials have been long-term.<ref name=Szatmari2003/> According to a Dr. Wollschlaeger, "a critical review of the literature has reported no adverse effects. Vinpocetine appears to be safe, without any adverse effects. The only reported side effect, in a very small number of cases, was a slightly upset stomach, which is almost always a side effect for some people taking herbs. We have not seen any adverse effects or drug-herb interactions, and it seems safe to take with other drugs, including diabetes drugs, and blood thinners like ]."<ref>{{Cite web|url=http://thyroid.about.com/cs/alternativehelp/a/vinpocetine.htm |title=Is Vinpocetine the Answer to Brain Fog, Cognitive and Memory Problems? |publisher=about.com |accessdate=2011-06-30}}</ref> | |||
| In July 2015, the ] (FTC) ruled that marketing claims for Procera AVH, which promoted the product as a “solution” to memory loss and cognitive decline, were false, misleading, unsubstantiated, and in violation of the FTC Act.<ref name="OCR070615"/><ref name="FTC070815">{{cite web |title=Supplement Marketers Will Relinquish $1.4 Million to Settle FTC Deceptive Advertising Charges |newspaper=Federal Trade Commission |url=https://www.ftc.gov/news-events/press-releases/2015/07/supplement-marketers-will-relinquish-14-million-settle-ftc |publisher=] |access-date=January 1, 2019 |date=July 8, 2015}}</ref><ref name="NLR072015">{{cite magazine |title=Procera AVH Marketers Can Forget About Claiming to Reverse Memory Loss |date=July 30, 2015 |url=https://www.natlawreview.com/article/procera-avh-marketers-can-forget-about-claiming-to-reverse-memory-loss |access-date=1 January 2019 |magazine=]}}</ref><ref name="NPI070815">{{cite web | vauthors = Myers S |title=Memory Supplement Marketers Settle FTC Case for $150M |url=https://www.naturalproductsinsider.com/claims/memory-supplement-marketers-settle-ftc-case-150m |website= Natural Products Insider |access-date=1 January 2019 |date=July 8, 2015}}</ref> BRL and its affiliated companies Brain Power Partners, Brain Power Founders, and MedHealth Direct (all based in ]) were fined $91 million. KeyView Labs, the ]-based company that purchased BRL in 2012, was fined $61 million.<ref name="OCR070615"/><ref name="NLR072015"/><ref name="FTC070815"/><ref name="NPI070815"/> Also named in the FTC complaint were George Reynolds (aka Josh Reynolds), founder and chief science officer of BRL, and John Arnold, the sole officer and employee of MedHealth. The FTC complaint charged Reynolds with making deceptive expert endorsements for Procera AVH.<ref name="TBT070915"/><ref name="OCR070615"/><ref name="NLR072015"/><ref name="FTC070815"/><ref name="NPI070815"/> The defendants in the case ultimately agreed to pay $1.4 million to settle the allegations of deceptive advertising brought by the FTC and California law enforcement officials. In addition, a permanent injunction barred the defendants from making similar deceptive claims about Procera AVH in the future and from misrepresenting the existence, results, or conclusions of any scientific study.<ref name="NLR072015"/><ref name="FTC070815"/> | |||
| The safety of vinpocetine in pregnant women has not been evaluated. | |||
| == References == | |||
| Vinpocetine has been implicated in one case to induce ],<ref>Shimizu Y, Saitoh K, Nakayama M, et al. . Medicine Online, Retrieved March 08, 2008.</ref> a condition in which ] are markedly decreased. Some people have anecdotally noted that their continued use of vinpocetine reduces immune function. ] warned that vinpocetine reduced immune function and could cause ] in the long term.<ref>''The Complete German Commission E Monographs, Therapeutic Guide to Herbal Medicines'', 1st ed. 1998, Integrative Medicine Communications, pub; Bk&CD-Rom edition, 1999.{{Page needed|date=September 2010}}</ref> | |||
| {{Reflist}} | |||
| Other alkaloids extracted from the periwinkle family, including ] and ] are powerful chemotherapeutic agents which impair formation of microtubules and thus growth of related cancers, intestinal epithelium and bone marrow. | |||
| ==Dosage== | |||
| {{Unreferenced section|date=September 2011}} | |||
| It is recommended that first-time users ingest only 2–5 mg of vinpocetine with meals to make sure they are not hypersensitive to it. Users may then increase the dosage to 10–40 mg a day (which may, although very rarely, cause some light side effects). | |||
| ==External links== | |||
| * ] | |||
| * from ] | |||
| * - ] review | |||
| * | |||
| ==References== | |||
| {{Reflist|2}} | |||
| {{Stimulants}} | |||
| {{Phosphodiesterase inhibitors}} | {{Phosphodiesterase inhibitors}} | ||
| {{Psychostimulants, agents used for ADHD and nootropics}} | |||
| ] | ] | ||
| Line 104: | Line 91: | ||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 05:29, 12 May 2024
Chemical compoundPharmaceutical compound
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Pregnancy category |
|
| Routes of administration | Oral, intravenous |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 56.6 ± 8.9% |
| Metabolism | hepatic |
| Elimination half-life | 2.54 ± 0.48 hours |
| Excretion | renal |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.050.917 |
| Chemical and physical data | |
| Formula | C22H26N2O2 |
| Molar mass | 350.462 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (what is this?) (verify) | |
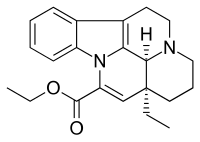
Vinpocetine (ethyl apovincaminate) is a synthetic derivative of the vinca alkaloid vincamine, differing by the removal of a hydroxyl group and by being the ethyl rather than the methyl ester of the underlying carboxylic acid. Vincamine is extracted from either the seeds of Voacanga africana or the leaves of Vinca minor (lesser periwinkle).
Medical uses
Vinpocetine has been used in many Asian and European countries for treatment of cerebrovascular disorders such as stroke and dementia for over three decades.
The FDA has tentatively ruled that vinpocetine, due to its synthetic nature and proposed therapeutic uses, is ineligible to be marketed as dietary supplement under the Federal Food, Drug, and Cosmetic Act. Despite this, vinpocetine remains widely available in dietary supplements often marketed as nootropics.
Vinpocetine does not fully support a benefit in either dementia or stroke. As of 2003, three controlled clinical trials had tested "older adults with memory problems".
Vinpocetine has also been studied for the prevention and recovery of acquired hearing loss in a phase II, longitudinal and prospective open clinical study on humans.
Side effects
Use during pregnancy may harm the baby or result in miscarriage.
Adverse effects of vinpocetine include flushing, nausea, dizziness, dry mouth, transient hypo- and hyper-tension, headaches, heartburn, and decreased blood pressure. FDA issued a statement in 2019 warning that "vinpocetine may cause a miscarriage or harm fetal development".
Mechanism of action
Vinpocetine’s mechanism of action has been postulated to involve three potential effects: blockage of sodium channels, reduction of cellular calcium influx, and antioxidant activity. Studies have also suggested that vinpocetine can inhibit PDE-1 in isolated rabbit aorta; inhibit IKK in vitro, preventing IκB degradation and the following translocation of NF-κB to the cell nucleus; and increase DOPAC, a metabolic breakdown product of dopamine, in isolated striatal nerve endings of rats.
Dietary supplement
The inclusion of vinpocetine in dietary supplements in the U.S. has come under scrutiny due to the lack of defined dosage parameters, unproven short- and long-term benefits, and risks to human health. In the U.S., vinpocetine supplements are marketed as sports supplements, brain enhancers, and weight loss supplements.
A 2015 analysis of 23 brands of vinpocetine dietary supplements sold at GNC and Vitamin Shoppe retail stores reported widespread labeling errors. Only 6 of the 23 supplement labels (26%) provided consumers with accurate dosages of vinpocetine (ranging from 0.3 to 32 mg per recommended daily serving), while 6 of 23 (26%) contained no vinpocetine at all, despite their labels claiming that the ingredient was in them. In total, 9 of the 23 products tested were mislabeled, and 17 of 23 (74%) did not provide any information on the quantity of vinpocetine.
In response to the study, then-senator Claire McCaskill, while at the time serving as the top Democrat on the Senate Special Committee on Aging, urged the FDA to suspend sales of vinpocetine supplements and asked 10 retailers to voluntarily stop selling vinpocetine products. McCaskill stated: "The way we regulate these supplements isn’t working—and it’s putting the lives and well-being of consumers at risk. We’ve seen products with false labels, tainted ingredients, wildly illegal claims, and, now, products containing synthesized ingredients that are classified as prescription drugs in other countries."
Lawsuits
Procera AVH is a dietary supplement containing undisclosed amounts of vinpocetine in combination with huperzine A and acetyl-l-carnitine. In 2012, manufacturer Brain Research Labs (BRL) agreed to pay $500,000 to settle a class action lawsuit which alleged that the company had falsely marketed Procera AVH as capable of improving brain function, in violation of the Consumer Fraud Act.
In July 2015, the U.S. Federal Trade Commission (FTC) ruled that marketing claims for Procera AVH, which promoted the product as a “solution” to memory loss and cognitive decline, were false, misleading, unsubstantiated, and in violation of the FTC Act. BRL and its affiliated companies Brain Power Partners, Brain Power Founders, and MedHealth Direct (all based in Laguna Beach, California) were fined $91 million. KeyView Labs, the Tampa, Florida-based company that purchased BRL in 2012, was fined $61 million. Also named in the FTC complaint were George Reynolds (aka Josh Reynolds), founder and chief science officer of BRL, and John Arnold, the sole officer and employee of MedHealth. The FTC complaint charged Reynolds with making deceptive expert endorsements for Procera AVH. The defendants in the case ultimately agreed to pay $1.4 million to settle the allegations of deceptive advertising brought by the FTC and California law enforcement officials. In addition, a permanent injunction barred the defendants from making similar deceptive claims about Procera AVH in the future and from misrepresenting the existence, results, or conclusions of any scientific study.
References
- ^ Office of the Commissioner (3 June 2019). "Statement on warning for women of childbearing age about possible safety risks of dietary supplements containing vinpocetine". FDA. Retrieved 5 June 2019.
- "Vinpocetine in Dietary Supplements". FDA. February 22, 2023. Retrieved June 9, 2023.
- Zhang YS, Li JD, Yan C (January 2018). "An update on vinpocetine: New discoveries and clinical implications". European Journal of Pharmacology. 819: 30–34. doi:10.1016/j.ejphar.2017.11.041. PMC 5766389. PMID 29183836.
- ^ Schmitt R (January 12, 2017). "Marketers exploit the aged with unproven brain-health claims". Newsweek. Retrieved January 18, 2016.
- Hank S (7 September 2016). "FDA rules vinpocetine not a legal dietary ingredient despite successful NDI filings". NutraIngredients. William Reed Business Media, England. Retrieved September 8, 2016.
- "FDA Concludes Vinpocetine Ineligible as a Dietary Ingredient". Nutraceuticals World. Rodman Media. September 20, 2016. Retrieved January 18, 2017.
- Schmitt R (February 3, 2017). "Dubious doses". Newsweek. Retrieved September 24, 2017.
- ^ Avula B, Chittiboyina AG, Sagi S, Wang YH, Wang M, Khan IA, et al. (March 2016). "Identification and quantification of vinpocetine and picamilon in dietary supplements sold in the United States". Drug Testing and Analysis. 8 (3–4): 334–343. doi:10.1002/dta.1853. PMID 26426301.
- ^ Cohen PA (October 2015). "Vinpocetine: An Unapproved Drug Sold as a Dietary Supplement". Mayo Clinic Proceedings. 90 (10): 1455. doi:10.1016/j.mayocp.2015.07.008. PMID 26434971.
- ^ "Supplement Marketers Will Relinquish $1.4 Million to Settle FTC Deceptive Advertising Charges". Federal Trade Commission. U.S. Federal Trade Commission. July 8, 2015. Retrieved January 1, 2019.
- ^ Erickson BE (October 31, 2016). "Vinpocetine: drug or dietary supplement?". Chemical & Engineering News. 94 (43): 16–17. ISSN 0009-2347. Retrieved December 28, 2018.
- Szatmari SZ, Whitehouse PJ (2003). "Vinpocetine for cognitive impairment and dementia". The Cochrane Database of Systematic Reviews. 2003 (1): CD003119. doi:10.1002/14651858.CD003119. PMC 8406981. PMID 12535455.
- ^ Bereczki D, Fekete I (January 2008). "Vinpocetine for acute ischaemic stroke". The Cochrane Database of Systematic Reviews. 2008 (1): CD000480. doi:10.1002/14651858.CD000480.pub2. PMC 7034523. PMID 18253980.
- McDaniel MA, Maier SF, Einstein GO (2003). ""Brain-specific" nutrients: a memory cure?". Nutrition. 19 (11–12): 957–975. doi:10.1016/S0899-9007(03)00024-8. PMID 14624946.
- Gutiérrez-Farfán I, Reyes-Legorreta C, Solís-Olguín M, Alatorre-Miguel E, Verduzco-Mendoza A, Durand-Rivera A (April 2021). "Evaluation of vinpocetine as a therapy in patients with sensorineural hearing loss: A phase II, open-label, single-center study". Journal of Pharmacological Sciences. 145 (4): 313–318. doi:10.1016/j.jphs.2021.01.010. PMID 33712282.
- National Toxicology Program (September 2013). "Chemical Information Review Document for Vinpocetine (CAS No. 42971-09-5)" (PDF). U.S. Department of Health and Human Services. Retrieved December 28, 2018.
- Office of the Commissioner (2019-06-03). "Statement on warning for women of childbearing age about possible safety risks of dietary supplements containing vinpocetine". FDA. Retrieved 2019-06-04.
- Hagiwara M, Endo T, Hidaka H (February 1984). "Effects of vinpocetine on cyclic nucleotide metabolism in vascular smooth muscle". Biochemical Pharmacology. 33 (3): 453–457. doi:10.1016/0006-2952(84)90240-5. PMID 6322804.
- Jeon KI, Xu X, Aizawa T, Lim JH, Jono H, Kwon DS, et al. (May 2010). "Vinpocetine inhibits NF-kappaB-dependent inflammation via an IKK-dependent but PDE-independent mechanism". Proceedings of the National Academy of Sciences of the United States of America. 107 (21): 9795–9800. doi:10.1073/pnas.0914414107. PMC 2906898. PMID 20448200.
- Medina AE (June 2010). "Vinpocetine as a potent antiinflammatory agent". Proceedings of the National Academy of Sciences of the United States of America. 107 (22): 9921–9922. Bibcode:2010PNAS..107.9921M. doi:10.1073/pnas.1005138107. PMC 2890434. PMID 20495091.
- Trejo F, Nekrassov V, Sitges M (August 2001). "Characterization of vinpocetine effects on DA and DOPAC release in striatal isolated nerve endings". Brain Research. 909 (1–2): 59–67. doi:10.1016/S0006-8993(01)02621-X. PMID 11478921. S2CID 38990597.
- French JM, King MD, McDougal OM (May 2016). "Quantitative Determination of Vinpocetine in Dietary Supplements". Natural Product Communications. 11 (5): 607–609. doi:10.1177/1934578X1601100512. PMC 5345962. PMID 27319129.
- ^ McGrory K (July 9, 2015). "Tampa diet supplement firm pays $1.4 million settlement over 'brain power' pill claims". Tampa Bay Times. Retrieved January 1, 2019.
- Hall H (September 18, 2012). "Procera AVH: A Pill to Restore Memory". Science Based Medicine. Retrieved January 1, 2019.
- ^ Almada B (July 6, 2015). "False claims for brain supplement draw $152 million penalty from FTC". Orange County Register. Retrieved January 1, 2019.
- ^ "Procera AVH Marketers Can Forget About Claiming to Reverse Memory Loss". National Law Review. July 30, 2015. Retrieved 1 January 2019.
- ^ Myers S (July 8, 2015). "Memory Supplement Marketers Settle FTC Case for $150M". Natural Products Insider. Retrieved 1 January 2019.