| Revision as of 01:58, 19 September 2012 edit71.20.8.226 (talk) →Brain tumors: clarity← Previous edit | Latest revision as of 00:52, 19 January 2025 edit undoJayCubby (talk | contribs)Extended confirmed users5,946 edits Changing short description from "Chemical compound used in plastics manufacturing" to "Chemicals used in plastics manufacturing"Tag: Shortdesc helper | ||
| Line 1: | Line 1: | ||
| {{Short description|Chemicals used in plastics manufacturing}} | |||
| {{Use dmy dates|date=September 2012}} | |||
| {{cs1 config|name-list-style=vanc|display-authors=3}} | |||
| {{chembox | |||
| {{Good article}} | |||
| | verifiedrevid = 476999384 | |||
| {{Use dmy dates|date=December 2020}} | |||
| | Name = Bisphenol A | |||
| {{Chembox | |||
| | ImageFile1_Ref = {{chemboximage|correct|??}} | |||
| |Watchedfields = changed | |||
| | ImageFile1 = Bisphenol A.svg | |||
| |verifiedrevid = 477162965 | |||
| | ImageSize1 = 240px | |||
| | |
|Name = Bisphenol A | ||
| |ImageFile1_Ref = {{chemboximage|correct|??}} | |||
| | ImageSize2 = 180px | |||
| | |
|ImageFile1 = Bisphenol-A-Skeletal.svg | ||
| |ImageSize1 = 240px | |||
| | IUPACName = 4,4'-(propane-2,2-diyl)diphenol | |||
| |ImageFile2 = Bisphenol A.png | |||
| | OtherNames = BPA, ''p'',''p'''-isopropylidenebisphenol,<br/> 2,2-bis(4-hydroxyphenyl)propane. | |||
| |ImageSize2 = 180px | |||
| | Section1 = {{Chembox Identifiers | |||
| |ImageName = Bisphenol A | |||
| | ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| |PIN = 4,4′-(Propane-2,2-diyl)diphenol | |||
| | ChEBI = 33216 | |||
| |OtherNames = {{unbulleted list|BPA|Diphenylolpropane|''p'',''p''-Isopropylidenebisphenol|2,2-Bis(4-hydroxyphenyl)propane|2,2-Di(4-phenylol)propane}} | |||
| | DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| |Section1={{Chembox Identifiers | |||
| | DrugBank = DB06973 | |||
| |IUPHAR_ligand = 7865 | |||
| | SMILES = Oc1ccc(cc1)C(c2ccc(O)cc2)(C)C | |||
| | |
|ChEBI_Ref = {{ebicite|correct|EBI}} | ||
| | |
|ChEBI = 33216 | ||
| | |
|DrugBank_Ref = {{drugbankcite|correct|drugbank}} | ||
| | |
|DrugBank = DB06973 | ||
| |SMILES = Oc1ccc(cc1)C(c2ccc(O)cc2)(C)C | |||
| | InChI = 1/C15H16O2/c1-15(2,11-3-7-13(16)8-4-11)12-5-9-14(17)10-6-12/h3-10,16-17H,1-2H3 | |||
| |UNII_Ref = {{fdacite|correct|FDA}} | |||
| | InChIKey = IISBACLAFKSPIT-UHFFFAOYAI | |||
| |UNII = MLT3645I99 | |||
| | SMILES1 = CC(C)(c1ccc(cc1)O)c2ccc(cc2)O | |||
| | |
|KEGG_Ref = {{keggcite|correct|kegg}} | ||
| | |
|KEGG = C13624 | ||
| |InChI = 1/C15H16O2/c1-15(2,11-3-7-13(16)8-4-11)12-5-9-14(17)10-6-12/h3-10,16-17H,1-2H3 | |||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| |InChIKey = IISBACLAFKSPIT-UHFFFAOYAI | |||
| | StdInChI = 1S/C15H16O2/c1-15(2,11-3-7-13(16)8-4-11)12-5-9-14(17)10-6-12/h3-10,16-17H,1-2H3 | |||
| |SMILES1 = CC(C)(c1ccc(cc1)O)c2ccc(cc2)O | |||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| |ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| | StdInChIKey = IISBACLAFKSPIT-UHFFFAOYSA-N | |||
| | |
|ChEMBL = 418971 | ||
| | |
|StdInChI_Ref = {{stdinchicite|correct|chemspider}} | ||
| |StdInChI = 1S/C15H16O2/c1-15(2,11-3-7-13(16)8-4-11)12-5-9-14(17)10-6-12/h3-10,16-17H,1-2H3 | |||
| | PubChem = 6623 | |||
| |StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| | EINECS = 201-245-8 | |||
| |StdInChIKey = IISBACLAFKSPIT-UHFFFAOYSA-N | |||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| |CASNo = 80-05-7 | |||
| | ChemSpiderID = 6371 | |||
| |CASNo_Ref = {{cascite|correct|CAS}} | |||
| | RTECS = SL6300000 | |||
| | |
|PubChem = 6623 | ||
| |EINECS = 201-245-8 | |||
| }} | |||
| |ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| | Section2 = {{Chembox Properties | |||
| |ChemSpiderID = 6371 | |||
| | C=15|H=16|O=2 | |||
| |RTECS = SL6300000 | |||
| | Appearance = White solid | |||
| |UNNumber = 2430 | |||
| | Density = 1.20 g/cm³ | |||
| | Solubility = 120–300 ppm (21.5 °C) | |||
| | MeltingPtCL = 158 | |||
| | MeltingPtCH = 159 | |||
| | BoilingPtC = 220 | |||
| | Boiling_notes = 4 mmHg | |||
| | Viscosity = | |||
| }} | }} | ||
| | |
|Section2={{Chembox Properties | ||
| |C=15 | H=16 | O=2 | |||
| | CrystalStruct = | |||
| |Appearance = White solid | |||
| | Dipole = | |||
| |Odour = Phenolic, medical | |||
| }} | |||
| |Density = 1.217 g/cm<sup>3</sup><ref>{{cite journal |last1=Lim |first1=Caitlin F. |last2=Tanski |first2=Joseph M. |title=Structural Analysis of Bisphenol-A and its Methylene, Sulfur, and Oxygen Bridged Bisphenol Analogs |journal=Journal of Chemical Crystallography |date=3 August 2007 |volume=37 |issue=9 |pages=587–595 |doi=10.1007/s10870-007-9207-8|bibcode=2007JCCry..37..587L |s2cid=97284173 }}</ref> | |||
| | Section7 = {{Chembox Hazards | |||
| |Solubility = 0.3 g/L (25 °C)<ref name=water-solubility>{{cite journal |last1=Shareef |first1=Ali |last2=Angove |first2=Michael J. |last3=Wells |first3=John D. |last4=Johnson |first4=Bruce B. |title=Aqueous Solubilities of Estrone, 17β-Estradiol, 17α-Ethynylestradiol, and Bisphenol A |journal=Journal of Chemical & Engineering Data |date=11 May 2006 |volume=51 |issue=3 |pages=879–881 |doi=10.1021/je050318c}}</ref> | |||
| | ExternalMSDS = | |||
| |MeltingPtC = 155 | |||
| | EUIndex = | |||
| |MeltingPt_ref =<ref name=mbt-mpt>{{cite journal |last1=Mitrofanova |first1=S. E. |last2=Bakirova |first2=I. N. |last3=Zenitova |first3=L. A. |last4=Galimzyanova |first4=A. R. |last5=Nefed'ev |first5=E. S. |title=Polyurethane varnish materials based on diphenylolpropane |journal=Russian Journal of Applied Chemistry |date=September 2009 |volume=82 |issue=9 |pages=1630–1635 |doi=10.1134/S1070427209090225|s2cid=98036316 }}</ref> | |||
| | EUClass = | |||
| |BoilingPtC = 250-252 | |||
| | NFPA-H = 3 | |||
| |BoilingPt_notes = at {{convert|13|Torr|atm}} | |||
| | NFPA-F = 0 | |||
| |BoilingPt_ref =<ref name=mbt-mpt /> | |||
| | NFPA-R = 0 | |||
| |LogP = 3.41<ref>{{cite journal |last1=Robinson |first1=Brian J. |last2=Hui |first2=Joseph P.M. |last3=Soo |first3=Evelyn C. |last4=Hellou |first4=Jocelyne |title=Estrogenic Compounds in Seawater and Sediment from Halifax Harbour, Nova Scotia, Canada |journal=Environmental Toxicology and Chemistry |date=2009 |volume=28 |issue=1 |pages=18–25 |doi=10.1897/08-203.1|pmid=18702564 |bibcode=2009EnvTC..28...18R |s2cid=13528747 }}</ref> | |||
| | RPhrases = {{R36}} {{R37}} {{R38}} {{R43}} | |||
| |VaporPressure = {{nowrap|5×10<sup>−6</sup> Pa}} (25 °C)<ref>{{cite web|url=http://www.speclab.com/compound/c80057.htm|website=speclab.com|title=Chemical Fact Sheet – Cas #80057 CASRN 80-05-7|date=1 April 2012|access-date=14 June 2012|archive-url=https://web.archive.org/web/20120212033315/http://www.speclab.com/compound/c80057.htm|archive-date=12 February 2012|url-status=dead}}</ref> | |||
| | SPhrases = {{S24}} {{S26}} {{S37}} | |||
| | FlashPt = {{convert|227|C|F}} | |||
| | autoignition = {{convert|600|C|F}} | |||
| }} | |||
| | Section8 = {{Chembox Related | |||
| | Function = | |||
| | OtherFunctn = | |||
| | OtherCpds = ]<br/>] | |||
| }} | |||
| }} | }} | ||
| |Section3={{Chembox Hazards | |||
| |Hazards_ref = <ref name="Sigma">{{Sigma-Aldrich|id=239658|name=Bisphenol A|access-date=22-05-2022}}</ref> | |||
| |NFPA-H = 2 | |||
| |NFPA-F = 1 | |||
| |NFPA-R = 0 | |||
| |GHSPictograms = {{GHS05}}{{GHS07}}{{GHS08}}{{GHS09}} | |||
| |GHSSignalWord = Danger | |||
| |HPhrases = {{H-phrases|317|318|335|360|H411}}<ref name="Sigma" /> | |||
| |PPhrases = {{P-phrases|201|202|261|273|302+352|304+340|305+351+338|308+313|333+313|363|403+233}}<ref name="Sigma" /> | |||
| |FlashPtC = 227 | |||
| |FlashPt_ref =<ref name="Sigma"/> | |||
| |AutoignitionPtC = 510 | |||
| |AutoignitionPt_ref=<ref name="Sigma"/> | |||
| }} | |||
| }} | |||
| '''Bisphenol A''' ('''BPA''') is a ] primarily used in the manufacturing of various ]s. It is a colourless solid which is ] in most common organic ]s, but has very poor solubility in water.<ref name=water-solubility/><ref name="Fiege" /> BPA is produced on an industrial scale by the ] of ] and ]. Global production in 2022 was estimated to be in the region of 10 million tonnes.<ref name=production /> | |||
| BPA's largest single application is as a ] in the production of ]s, which accounts for 65–70% of all BPA production.<ref name=EU2008 /><ref name="Tom2021"/> The manufacturing of ]s and ]s account for 25–30% of BPA use.<ref name=EU2008/><ref name="Tom2021" /> The remaining 5% is used as a major component of several ], and as a minor additive in ], ], ], and several other materials. It is not a ],<ref>{{cite book |doi=10.1002/14356007.a20_439|chapter=Plasticizers|title=Ullmann's Encyclopedia of Industrial Chemistry|year=2000| vauthors = Cadogan DF, Howick CJ |isbn=3527306730}}</ref> although it is often wrongly labelled as such. | |||
| '''Bisphenol A''' ('''BPA''') is an ] with the ] (CH<sub>3</sub>)<sub>2</sub>C(C<sub>6</sub>H<sub>4</sub>OH)<sub>2</sub>. It is a colorless solid that is soluble in organic solvents, but poorly soluble in water. Having two ] ]s, it is used to make ] polymers and ], along with other materials used to make plastics. {{nowrap|Bisphenol A}} has a vapor pressure of {{nowrap|5*10<sup>-6</sup> Pa}}.<ref>, 1 April 2012</ref> | |||
| The health effects of BPA have been the subject of prolonged public and scientific debate.<ref name=WHO /><ref name=German2011 /><ref name=GLP /> BPA is a ], exhibiting hormone-like properties that mimic the effects of ] in the body.<ref>{{cite journal| vauthors = Egan M |title=Sarah A. Vogel. Is It Safe? BPA and the Struggle to Define the Safety of Chemicals|location=Berkeley|publisher=University of California Press |date=2013 |journal=Isis |volume=105 |issue=1 |pages=254 |doi=10.1086/676809 |issn=0021-1753}}</ref> Although the effect is very weak,<ref name=Xenochemicals /> the pervasiveness of BPA-containing materials raises concerns, as exposure is effectively lifelong. Many BPA-containing materials are non-obvious but commonly encountered,<ref name="Covaci">{{cite journal | vauthors = Geens T, Aerts D, Berthot C, Bourguignon JP, Goeyens L, Lecomte P, Maghuin-Rogister G, Pironnet AM, Pussemier L, Scippo ML, Van Loco J, Covaci A | title = A review of dietary and non-dietary exposure to bisphenol-A | journal = Food and Chemical Toxicology | volume = 50 | issue = 10 | pages = 3725–3740 | date = October 2012 | pmid = 22889897 | doi = 10.1016/j.fct.2012.07.059 | url = https://dipot.ulb.ac.be/dspace/bitstream/2013/195906/1/Elsevier_179533.pdf }}</ref> and include coatings for the inside of ]s,<ref>{{cite journal | vauthors = Noonan GO, Ackerman LK, Begley TH | title = Concentration of bisphenol A in highly consumed canned foods on the U.S. market | journal = Journal of Agricultural and Food Chemistry | volume = 59 | issue = 13 | pages = 7178–7185 | date = July 2011 | pmid = 21598963 | doi = 10.1021/jf201076f | bibcode = 2011JAFC...59.7178N }}</ref> clothing designs,<ref name="ir.rcees.ac.cn">{{cite journal | vauthors = Xue J, Liu W, Kannan K | title = Bisphenols, Benzophenones, and Bisphenol A Diglycidyl Ethers in Textiles and Infant Clothing | journal = Environmental Science & Technology | volume = 51 | issue = 9 | pages = 5279–5286 | date = May 2017 | pmid = 28368574 | doi = 10.1021/acs.est.7b00701 | bibcode = 2017EnST...51.5279X | url = http://ir.rcees.ac.cn/handle/311016/39260 | access-date = 12 April 2022 | archive-date = 29 December 2022 | archive-url = https://web.archive.org/web/20221229150022/https://ir.rcees.ac.cn/handle/311016/39260 | url-status = dead }}</ref> shop receipts,<ref name=paper1/> and dental fillings.<ref name="teeth">{{cite journal | vauthors = Ahovuo-Saloranta A, Forss H, Walsh T, Nordblad A, Mäkelä M, Worthington HV | title = Pit and fissure sealants for preventing dental decay in permanent teeth | journal = The Cochrane Database of Systematic Reviews | volume = 2017 | pages = CD001830 | date = July 2017 | issue = 7 | pmid = 28759120 | pmc = 6483295 | doi = 10.1002/14651858.CD001830.pub5 }}</ref> BPA has been investigated by public health agencies in many countries, as well as by the ].<ref name=WHO /> While normal exposure is below the level currently associated with risk, several jurisdictions have taken steps to reduce exposure on a precautionary basis, in particular by banning BPA from baby bottles. There is some evidence that BPA exposure in infants has decreased as a result of this.<ref name="auto"/> BPA-free plastics have also been introduced, which are manufactured using alternative bisphenols such as ] and ], but there is also controversy around whether these are actually safer.<ref>{{cite journal | vauthors = Thoene M, Dzika E, Gonkowski S, Wojtkiewicz J | title = Bisphenol S in Food Causes Hormonal and Obesogenic Effects Comparable to or Worse than Bisphenol A: A Literature Review | journal = Nutrients | volume = 12 | issue = 2 | pages = 532 | date = February 2020 | pmid = 32092919 | pmc = 7071457 | doi = 10.3390/nu12020532 | doi-access = free }}</ref><ref name=Other>{{cite journal |last1=Chen |first1=Da |last2=Kannan |first2=Kurunthachalam |last3=Tan |first3=Hongli |last4=Zheng |first4=Zhengui |last5=Feng |first5=Yong-Lai |last6=Wu |first6=Yan |last7=Widelka |first7=Margaret |title=Bisphenol Analogues Other Than BPA: Environmental Occurrence, Human Exposure, and Toxicity—A Review |journal=Environmental Science & Technology |date=7 June 2016 |volume=50 |issue=11 |pages=5438–5453 |doi=10.1021/acs.est.5b05387|pmid=27143250 |bibcode=2016EnST...50.5438C}}</ref><ref>{{cite journal |last1=Eladak |first1=Soria |last2=Grisin |first2=Tiphany |last3=Moison |first3=Delphine |last4=Guerquin |first4=Marie-Justine |last5=N'Tumba-Byn |first5=Thierry |last6=Pozzi-Gaudin |first6=Stéphanie |last7=Benachi |first7=Alexandra |last8=Livera |first8=Gabriel |last9=Rouiller-Fabre |first9=Virginie |last10=Habert |first10=René |date=2015 |title=A new chapter in the bisphenol A story: bisphenol S and bisphenol F are not safe alternatives to this compound |journal=Fertility and Sterility |language=en |volume=103 |issue=1 |pages=11–21 |doi=10.1016/j.fertnstert.2014.11.005|pmid=25475787 |doi-access=free }}</ref> | |||
| BPA exhibits hormone-like properties that raise concern about its suitability in consumer products and food containers. Since 2008, several governments have questioned its safety, which prompted some retailers to withdraw ] products. A 2010 report from the United States ] (FDA) warned of possible hazards to fetuses, infants, and young children.<ref name="U.S. Food and Drug Administration">{{vcite web|url=http://www.fda.gov/downloads/NewsEvents/PublicHealthFocus/UCM197778.pdf|title=Update on Bisphenol A for Use in Food Contact Applications: January 2010 |date=15 January 2010 |publisher=]|accessdate=15 January 2010}}</ref> In September 2010, Canada became the first country to declare BPA a toxic substance.<ref>{{vcite journal |url=http://www.gazette.gc.ca/rp-pr/p2/2010/2010-10-13/pdf/g2-14421.pdf |journal=] Part II |volume=144 |issue=21 |date=13 October 2010 |pages=1806–18 }}</ref><ref>{{vcite news|url=http://www.theglobeandmail.com/news/national/canada-first-to-declare-bisphenol-a-toxic/article1755272/ |title=Canada first to declare bisphenol A toxic |author=Martin Mittelstaedt |work=Globe and Mail |location=Canada |date=13 October 2010}}</ref> The ], Canada, and recently the United States have banned BPA use in baby bottles.<ref name="urlFDA Bans Chemical BPA From Sippy Cups And Baby Bottles">{{cite web | url = http://www.npr.org/blogs/thesalt/2012/07/17/156916616/fda-bans-chemical-bpa-in-sippy-cups-and-baby-bottles | title = FDA Bans Chemical BPA From Sippy Cups And Baby Bottles | publisher = National Public Radio | author = Hamilton J | date = 12 July 2012 | work = The Salt: NPR Food Blog }}</ref> | |||
| == |
== History == | ||
| Bisphenol A was first reported in 1891 by the Russian ] ].<ref>See: | |||
| World production capacity of this compound was 1 million tons in the 80s,<ref name="Fiege"/> and more than 2.2 million tons in 2009.<ref>{{vcite news |url=http://www.reuters.com/article/idUSTRE65L6JN20100622?loomia_ow=t0:s0:a49:g43:r3:c0.084942:b35124310:z0 |title=Experts demand European action on plastics chemical |publisher=Reuters |date=22 June 2010}}</ref> In 2003, U.S. consumption was 856,000 tons, 72% of which was used to make polycarbonate plastic and 21% going into epoxy resins.<ref name="CERHR">{{vcite news |title=CERHR Expert Panel Report for Bisphenol A |url=http://cerhr.niehs.nih.gov/chemicals/bisphenol/BPAFinalEPVF112607.pdf |format=PDF |accessdate=18 April 2008 |date=26 November 2007 |author=National Toxicology Program, U.S. Department of Health and Human Services |archiveurl=http://web.archive.org/web/20080218195117/http://cerhr.niehs.nih.gov/chemicals/bisphenol/BPAFinalEPVF112607.pdf <!-- Bot retrieved archive --> |archivedate=18 February 2008}}</ref> In the U.S., less than 5% of the BPA produced is used in food contact applications,<ref name="epa-action-plan"/> but remains in the canned food industry and printing applications such as sales receipts.<ref name="consumerreports">{{vcite news |url=http://www.consumerreports.org/cro/magazine-archive/december-2009/food/bpa/overview/bisphenol-a-ov.htm |work=Consumer Reports |title=Concern over canned foods |date=December 2009 |accessdate=2 February 2012}}</ref><ref>{{vcite news |url=http://www.foxnews.com/health/2011/11/23/soaring-bpa-levels-found-in-people-who-eat-canned-foods/ |publisher=Fox News |date=23 November 2011 |title=Soaring BPA Levels Found in People Who Eat Canned Foods}}</ref> | |||
| * А. Дианина (1891) (On condensation products of ketones with phenols), ''Журнал Русского физико-химического общества'' (Journal of the Russian Physical Chemistry Society), '''23''' : 488-517, 523–546, 601–611; see especially pages 491-493 ("Диметилдифенолметань" (dimethyldiphenolmethane)). | |||
| Bisphenol A was first synthesized by the ]n ] ] in 1891.<ref name="dianin">{{vcite journal | author=Dianin | title = Zhurnal russkogo fiziko-khimicheskogo obshchestva | volume = 23 | year = 1891 | pages = 492–}}</ref><ref>{{vcite journal | author = Zincke T | title = Ueber die Einwirkung von Brom und von Chlor auf Phenole: Substitutionsprodukte, Pseudobromide und Pseudochloride | journal=] | year = 1905 | pages = 75–99 | doi = 10.1002/jlac.19053430106 | volume = 343}}</ref> This compound is synthesized by the ] of ] (hence the suffix A in the name)<ref>{{vcite book | last=Uglea | first=Constantin V. | coauthors=Ioan I. Negulescu | title=Synthesis and Characterization of Oligomers | year=1991 | publisher=] | page=103 | isbn=0-8493-4954-0}}</ref> with two ] of ]. The reaction is ] by a strong acid, such as ] (HCl) or a ]. Industrially, a large excess of phenol is used to ensure full condensation; the product mixture of the ] (acetone and phenol) may also be used as starting material:<ref name="Fiege"/> | |||
| * Reprinted in condensed form in: A. Dianin (1892) (Condensation products of ketones and phenols), ''Berichte der Deutschen chemischen Gesellschaft zu Berlin'', '''25''', part 3 : 334-337. {{doi|10.1002/cber.18920250333}}</ref> | |||
| In 1934, workers at ] reported the coupling of BPA and ]. Over the following decade, coatings and resins derived from similar materials were described by workers at the companies of DeTrey Freres in ] and DeVoe and Raynolds in the US. This early work underpinned the development of ], which in turn motivated production of BPA.<ref name="UllmannEpox">{{Ullmann| vauthors = Pham HQ, Marks MJ |chapter=Epoxy Resins|year=2012|doi=10.1002/14356007.a09_547.pub2|isbn=978-3527306732}}</ref> The utilization of BPA further expanded with discoveries at ] and ] on ] ]s. These plastics first appeared in 1958, being produced by ], General Electric, and Bayer.<ref name=Ullmann>{{Ullmann|first = Volker | last = Serini |chapter=Polycarbonates|year=2000|doi=10.1002/14356007.a21_207}}</ref> | |||
| :] | |||
| The British biochemist Edward ] tested BPA as an artificial ] in the early 1930s.<ref name="Vogel2009">{{cite journal | vauthors = Vogel SA | title = The politics of plastics: the making and unmaking of bisphenol a "safety" | journal = American Journal of Public Health | volume = 99 | issue = Suppl 3 | pages = S559–S566 | date = November 2009 | pmid = 19890158 | pmc = 2774166 | doi = 10.2105/AJPH.2008.159228 }}</ref><ref>{{cite journal|vauthors=Dodds EC, Lawson W | year = 1936| title = Synthetic Œstrogenic Agents without the Phenanthrene Nucleus| journal=Nature| volume = 137| issue =3476| page = 996|bibcode=1936Natur.137..996D| doi=10.1038/137996a0| s2cid = 4171635| doi-access =free}}</ref><ref name="W. Lawson, 1938 pp. 222">{{cite journal | vauthors = Dodds EC, Lawson W | year = 1938| title = Molecular Structure in Relation to Oestrogenic Activity. Compounds without a Phenanthrene Nucleus| journal = Proceedings of the Royal Society of London B: Biological Sciences | volume = 125 | issue = 839| pages = 222–232 | doi=10.1098/rspb.1938.0023| bibcode=1938RSPSB.125..222D| doi-access = free}}</ref> Subsequent work found that it bound to ] tens of thousands of times more weakly than ], the major natural female sex hormone.<ref>{{cite journal |last1=Kwon |first1=Jung-Hwan |last2=Katz |first2=Lynn E. |last3=Liljestrand |first3=Howard M. |title=Modeling binding equilibrium in a competitive estrogen receptor binding assay |journal=Chemosphere |date=October 2007 |volume=69 |issue=7 |pages=1025–1031 |doi=10.1016/j.chemosphere.2007.04.047|pmid=17559906 |bibcode=2007Chmsp..69.1025K }}</ref><ref name=Xenochemicals>{{cite journal |last1=Blair |first1=R. M. |title=The Estrogen Receptor Relative Binding Affinities of 188 Natural and Xenochemicals: Structural Diversity of Ligands |journal=Toxicological Sciences |date=1 March 2000 |volume=54 |issue=1 |pages=138–153 |doi=10.1093/toxsci/54.1.138|pmid=10746941 | doi-access = free}}</ref> Dodds eventually developed a structurally similar compound, ] (DES), which was used as a synthetic estrogen drug in women and animals until it was banned due to its risk of causing cancer; the ban on use of DES in humans came in 1971 and in animals, in 1979.<ref name=Vogel2009 /> BPA was never used as a drug.<ref name="Vogel2009" /> | |||
| A large number of ]s undergo analogous condensation reactions. Commercial production of BPA requires distillation – either extraction of BPA from many resinous byproducts under ], or solvent-based extraction using additional phenol followed by distillation.<ref name="Fiege">{{vcite book | author = Fiege H, Voges H-W, Hamamoto T, Umemura S, = Iwata T, Miki H, Fujita Y, Buysch H-J, = Garbe D, Paulus W | title = Phenol Derivatives | series = Ullmann's Encyclopedia of Industrial Chemistry | publisher=Wiley-VCH | location = Weinheim | year = 2002 | doi = 10.1002/14356007.a19_313}}</ref> | |||
| == |
== Production == | ||
| The synthesis of BPA still follows Dianin's general method, with the fundamentals changing little in 130 years. The ] of ] (hence the suffix 'A' in the name)<ref>{{cite book | vauthors = Uglea CV, Negulescu II | title=Synthesis and Characterization of Oligomers | year=1991 | publisher=] | page=103 | isbn=978-0-8493-4954-6}}</ref> with two ] of ] is ] by a strong acid, such as concentrated ], ], or a solid acid ] such as the ] form of ].<ref>{{cite journal | vauthors = De Angelis A, Ingallina P, Perego C |title=Solid Acid Catalysts for Industrial Condensations of Ketones and Aldehydes with Aromatics |journal=Industrial & Engineering Chemistry Research |date=March 2004 |volume=43 |issue=5 |pages=1169–1178 |doi=10.1021/ie030429+}}</ref> An excess of phenol is used to ensure full condensation and to limit the formation of byproducts, such as ]. BPA is fairly cheap to produce, as the synthesis benefits from a high ] and large amounts of both starting materials are available from the ].<ref name="Fiege" /> As the only ] is water, it may be considered an industrial example of ]. Global production in 2022 was estimated to be in the region of 10 million tonnes.<ref name=production>{{cite journal | vauthors = Abraham A, Chakraborty P | title = A review on sources and health impacts of bisphenol A | journal = Reviews on Environmental Health | volume = 35 | issue = 2 | pages = 201–210 | date = June 2020 | pmid = 31743105 | doi = 10.1515/reveh-2019-0034 | s2cid = 208186123}}</ref> | |||
| {{further2|]}} | |||
| Bisphenol A is used primarily to make plastics, and products using bisphenol A-based plastics have been in commercial use since 1957.<ref name="infosheet">{{vcite web | title=Bisphenol A Information Sheet | date=October 2002 | publisher=Bisphenol A Global Industry Group | url=http://www.bisphenol-a.org/pdf/DiscoveryandUseOctober2002.pdf | accessdate=7 December 2010 }}</ref> At least 3.6 million tonnes (8 billion pounds) of BPA are used by manufacturers yearly.<ref name="USNews3">{{vcite news|url=http://health.usnews.com/health-news/family-health/heart/articles/2009/06/10/studies-report-more-harmful-effects-from-bpa.html|title=Studies Report More Harmful Effects From BPA|date=10 June 2009|work=]|accessdate=28 October 2010}}</ref> It is a key ] in production of ] resins<ref>{{vcite web | |||
| |url=http://www.californiaprogressreport.com/2009/07/committee_succe.html | |||
| |title=Lawmakers to press for BPA regulation | |||
| |author = Replogle J | |||
| |date = 17 July 2009 | |||
| |publisher=California Progress Report | |||
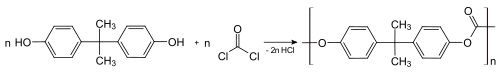
| |accessdate=31 January 2012}}</ref><ref>{{vcite news|url=http://www.thestar.com/article/415296|title=Ridding life of bisphenol A a challenge|last=Ubelacker |first=Sheryl |date=16 April 2008|work=Toronto Star |accessdate=2 August 2009}}</ref> and in the most common form of ] plastic.<ref name="Fiege"/><ref>{{vcite book|last=Kroschwitz|first=Jacqueline I.|title=Kirk-Othmer encyclopedia of chemical technology|edition=5|volume=5|page=8|isbn=0-471-52695-9}}</ref><ref>{{vcite web|url=http://www.alliancepoly.com/polycarbonate.asp|title=Polycarbonate (PC) Polymer Resin|publisher=Alliance Polymers, Inc|accessdate=2 August 2009}}</ref> Bisphenol A and ] react to give polycarbonate under biphasic conditions; the hydrochloric acid is scavenged with aqueous base: | |||
| :] | |||
| :] | |||
| Usually, the addition of acetone takes place at the ] on both phenols, however minor amounts of the ortho-para (up to 3%) and ortho-ortho isomers are also produced, along with several other minor by‑products.<ref name=terasaki>{{cite journal | vauthors = Terasaki M, Nomachi M, Edmonds JS, Morita M | title = Impurities in industrial grade 4,4'-isopropylidene diphenol (bisphenol A): possible implications for estrogenic activity | journal = Chemosphere | volume = 55 | issue = 6 | pages = 927–931 | date = May 2004 | pmid = 15041297 | doi = 10.1016/j.chemosphere.2003.11.063 | bibcode = 2004Chmsp..55..927T }}</ref> These are not always removed and are known impurities in commercial samples of BPA.<ref>{{cite journal | vauthors = Pahigian JM, Zuo Y | title = Occurrence, endocrine-related bioeffects and fate of bisphenol A chemical degradation intermediates and impurities: A review | journal = Chemosphere | volume = 207 | pages = 469–480 | date = September 2018 | pmid = 29807346 | doi = 10.1016/j.chemosphere.2018.05.117 | s2cid = 44172964 | bibcode = 2018Chmsp.207..469P | doi-access = free }}</ref><ref name=terasaki/> | |||
| ] may be used in place of phosgene. ] is eliminated instead of hydrochloric acid. This transesterification process avoids the toxicity and handling of phosgene.<ref name="ioc">{{cite book |last=Wittcoff |first=Harold |last2=Reuben |first2=B. G. |last3=Plotkin |first3=Jeffrey S. |year=2004 |title=Industrial Organic Chemicals |publisher=Wiley-IEEE |isbn=978-0-471-44385-8 |pages=278 |url=http://books.google.com/?id=4KHzc-nYPNsC&pg=PA278&dq=%22Diphenyl+carbonate%22 |accessdate=1 February 2012}}</ref> | |||
| ==Properties== | |||
| Polycarbonate plastic, which is clear and nearly shatter-proof, is used to make a variety of common products including baby and water bottles, sports equipment, medical and dental devices, ] and sealants, CDs and DVDs, household electronics, and eyeglass lenses.<ref name="Fiege"/> BPA is also used in the synthesis of ]s and ] ], as an ] in some ]s, and as a ] inhibitor in ]. Epoxy resins containing bisphenol A are used as coatings on the inside of almost all food and ]s,<ref name="C&ENews">{{vcite journal|author = Erickson BE | date = 2 June 2008 |title=Bisphenol A under scrutiny|journal=Chemical and Engineering News|publisher=American Chemical Society|volume=86|issue=22|pages=36–39|url=http://pubs.acs.org/isubscribe/journals/cen/86/i22/html/8622gov1.html}}</ref> however, due to BPA health concerns, in Japan epoxy coating was mostly replaced by ].<ref>{{vcite web|url=http://www.foodnavigator.com/Financial-Industry/Consumers-fear-the-packaging-a-BPA-alternative-is-needed-now|title=Consumers fear the packaging – a BPA alternative is needed now|last=Byrne|first=Jane|date=22 September 2008|accessdate=5 January 2010}}</ref> | |||
| BPA has a fairly high melting point but can be easily dissolved in a broad range of organic solvents including ], ] and ].<ref>{{cite book |title=CRC handbook of chemistry and physics : a ready-reference book of chemical and physical data. |date=2017 |publisher=CRC Press, Inc.|location=Boca Raton, Florida |isbn=9781498754293 |pages=3–56 |edition=2016-2017, 97th |url=https://books.google.com/books?id=VVezDAAAQBAJ&dq=crc+handbook+of+chemistry+and+physics+%2280-05-7%22&pg=SA3-PA56|last1=Haynes |first1=William M. }}</ref> It may be purified by ] from acetic acid with water.<ref>{{cite book |last1=Perrin |first1=Douglas Dalzell Perrin |last2=Armarego |first2=W. L. F. |title=Purification of laboratory chemicals |year=1988 |publisher=Butterworth-Heinemann |isbn=9780080347141 |page=208 |url=https://books.google.com/books?id=vLFXAAAAMAAJ}}</ref> Crystals form in the ] ] P 2<sub>1</sub>/n (where n indicates the glide plane); within this individual molecules of BPA are arraigned with a 91.5° ] between the phenol rings.<ref>{{cite web |title=2,2-bis(4-Hydroxyphenyl)propane |url=https://www.ccdc.cam.ac.uk/structures/Search?Ccdcid=1122458&DatabaseToSearch=Published |website=www.ccdc.cam.ac.uk |publisher=The Cambridge Crystallographic Data Centre |access-date=29 June 2022}}</ref><ref>{{cite journal |last1=Okada |first1=Kenji |title=X-ray crystal structure analyses and atomic charges of color former and developer. I. Color developers |journal=Journal of Molecular Structure |date=July 1996 |volume=380 |issue=3 |pages=223–233 |doi=10.1016/0022-2860(95)09168-8|bibcode=1996JMoSt.380..223O }}</ref><ref>{{cite journal |last1=Wolak |first1=J. E. |last2=Knutson |first2=J. |last3=Martin |first3=J. D. |last4=Boyle |first4=P. |last5=Sargent |first5=Andrew L. |last6=White |first6=Jeffery L. |title=Dynamic Disorder and Conformer Exchange in the Crystalline Monomer of Polycarbonate |journal=The Journal of Physical Chemistry B |date=1 December 2003 |volume=107 |issue=48 |pages=13293–13299 |doi=10.1021/jp036527q}}</ref> ] data is available from ].<ref>{{cite web |title=4,4'-isopropylidenediphenol |url=https://sdbs.db.aist.go.jp/CompoundView.aspx?sdbsno=1716 |website=sdbs.db.aist.go.jp |publisher=Spectral Database for Organic Compounds (SDBS) |access-date=8 August 2024}}</ref> | |||
| == Uses and applications == | |||
| Bisphenol A is also a precursor to the ] ], and formerly was used as a ].<ref>{{vcite web|url=http://pesticideinfo.org/Detail_Chemical.jsp?Rec_Id=PC33756 |title=Bisphenol A |publisher=Pesticideinfo.org |accessdate=23 October 2011}}</ref> Bisphenol A is a preferred color developer in ] and ],<ref>{{cite patent |country=US |title=Thermal paper with security features |number=6562755}}</ref> with the most common public exposure coming from some<ref>{{vcite web|title=More evidence that BPA laces store receipts|url=http://www.sciencenews.org/view/generic/id/61490/title/Science_%2B_the_Public__More_evidence_that_BPA_laces_store_receipts|quote=Bill Van Den Brandt of Appleton Papers says that the receipts paper made by his company (which bills itself as the nation's leading producer of carbonless and thermal papers) is BPA-free.|date=27 July 2001|accessdate=3 August 2010|author=Raloff, Janet|publisher=Science News}}</ref> thermal ] receipt paper.<ref>{{vcite web|title=Concerned about BPA: Check your receipts|url=http://www.sciencenews.org/view/generic/id/48084/title/Science_%2B_the_Public__Concerned_about_BPA_Check_your_receipts|date=7 October 2009|accessdate=3 August 2010|author=Raloff, Janet|publisher=Science News}}</ref><ref name="ReferenceA">{{vcite doi|10.1016/S0045-6535(00)00507-5}}</ref> BPA-based products are also used in ] and for lining water pipes.<ref name="epa-action-plan">{{vcite web|url=http://www.epa.gov/oppt/existingchemicals/pubs/actionplans/bpa_action_plan.pdf|title=Bisphenol A Action Plan |date=29 March 2010|publisher=U.S. Environmental Protection Agency|accessdate=12 April 2010}}</ref> | |||
| ] water bottle.]] | |||
| === |
===Main uses=== | ||
| ==== Polycarbonates ==== | |||
| {{Main|Resin identification code}} | |||
| {{main|Polycarbonate}} | |||
| ] plastics may leak bisphenol A|left]] ] plastics may leak bisphenol A|right]] | |||
| About 65–70% of all bisphenol A is used to make ] plastics,<ref name="EU2008">{{cite book |author1=European Commission. Joint Research Centre. Institute for Health Consumer Protection |title=Updated European Union risk assessment report : 4,4'-isopropylidenediphenol (bisphenol-A) : environment addendum of February 2008 |date=2010 |publisher=Publications Office |isbn=9789279175428 |page=6 |doi=10.2788/40195 |doi-access=free}}</ref><ref name="Tom2021">{{cite journal | vauthors = Vasiljevic T, Harner T | title = Bisphenol A and its analogues in outdoor and indoor air: Properties, sources and global levels | journal = The Science of the Total Environment | volume = 789 | pages = 148013 | date = May 2021 | pmid = 34323825 | doi = 10.1016/j.scitotenv.2021.148013 | bibcode = 2021ScTEn.78948013V | doi-access = free}}</ref> which can consist of nearly 90% BPA by mass. ] is achieved by a reaction with ], conducted under biphasic conditions; the hydrochloric acid is scavenged with aqueous base.<ref name="UllmannPolyC">{{Ullmann|first=Volker|last1=Serini |title=Polycarbonates|year=2000|doi=10.1002/14356007.a21_207}}</ref> This process converts the individual molecules of BPA into large polymer chains, effectively trapping them. | |||
| There are ] used in packaging applications. Currently there are no BPA labeling requirements for plastics. | |||
| :] | |||
| "In general, plastics that are marked with recycle codes 1, 2, 4, 5, and 6 are very unlikely to contain BPA. Some, but not all, plastics that are marked with recycle codes 3 or 7 may be made with BPA."<ref>{{vcite web|url=http://www.hhs.gov/safety/bpa/ |title=Bisphenol A (BPA) Information for Parents |publisher=Hhs.gov |date=15 January 2010 |accessdate=23 October 2011}}</ref> | |||
| ==== Epoxy and vinyl ester resins ==== | |||
| Type 7 is the catch-all "other" class, and some type 7 plastics, such as ] (sometimes identified with the letters "PC" near the ]) and epoxy resins, are made from bisphenol A monomer.<ref name="Fiege"/><!--When such plastics are exposed to hot liquids, bisphenol A leaks out 55 times faster than it does under normal conditions.{{Clarify|date=March 2009}}--><!-- Unit incomplete. Nanogrammes per hour per how much of the plastic? That much per mg would be a lot, per tonne not much. Also need to know what "hot" means, and what "normal conditions" means.--><ref name="sciam2008">{{vcite journal|author=Biello D | title=Plastic (not) fantastic: Food containers leach a potentially harmful chemical | journal=Scientific American | volume=2 | date=19 February 2008 | url=http://www.sciam.com/article.cfm?id=plastic-not-fantastic-with-bisphenol-a | accessdate=9 April 2008}}</ref> | |||
| About 25–30% of all BPA is used in the manufacture of ]s and ]s.<ref name="EU2008" /><ref name="Tom2021" /> For epoxy resin, it is first converted to its ] (usually abbreviated BADGE or DGEBA).<ref>{{cite journal | vauthors = Ng F, Couture G, Philippe C, Boutevin B, Caillol S | title = Bio-Based Aromatic Epoxy Monomers for Thermoset Materials | journal = Molecules | volume = 22 | issue = 1 | pages = 149 | date = January 2017 | pmid = 28106795 | pmc = 6155700 | doi = 10.3390/molecules22010149 | doi-access = free}}</ref><ref>{{cite book| vauthors = Kroschwitz JI |title=Kirk-Othmer Encyclopedia of Chemical Technology|year=1998|edition=5|volume=5|page=8|publisher=Wiley |isbn=978-0-471-52695-7}}</ref> This is achieved by a reaction with ] under basic conditions. | |||
| :] | |||
| Type 3 (]) also may contain bisphenol A as an antioxidant in ].<ref name="Fiege"/> This refers to "flexible PVC", but not for rigids such as pipe, windows, and siding. | |||
| {{Clear}} | |||
| Some of this is further reacted with ] to form ], which is used to make vinyl ester resins. Alternatively, and to a much lesser extent, BPA may be ] and then converted to its di] and di] derivatives (bis-EMA, or EBPADMA). These may be incorporated at low levels in vinyl ester resins to change their physical properties<ref>{{cite journal | vauthors = Gonçalves F, Kawano Y, Pfeifer C, Stansbury JW, Braga RR | title = Influence of BisGMA, TEGDMA, and BisEMA contents on viscosity, conversion, and flexural strength of experimental resins and composites | journal = European Journal of Oral Sciences | volume = 117 | issue = 4 | pages = 442–446 | date = August 2009 | pmid = 19627357 | doi = 10.1111/j.1600-0722.2009.00636.x }}</ref> and see common use in ]s and ].<ref>{{cite journal |last1=Sideridou |first1=I. |last2=Tserki |first2=V. |last3=Papanastasiou |first3=G. |title=Effect of chemical structure on degree of conversion in light-cured dimethacrylate-based dental resins |journal=Biomaterials |date=April 2002 |volume=23 |issue=8 |pages=1819–1829 |doi=10.1016/S0142-9612(01)00308-8|pmid=11950052 }}</ref><ref>{{cite journal |last1=Sideridou |first1=Irini D. |last2=Achilias |first2=Dimitris S. |title=Elution study of unreacted Bis-GMA, TEGDMA, UDMA, and Bis-EMA from light-cured dental resins and resin composites using HPLC |journal=Journal of Biomedical Materials Research Part B: Applied Biomaterials |date=July 2005 |volume=74B |issue=1 |pages=617–626 |doi=10.1002/jbm.b.30252|pmid=15889433}}</ref> | |||
| ==History== | |||
| ===Minor uses=== | |||
| Bisphenol A was discovered in 1891 by Russian chemist ]. In the early 1930s the British chemist Charles Edward Dodds recognized BPA as an artificial estrogen.<ref name="Erler_Novak_2010">{{cite journal | author = Erler C, Novak J | title = Bisphenol a exposure: human risk and health policy | journal = J Pediatr Nurs | volume = 25 | issue = 5 | pages = 400–7 | year = 2010 | month = October | pmid = 20816563 | doi = 10.1016/j.pedn.2009.05.006 }}</ref> During that time BPA had two initial uses. The first use of BPA was to enhance the growth of cattle and poultry. The second use of BPA in the mid 1930s was as an estrogen replacement for women. BPA was only briefly used as an estrogen replacement and was replaced by ] (DES).<ref name="Erler_Novak_2010"/> Based on research by chemists at Bayer and General Electric, BPA has been used since the 1950s to harden polycarbonate plastics and make epoxy resin, and in the lining of food and beverage containers.<ref name="timeBPA1"> | |||
| The remaining 5% of BPA is used in a wide range of applications, many of which involve plastic.<ref name=potential>{{cite journal | vauthors = Geens T, Goeyens L, Covaci A | title = Are potential sources for human exposure to bisphenol-A overlooked? | journal = International Journal of Hygiene and Environmental Health | volume = 214 | issue = 5 | pages = 339–347 | date = September 2011 | pmid = 21570349 | doi = 10.1016/j.ijheh.2011.04.005 | bibcode = 2011IJHEH.214..339G }}</ref> BPA is a main component of several ], the production of these is low compared to other plastics but still equals several thousand tons a year. Comparatively minor amounts of BPA are also used as additives or modifiers in some ]. These materials are much more common but their BPA content will be low. | |||
| {{vcite news|url=http://www.time.com/time/specials/packages/article/0,28804,1976909_1976908_1976938-2,00.html | |||
| |title=The Perils of Plastic – Environmental Toxins – TIME | |||
| |author = Walsh B | |||
| |date=1 April 2010 | |||
| |work=TIME | |||
| |accessdate=2 July 2010}}</ref><ref name="pmid20816563">{{cite journal | author = Erler C, Novak J | title = Bisphenol a exposure: human risk and health policy | journal = J Pediatr Nurs | volume = 25 | issue = 5 | pages = 400–7 | year = 2010 | month = October | pmid = 20816563 | doi = 10.1016/j.pedn.2009.05.006 | url = }}</ref> The first evidence of the ] of bisphenol A came from experiments on rats conducted in the 1930s,<ref> | |||
| {{vcite journal | |||
| | author=Dodds EC, Lawson W | |||
| | year = 1936 | |||
| | title = Synthetic Œstrogenic Agents without the Phenanthrene Nucleus | |||
| | url = | |||
| | journal=Nature | |||
| | volume = 137 | |||
| | issue =3476 | |||
| | page = 996 | |||
| |bibcode=1936Natur.137..996D | |||
| | doi=10.1038/137996a0}}</ref><ref name="W. Lawson, 1938 pp. 222">E. C. Dodds and W. Lawson, ''Proceedings of the Royal Society of London, Series B, Biological Sciences'', 125, No. 839 (27-IV-1938), pp. 222–232.</ref> but it was not until 1997 that adverse effects of low-dose exposure on laboratory animals were first proposed (]).<ref name="C&ENews"/> Modern studies began finding possible connections to health issues caused by exposure to BPA during pregnancy and during development. See ]. Research is ongoing and the debate continues as to whether BPA should be banned or not, and to what extent, all over the world. In 2010 Canada's department of the environment declared BPA to be a ''"toxic substance"''.<ref name="gazette.gc.ca">{{vcite web |url=http://www.gazette.gc.ca/rp-pr/p2/2010/2010-10-13/html/sor-dors194-eng.html |title=Order Adding a Toxic Substance to Schedule 1 to the Canadian Environmental Protection Act, 1999 |work=] |date=23 September 2010 |accessdate=2 February 2012}}</ref> | |||
| == |
====Plastics==== | ||
| ;As a major component | |||
| Bisphenol A is a weak ], which can mimic ] and may lead to negative health effects.<ref> | |||
| * Polycyanurates can be produced from BPA by way of its di] (BADCy).<ref name=potential /> This is formed by a reaction between BPA and ].<ref>{{cite book |last1=Hamerton |first1=Ian |title=Chemistry and technology of cyanate ester resins |date=1994 |publisher=Blackie Academic & Professional |location=London |isbn=978-0-7514-0044-1 |edition=1st}}</ref> Examples include ], which is one of a number of resins used in the production of ]. | |||
| {{vcite book | |||
| * ]s such as Ultem can be produced from BPA via a nitro-displacement of appropriate bisnitroimides.<ref>{{cite journal | vauthors = Takekoshi T, Kochanowski JE, Manello JS, Webber MJ |title=Polyetherimides. I. Preparation of dianhydrides containing aromatic ether groups |journal=Journal of Polymer Science: Polymer Chemistry Edition |date=June 1985 |volume=23 |issue=6 |pages=1759–1769 |doi=10.1002/pol.1985.170230616|bibcode=1985JPoSA..23.1759T}}</ref><ref>{{cite book |last1=Lau |first1=Kreisler S.Y. |title=Handbook of thermoset plastics |date=2014 |publisher=William Andrew |location=San Diego |isbn=978-1-4557-3107-7 |pages=319–323 |edition=3rd |chapter=10 - High-Performance Polyimides and High Temperature Resistant Polymers}}</ref> These ] ] plastics have exceptional resistance to mechanical, thermal and chemical damage. They are used in medical devices and other high performance instrumentation. | |||
| |author = Gore AC | |||
| * ]s may be produced from a number of biphenols, including BPA.<ref>{{cite journal | vauthors = Vijayakumar CT, Shamim Rishwana S, Surender R, David Mathan N, Vinayagamoorthi S, Alam S |title=Structurally diverse benzoxazines: synthesis, polymerization, and thermal stability |journal=Designed Monomers and Polymers |date=2 January 2014 |volume=17 |issue=1 |pages=47–57 |doi=10.1080/15685551.2013.797216|s2cid=94255723 |doi-access=free }}</ref><ref>{{cite journal | vauthors = Ghosh NN, Kiskan B, Yagci Y |title=Polybenzoxazines—New high performance thermosetting resins: Synthesis and properties |journal=Progress in Polymer Science |date=November 2007 |volume=32 |issue=11 |pages=1344–1391 |doi=10.1016/j.progpolymsci.2007.07.002}}</ref> | |||
| |title=Endocrine-Disrupting Chemicals: From Basic Research to Clinical Practice | |||
| * ]s can be produced from BPA and ] forming poly(bisphenol-A sulfone) (PSF). It is used as a high performance alternative to polycarbonate.<ref name=potential /><ref name="q156">{{cite book | title=Kirk-Othmer Encyclopedia of Chemical Technology | publisher=Wiley | date=2001-01-26 | isbn=978-0-471-48494-3 | doi=10.1002/0471238961.0118151323080920.a01 | page=}}</ref> | |||
| |publisher=Humana Press | |||
| *Bisphenol-A formaldehyde resins are a subset of ]s. They are used in the production of ]s<ref name=potential /> | |||
| |date=8 June 2007|series=Contemporary Endocrinology|isbn=978-1-58829-830-0}}</ref><ref>{{vcite journal | |||
| | author = O’Connor JC, Chapin RE | |||
| | title = Critical evaluation of observed adverse effects of endocrine active substances on reproduction and development, the immune system, and the nervous system | |||
| | journal=Pure Appl. Chem | |||
| | volume = 75 | |||
| | issue = 11–12 | |||
| | pages = 2099–2123 | |||
| |year=2003 | |||
| | url = http://www.iupac.org/publications/pac/2003/pdf/7511x2099.pdf | |||
| | accessdate =28 February 2007 | |||
| | doi = 10.1351/pac200375112099}}</ref><ref name="pmid18197296">{{vcite journal | author = Okada H, Tokunaga T, Liu X, Takayanagi S, Matsushima A, Shimohigashi Y | title = Direct evidence revealing structural elements essential for the high binding ability of bisphenol A to human estrogen-related receptor-gamma | journal = Environ. Health Perspect. | volume = 116 | issue = 1 | pages = 32–8 | year = 2008 | month = January | pmid = 18197296 | pmc = 2199305 | doi = 10.1289/ehp.10587 | url = }}</ref><ref name="JAMAVS">{{vcite journal |author=vom Saal FS, Myers JP |title=Bisphenol A and Risk of Metabolic Disorders |journal=] |volume=300 |issue=11 |pages=1353–5 |year=2008 |pmid=18799451|doi=10.1001/jama.300.11.1353 |url=http://jama.ama-assn.org/cgi/content/full/300.11.1353}}</ref> Early developmental stages appear to be the period of greatest sensitivity to its effects,<ref name="HealthCanada"> | |||
| ], 2008.</ref> and some studies have linked prenatal exposure to later physical and neurological difficulties. Regulatory bodies have determined safety levels for humans, but those safety levels are currently being questioned or are under review as a result of new scientific studies.<ref name="EHP">{{vcite journal |author=Ginsberg G, Rice DC | |||
| |title=Does Rapid Metabolism Ensure Negligible Risk from Bisphenol A? |journal=] |volume= 117 |issue=11 |pages=1639–1643 |year=2009 |doi=10.1289/ehp.0901010 |url=http://www.ehponline.org/members/2009/0901010/0901010.html |pmid=20049111 |pmc=2801165}}</ref><ref>{{cite pmid|19931376}}</ref> A 2011 study that investigated the number of chemicals pregnant women are exposed to in the U.S. found BPA in 96% of women.<ref> | |||
| {{vcite web |url=http://www.sciencedaily.com/releases/2011/01/110114081653.htm |title=99% of pregnant women in US test positive for multiple chemicals including banned ones, study suggests |doi=10.1289/ehp.1002727 |publisher=ScienceDaily |date=14 January 2011 |accessdate=1 February 2012}}</ref> | |||
| ;As a minor component | |||
| Overall, empirical evidence supporting the negative health effects of BPA varies significantly across studies. Opinions vary greatly about the health effects of BPA. Some studies conclude that BPA poses no health risks while others state that BPA causes a number of adverse health effects. In general, the European’s Scientific Committee on Food, the EUs European Chemicals Bureau, the European Food Safety Authority, and the US ] have concluded that current levels of BPA present no risk to the general population. However, experts in the field of endocrine disruptors have stated that the entire population may suffer adverse health effects from current BPA levels.<ref name="test">] released a statement citing the adverse effects of endocrine-disrupting chemicals, and the controversy surrounding BPA.<ref>{{vcite web |url=http://www.endo-society.org/advocacy/policy/upload/Endocrine-Disrupting-Chemicals-Position-Statement.pdf |title=Endocrine Society Position Statement Endocrine Disrupting Chemicals |publisher=Endocrine Society |date=11 June 2009 |accessdate=6 April 2012}}</ref> | |||
| * ] can incorporate BPA and its derivatives as hard segment chain extenders, particularly in ]s.<ref>{{cite journal | vauthors = Laza JM, Veloso A, Vilas JL |title=Tailoring new bisphenol a ethoxylated shape memory polyurethanes |journal=Journal of Applied Polymer Science |date=10 January 2021 |volume=138 |issue=2 |pages=49660 |doi=10.1002/app.49660|s2cid=224955435 }}</ref><ref>{{cite book |last1=Król |first1=Piotr |title=Linear polyurethanes : synthesis methods, chemical structures, properties and applications |date=2008 |publisher=VSP |location=Leiden |isbn=9789004161245 |pages=11–14}}</ref> | |||
| In 2012 the FDA did ban the use of BPA in baby bottles, however the ] called the ban "purely cosmetic". In a statement they said,“If the agency truly wants to prevent people from being exposed to this toxic chemical associated with a variety of serious and chronic conditions it should ban its use in cans of infant formula, food and beverages." The ] called the move inadequate saying, the FDA needs to ban BPA from all food packaging.<ref name="commondreams">http://www.commondreams.org/headline/2012/07/17-4</ref> In a statement a FDA spokesman said the agency's action was not based on safety concerns and that "the agency continues to support the safety of BPA for use in products that hold food."<ref name="huffpo">http://www.huffingtonpost.com/2012/07/17/fda-bans-bpa-baby-bottles_n_1679795.html</ref> | |||
| * ] can contain BPA and its derivatives through multiple routes. BPA is sometimes used as an antioxidant in ],<ref>{{cite web |title=European Union Summary Risk Assessment Report - Bis (2-ethylhexyl) Phthalate (DEHP) |url=https://publications.jrc.ec.europa.eu/repository/handle/JRC45844 |website=Joint Research Centre (JRC) Publications Repository |date=16 July 2008 |publisher=European Commission |issn=1018-5593|access-date=24 November 2021}}{{open access}}</ref> which are extensively used as ]s for PVC. BPA has also been used as an antioxidant to protect sensitive PVC ]s. Historically 5–10% by weight of BPA was included in barium-cadmium types, although these have largely been phased out due health concerns surrounding the ]. BPA diglycidyl ether (BADGE) is used as an acid scavenger, particularly in PVC ]s, such as organosols or ]s,<ref>{{cite journal | vauthors = Shah AC, Poledna DJ |title=Review of PVC dispersion and blending resin products |journal=Journal of Vinyl and Additive Technology |date=September 2003 |volume=9 |issue=3 |pages=146–154 |doi=10.1002/vnl.10076|s2cid=98016356 }}</ref><ref>{{cite journal | vauthors = Shah AC, Poledna DJ |title=Review of specialty PVC resins |journal=Journal of Vinyl and Additive Technology |date=September 2002 |volume=8 |issue=3 |pages=214–221 |doi=10.1002/vnl.10365|s2cid=97146596 }}</ref> which are used as coatings for the inside of food cans, as well as embossed clothes designs produced using ] or ] machines.<ref name="ir.rcees.ac.cn"/> | |||
| * BPA is used to form a number of ]s used in plastics.<ref name=UllmannBr>{{ Ullmann | vauthors = Dagani MJ, Barda HJ, Benya TJ, Sanders DC | title = Bromine Compounds | doi = 10.1002/14356007.a04_405 }}</ref> Bromination of BPA forms ] (TBBPA), which is mainly used as a reactive component of polymers, meaning that it is incorporated into the polymer backbone. It is used to prepare fire-resistant ]s by replacing some bisphenol A. A lower grade of TBBPA is used to prepare ]s, used in ]s. TBBPA is also converted to tetrabromobisphenol-A-bis(2,3,-dibromopropyl ether) (TBBPA-BDBPE) which can be used as a flame retardant in ]. TBBPA-BDBPE is not chemically bonded to the polymer and can leach out into the environment.<ref>{{cite journal |last1=Gauthier |first1=Lewis T. |last2=Laurich |first2=Bruce |last3=Hebert |first3=Craig E. |last4=Drake |first4=Christine |last5=Letcher |first5=Robert J. |title=Tetrabromobisphenol-A-Bis(dibromopropyl ether) Flame Retardant in Eggs, Regurgitates, and Feces of Herring Gulls from Multiple North American Great Lakes Locations |journal=Environmental Science & Technology |date=20 August 2019 |volume=53 |issue=16 |pages=9564–9571 |doi=10.1021/acs.est.9b02472|pmid=31364365 |bibcode=2019EnST...53.9564G |s2cid=198998658 }}</ref> The use of these compounds is diminishing due to restrictions on ]s. The reaction of BPA with ] and ] forms ] (BADP), which is used as a liquid flame retarder in some high performance ]s such as polycarbonate/] mixtures.<ref>{{cite journal |last1=Pawlowski |first1=Kristin H |last2=Schartel |first2=Bernhard |title=Flame retardancy mechanisms of triphenyl phosphate, resorcinol bis(diphenyl phosphate) and bisphenol A bis(diphenyl phosphate) in polycarbonate/acrylonitrile–butadiene–styrene blends |journal=Polymer International |date=November 2007 |volume=56 |issue=11 |pages=1404–1414 |doi=10.1002/pi.2290}}</ref> | |||
| ====Other applications==== | |||
| The ] (EPA) also holds the position that BPA is not a health concern. In 2011, Andrew Wadge, the chief scientist of the United Kingdom's ], commented on an EPA study on dietary exposure of adult humans to BPA,<ref name="Teeguarden">{{vcite journal |author=Teeguarden JG, Calafat AM, Ye X, Doerge DR, Churchwell MI, Gunawan R, Graham MK. |year=2011 |title=Twenty-four hour human urine and serum profiles of bisphenol a during high-dietary exposure |url=http://www.ncbi.nlm.nih.gov/pubmed/21705716 |journal=] |volume=125 |pages=318–20}}</ref> saying, "This corroborates other independent studies and adds to the evidence that BPA is rapidly absorbed, detoxified, and eliminated from humans – therefore is not a health concern."<ref>{{vcite web |url=http://blogs.food.gov.uk/science/entry/small_pond_same_big_issues |title=Small pond, same big issues |publisher=] |first=Andrew |last=Wage |date=27 July 2011 |accessdate=3 August 2011 }}</ref> In the study 20 subjects were tested for BPA every hour for twenty-four hours while consuming three meals consisting of canned food.<ref name="Teeguarden"/> This study has been criticized, however, as lacking data and having flawed assumptions.<ref>{{cite journal |author=Vom Saal FS, Prins GS, Welshons WV. |year=2012 |title=Report of very low real-world exposure to bisphenol A is unwarranted based on a lack of data and flawed assumptions. Toxicol Sci. 2012 Jan;125(1):318-20 |journal=] |volume=125 |pages=321–5 |pmid=22020768 |doi=10.1093/toxsci/kfr273 |issue=1}}</ref> | |||
| * BPA is used as an antioxidant in several fields, particularly in ]s.<ref>{{cite journal | vauthors = Lamprea K, Bressy A, Mirande-Bret C, Caupos E, Gromaire MC | title = Alkylphenol and bisphenol A contamination of urban runoff: an evaluation of the emission potentials of various construction materials and automotive supplies | journal = Environmental Science and Pollution Research International | volume = 25 | issue = 22 | pages = 21887–21900 | date = August 2018 | pmid = 29796891 | doi = 10.1007/s11356-018-2272-z | bibcode = 2018ESPR...2521887L | s2cid = 44140721 | url = https://hal.science/hal-01813100/file/Lamprea2018_ESPR.pdf }}</ref> | |||
| *BPA is used as a developing agent in ] (shop receipts).<ref name=paper1>{{cite journal | vauthors = Björnsdotter MK, de Boer J, Ballesteros-Gómez A | title = Bisphenol A and replacements in thermal paper: A review | journal = Chemosphere | volume = 182 | pages = 691–706 | date = September 2017 | pmid = 28528315 | doi = 10.1016/j.chemosphere.2017.05.070 | bibcode = 2017Chmsp.182..691B | hdl = 1871.1/0c9480c5-48ce-4955-8d53-39b8b246802f | url = https://research.vu.nl/en/publications/0c9480c5-48ce-4955-8d53-39b8b246802f | hdl-access = free }}</ref> Recycled paper products can also contain BPA,<ref>{{cite journal | vauthors = Liao C, Kannan K | title = Widespread occurrence of bisphenol A in paper and paper products: implications for human exposure | journal = Environmental Science & Technology | volume = 45 | issue = 21 | pages = 9372–9379 | date = November 2011 | pmid = 21939283 | doi = 10.1021/es202507f | bibcode = 2011EnST...45.9372L }}</ref> although this can depend strongly on how it is recycled. ] can remove 95% of BPA,<ref name=EU2008 /> with the pulp produced used to make newsprint, toilet paper and facial tissues. If deinking is not performed then the BPA remains in the fibers, paper recycled this way is usually made into ].<ref name=EU2008 /> | |||
| * ] BPA finds minor use as a 'levelling agent' in tin ]. | |||
| * Several drug candidates have also been developed from bisphenol A, including ], ], and ]. | |||
| ==BPA substitutes== | |||
| ===Expert panel conclusions=== | |||
| {{see also|Bisphenol}} | |||
| In 2006, the US Government sponsored an assessment of the scientific literature on BPA. 38 opponents of bisphenol A gathered in Chapel Hill, North Carolina to review several hundred studies on BPA, many conducted by members of the group. At the end of the meeting, the group issued the Chapel Hill Consensus Statement, which stated "BPA at concentrations found in the human body is associated with organizational changes in the prostate, breast, testis, mammary glands, body size, brain structure and chemistry, and behavior of laboratory animals."<ref>Vogel, S. (2009). "The Politics of Plastics: The Making and Unmaking of Bisphenol A ‘Safety’". American Journal of Public Health 99 (S3): 559-566.</ref> | |||
| Concerns about the health effects of BPA have led some manufacturers replacing it with other bisphenols, such as ] and ]. These are produced in a similar manner to BPA, by replacing acetone with other ]s, which undergo analogous condensation reactions.<ref name="Fiege">{{Ullmann| vauthors = Fiege H, Voges HW, Hamamoto T, Umemura S, Iwata T, Miki H, Fujita Y, Buysch HJ, Garbe D, Paulus W |year=2000 |doi=10.1002/14356007.a19_313|title=Phenol Derivatives |isbn=978-3527306732}}</ref> Thus, in ], the F signifies ]. | |||
| Health concerns have also been raised about these substitutes.<ref>{{cite journal | vauthors = Rochester JR, Bolden AL | title = Bisphenol S and F: A Systematic Review and Comparison of the Hormonal Activity of Bisphenol A Substitutes | journal = Environmental Health Perspectives | volume = 123 | issue = 7 | pages = 643–650 | date = July 2015 | pmid = 25775505 | pmc = 4492270 | doi = 10.1289/ehp.1408989| bibcode = 2015EnvHP.123..643R }}</ref><ref name=Other /> Alternative polymers, such as ] have been developed to give the same properties as polycarbonate (durable, clear) without using BPA or its analogues. | |||
| {| class="wikitable" | |||
| The Chapel Hill Consensus Statement claimed that average levels in people are above those that cause harm to many animals in laboratory experiments. They noted that while BPA is not persistent in the environment or in humans, ] surveys indicate that exposure is continuous, however, which is problematic because acute animal exposure studies are used to estimate daily human exposure to BPA, and no studies that had examined BPA ] in animal models had followed continuous low-level exposures. They added that measurement of BPA levels in serum and other body fluids suggests the possibilities that BPA intake is much higher than accounted for, and/or that BPA can bioaccumulate in some conditions (such as pregnancy).<ref>{{vcite journal |author=vom Saal FS |title=Chapel Hill bisphenol A expert panel consensus statement: integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure |journal=Reprod. Toxicol. |volume=24 |issue=2 |pages=131–8 |year=2007 |pmid=17768031 |pmc=2967230 |doi=10.1016/j.reprotox.2007.07.005 |url= |author-separator=, |author2=Akingbemi BT |author3=Belcher SM |display-authors=3 |last4=Birnbaum |first4=Linda S. |last5=Crain |first5=D. Andrew |last6=Eriksen |first6=Marcus |last7=Farabollini |first7=Francesca |last8=Guillette |first8=Louis J. |last9=Hauser |first9=Russ}}</ref> A 2011 study, the first to examine BPA in a continuous low-level exposure throughout the day, did find an increased absorption and accumulation of BPA in the blood of mice.<ref>{{vcite web |url=http://www.sciencedaily.com/releases/2011/06/110606075708.htm |title=Bisphenol A (BPA) accumulates more rapidly within the body than previously thought |doi=10.1289/ehp.1003385 |publisher=Sciencedaily.com |date=6 June 2011 |accessdate=1 February 2012}}</ref> | |||
| ! Structural formula | |||
| ! Name | |||
| In 2007 studies indicating harm reported a variety of deleterious effects in rodent offspring exposed in the uterus: abnormal weight gain, insulin resistance, prostate cancer, and excessive mammary gland development.<ref>{{vcite web |url=http://pubs.acs.org/cen/government/85/8516gov2.html |title=Chemical & Engineering News: Government & Policy – Bisphenol A On Trial |publisher=Pubs.acs.org |accessdate=23 October 2011}}</ref> | |||
| ! ] | |||
| ! colspan="2" | ]s | |||
| A panel convened by the U.S. ] in 2007 noted that many of the studies referenced by the Chapel Hill group had methodological problems. This panel could not rule out "some concern" about BPA's effects on fetal and infant brain development and behavior.<ref name="CERHR"/> The concern over the effect of BPA on infants was also heightened by the fact that infants and children are estimated to have the highest daily intake of BPA.<ref>{{vcite web |url=http://www.center4research.org/2010/04/are-bisphenol-a-bpa-plastic-products-safe-for-infants-and-children/ |title=Are Bisphenol A (BPA) Plastic Products Safe for Infants and Children? |publisher=National Research Center for Women & Families |author=Diana Zuckerman, PhD, Paul Brown, BS, and Laura Walls, BA |date=November 2009 |accessdate=2 February 2012}}</ref> A 2008 report by the U.S. ] (NTP) later agreed with the panel, expressing "some concern for effects on the brain, behavior, and prostate gland in fetuses, infants, and children at current human exposures to bisphenol A," and "''minimal'' concern for effects on the mammary gland and an earlier age for puberty for females in fetuses, infants, and children at current human exposures to bisphenol A." The NTP had "''negligible'' concern that exposure of pregnant women to bisphenol A will result in fetal or neonatal mortality, birth defects, or reduced birth weight and growth in their offspring."<ref name="NTP08">{{vcite web |url=http://www.niehs.nih.gov/news/sya/sya-bpa/ |title=Since you asked – Bisphenol A (BPA): Questions and Answers about Bisphenol A |publisher=National Institute of Environmental Health Sciences |author=John Bucher, PhD, Mike Shelby, PhD |accessdate=2 February 2012}}</ref> | |||
| ===Obesity=== | |||
| A 2008 review has concluded that obesity may be increased as a function of BPA exposure, which "...merits concern among scientists and public health officials."<ref>{{vcite doi|10.1097/MED.0b013e32830ce95c}}</ref> A 2009 review of available studies has concluded that "perinatal BPA exposure acts to exert persistent effects on body weight and adiposity".<ref>{{cite pmid|19433248}}</ref> Another 2009 review has concluded that "Eliminating exposures to (BPA) and improving nutrition during development offer the potential for reducing obesity and associated diseases".<ref>{{vcite doi|10.1016/j.mce.2009.02.025}}</ref> Other reviews have come with similar conclusions.<ref>{{cite pmid|19433252}}</ref><ref>{{cite pmid|19433244}}</ref> A later study on rats has suggested that perinatal exposure to drinking water containing 1 mg/L of BPA increased adipogenesis in females at weaning.<ref>{{vcite doi|10.1289/ehp.11342}}</ref> Another study suggested that larger size-for-age was due to a faster growth rate rather than obesity.<ref>{{cite pmid|20351315}}</ref> | |||
| ===Neurological issues=== | |||
| A panel convened by the U.S. ] determined that there was "some concern" about BPA's effects on fetal and infant brain development and behavior.<ref name="CERHR"/> A 2008 report by the U.S. ] (NTP) later agreed with the panel, expressing "some concern for effects on the brain".<ref name="NTP08"/> In January 2010 the FDA expressed the same level of concern. | |||
| A 2007 review has concluded that BPA, like other xenoestrogens, should be considered as a player within the nervous system that can regulate or alter its functions through multiple pathways.<ref>{{cite pmid|17868795}}</ref> A 2007 review has concluded that low doses of BPA during development have persistent effects on brain structure, function and behavior in rats and mice.<ref>{{vcite doi|10.1016/j.reprotox.2007.06.004}}</ref> A 2008 review concluded that low-dose BPA maternal exposure causes long-term consequences at the level of neurobehavioral development in mice.<ref>{{cite pmid|18949834}}</ref> A 2008 review has concluded that neonatal exposure to Bisphenol-A (BPA) can affect sexually dimorphic brain morphology and neuronal adult phenotypes in mice.<ref>{{cite pmid|17822772}}</ref> A 2008 review has concluded that BPA altered ] in the ] and even nanomolar dosage could induce significant effects on memory processes.<ref>{{cite pmid|17822775}}</ref> A 2009 review raised concerns about BPA effect on ].<ref name="pmid18394690">{{cite journal | author = Gore AC | title = Developmental programming and endocrine disruptor effects on reproductive neuroendocrine systems | journal = Front Neuroendocrinol | volume = 29 | issue = 3 | pages = 358–74 | year = 2008 | month = June | pmid = 18394690 | pmc = 2702520 | doi = 10.1016/j.yfrne.2008.02.002 }}</ref> | |||
| A 2008 study by the ] demonstrated that adverse neurological effects occur in ] regularly exposed to bisphenol A at levels equal to the ]'s (EPA) maximum safe dose of 50 µg/kg/day.<ref name="pmid18768812">{{vcite journal |author=Leranth C, Hajszan T, Szigeti-Buck K, Bober J, Maclusky NJ |title=Bisphenol A prevents the synaptogenic response to estradiol in hippocampus and prefrontal cortex of ovariectomized nonhuman primates |journal=Proc. Natl. Acad. Sci. U.S.A. |volume= 105|issue= 37|pages= 14187–91|year=2008 |month=September |pmid=18768812 |doi=10.1073/pnas.0806139105 |url= |pmc=2544599|bibcode = 2008PNAS..10514187L }}</ref><ref name="Layton2">{{vcite news|url=http://www.washingtonpost.com/wp-dyn/content/article/2008/09/03/AR2008090303397.html?hpid=topnews|title=Chemical in Plastic Is Connected to Health Problems in Monkeys|last=Layton|first=Lindsey|date=4 September 2008|work=Washington Post |pages=A02|accessdate=6 September 2008}}</ref> This research found a connection between BPA and interference with brain cell connections vital to memory, learning, and mood. | |||
| A 2010 study with rats prenatally exposed to 40 µg/kg bw BPA has concluded that ] and its actions in the brain are sensitive to the programming effects of BPA.<ref>{{cite pmid|20219646}}</ref><!--what does that mean? it makes no sense, more information is needed for readers.--> | |||
| ====Disruption of the dopaminergic system==== | |||
| A 2005 review concluded that prenatal and neonatal exposure to BPA in mice can potentiate the central ], resulting in the supersensitivity to the drugs-of-abuse-induced reward effects and ].<ref>{{cite pmid|16045194}}</ref> | |||
| A 2008 review has concluded that BPA mimics estrogenic activity and affects various dopaminergic processes to enhance mesolimbic dopamine activity resulting in hyperactivity, attention deficits, and a heightened sensitivity to drugs of abuse.<ref>{{cite pmid|18555207}}</ref> | |||
| A 2009 study on rats has concluded that prenatal and neonatal exposure to low-dose BPA causes deficits in development at dorsolateral ] via altering the function of dopaminergic receptors.<ref>{{cite pmid|19162132}}</ref> Another 2009 study has found associated changes in the dopaminergic system.<ref name="Tanida">{{cite pmid|19481886}}</ref><!--and the implications are?...--> | |||
| ===Thyroid function=== | |||
| A 2007 review has concluded that bisphenol-A has been shown to bind to thyroid hormone receptor and perhaps have selective effects on its functions.<ref>{{cite pmid|17956155}}</ref> | |||
| A 2009 review about environmental chemicals and thyroid function raised concerns about BPA effects on ] and concluded that "available evidence suggests that governing agencies need to regulate the use of thyroid-disrupting chemicals, particularly as such uses relate exposures of pregnant women, neonates and small children to the agents".<ref>{{cite pmid|19625957}}</ref> | |||
| A 2009 review summarized BPA adverse effects on thyroid hormone action.<ref>{{vcite doi|10.1248/jhs.55.147}}</ref> | |||
| ===Cancer research=== | |||
| According to the WHO's INFOSAN, carcinogenicity studies conducted under the US National Toxicology Program, have shown increases in leukaemia and testicular interstitial cell tumours in male rats. However, according to the note "these studies have not been considered as convincing evidence of a potential cancer risk because of the doubtful statistical significance of the small differences in incidences from controls."<ref name="infosan">{{vcite web|url=http://www.who.int/entity/foodsafety/publications/fs_management/No_05_Bisphenol_A_Nov09_en.pdf|title=BISPHENOL A (BPA) – Current state of knowledge and future actions by WHO and FAO|date=27 November 2009|accessdate=2 December 2009}}</ref> | |||
| A 2010 review at Tufts University Medical School concluded that Bisphenol A may increase cancer risk.<ref>{{vcite doi|10.1038/nrendo.2010.87}}</ref> | |||
| ====Breast cancer==== | |||
| {{Further|Risk factors of breast cancer#Bisphenol A}} | |||
| A 2008 review stated that "evidence from animal models is accumulating that perinatal exposure to (...) low doses of (..) BPA, alters breast development and increases breast cancer risk".<ref>{{vcite doi|10.2533/chimia.2008.406}}</ref> Another 2008 review concluded that "animal experiments and epidemiological data strengthen the hypothesis that fetal exposure to xenoestrogens may be an underlying cause of the increased incidence of breast cancer observed over the last 50 years".<ref>{{cite pmid|18226065}}</ref> <!-- not a good source A 2009 review, funded by the "Breast Cancer Fund", has recommended "a federal ban on the manufacture, distribution and sale of consumer products containing bisphenol A".<ref>{{cite pmid|19267127}}</ref> --> | |||
| A 2009 in vitro study has concluded that BPA is able to induce neoplastic transformation in human breast epithelial cells.<ref>{{cite pmid|19933552}}</ref> Another 2009 study concluded that maternal oral exposure to low concentrations of BPA during lactation increases mammary carcinogenesis in a rodent model.<ref>{{vcite doi|10.1289/ehp.11751}}</ref> | |||
| A 2010 study with the mammary glands of the offspring of pregnant rats treated orally with 0, 25 or 250 µg BPA/kg body weight has found that key proteins involved in signaling pathways such as cellular proliferation were regulated at the protein level by BPA.<ref>{{cite pmid|20219716}}</ref> | |||
| A 2010 study has found that BPA may reduce sensitivity to chemotherapy treatment of specific tumors.<ref>{{cite pmid|19796866}}</ref> | |||
| ====Neuroblastoma==== | |||
| In vitro studies have suggested that BPA can promote the growth of ] cells.<ref>{{cite pmid|19361625}}</ref><ref>{{vcite doi|10.1016/j.etap.2007.05.003}}</ref> A 2010 in vitro study has concluded that BPA potently promotes invasion and ] of neuroblastoma cells through overexpression of ] and ] as well as downregulation of ].<ref>{{cite pmid|19956873}}</ref> | |||
| ====Prostate development and cancer==== | |||
| A 1997 study in mice has found that neonatal BPA exposure of 2 μg/kg increased adult prostate weight.<ref>{{cite pmid|9074884}}</ref> A 2005 study in mice has found that neonatal BPA exposure at 10 μg/kg disrupted the development of the fetal mouse prostate.<ref>{{vcite doi|10.1073/pnas.0502544102}}</ref> | |||
| A 2006 study in rats has shown that neonatal bisphenol A exposure at 10 μg/kg levels increases prostate gland susceptibility to adult-onset precancerous lesions and hormonal carcinogenesis.<ref>{{cite pmid|16740699}}</ref> | |||
| A 2007 in vitro study has found that BPA within the range of concentrations currently measured in human serum is associated with permanent increases in prostate size.<ref>{{cite pmid|17589598}}</ref> A 2009 study has found that newborn rats exposed to a low-dose of BPA (10 µg/kg) showed increased prostate cancer susceptibility when adults.<ref>{{vcite doi| 10.1016/j.fertnstert.2007.12.023}}</ref> | |||
| ====DNA methylation==== | |||
| At least one study has suggested that bisphenol A suppresses ]<ref>{{vcite book |last=Bagchi |first=Debasis |title=Genomics, Proteomics and Metabolomics in Nutraceuticals and Functional Foods |year=2010 |publisher=Wiley |page=319 |isbn=0-8138-1402-2}}</ref> which is linked to ] changes.<ref>{{vcite doi|10.1073/pnas.0703739104}}</ref> | |||
| ===Reproductive system and sexual behavior research=== | |||
| A 2007 study using pregnant mice showed that BPA changes the expression of key developmental ] that form the uterus, which may impact female reproductive tract development and future fertility of female fetuses.<ref name="pmid17093138">{{cite journal | author = Smith CC, Taylor HS | title = Xenoestrogen exposure imprints expression of genes (Hoxa10) required for normal uterine development | journal = FASEB J. | volume = 21 | issue = 1 | pages = 239–46 | year = 2007 | month = January | pmid = 17093138 | doi = 10.1096/fj.06-6635com | laysummary = http://www.sciencedaily.com/releases/2007/02/070215145120.htm | laysource = Science Daily }}</ref> | |||
| A series of studies made in 2009 found: | |||
| * Mouse ovary anomalies from exposure as low as 1 µg/kg, concluded that BPA exposure causes long-term adverse reproductive and carcinogenic effects if exposure occurs during prenatal critical periods of differentiation.<ref name="pmid19590677">{{cite pmid|19590677}}</ref> | |||
| * Neonatal exposure of as low as 50 µg/kg disrupts ovarian development in mice.<ref name="pmid19535786">{{cite pmid|19535786}}</ref><ref>{{cite pmid|19696011}}</ref> | |||
| * Neonatal BPA exposition of as low as 50 µg/kg permanently alters the hypothalamic estrogen-dependent mechanisms that govern sexual behavior in the adult female rat.<ref>{{vcite doi|10.1016/j.reprotox.2009.06.012}}</ref> | |||
| * Prenatal exposure to BPA at levels of (10 μg/kg/day) affects behavioral sexual differentiation in male monkeys.<ref>{{vcite doi|10.1016/j.psyneuen.2009.03.005}}</ref> | |||
| * In placental JEG3 cells ''in vitro'' BPA may reduce estrogen synthesis.<ref>{{vcite doi|10.1016/j.toxlet.2009.06.853 }}</ref> | |||
| * BPA exposure disrupted the ] when administered to immature, but not to adult, rats.<ref>{{cite pmid|19497385}}</ref> | |||
| * Exposure to BPA in the workplace was associated with self-reported adult male sexual dysfunction.<ref>{{vcite doi | 10.1093/humrep/dep381}}</ref> | |||
| A 2009 rodent study, funded by EPA and conducted by some of its scientists, concluded that, compared with ], low-dose exposures of bisphenol A (BPA) showed no effects on several reproductive functions and behavioral activities measured in female rats.<ref>{{vcite doi|10.1093/toxsci/kfp266}}</ref> That study was criticized as flawed for using polycarbonate cages in the experiment (since polycarbonate contains BPA) and the claimed resistance of the rats to estradiol,<ref name="Vom2010">{{vcite doi|10.1093/toxsci/kfq048}}</ref> but that claim was contested by the authors and others.<ref name="pmid20207694">{{vcite journal | author=Gray LE, Ryan B, Hotchkiss AK, Crofton KM | title = Rebuttal of "Flawed Experimental Design Reveals the Need for Guidelines Requiring Appropriate Positive Controls in Endocrine Disruption Research" by (Vom Saal 2010) | journal= Toxicol. Sci. | volume = 115| issue = 2| page = 614| year = 2010 | month = March | pmid = 20207694 | doi = 10.1093/toxsci/kfq073 | issn = }}</ref> Another 2009 rodent study found that BPA exposure during pregnancy has a lasting effect on one of the genes that are responsible for uterine development and subsequent fertility in both mice and humans (HOXA10). The authors concluded, "We don't know what a safe level of BPA is, so pregnant women should avoid BPA exposure."<ref>{{vcite web |url=http://www.sciencedaily.com/releases/2009/06/090610124428.htm |title=Bisphenol A Exposure In Pregnant Mice Permanently Changes DNA Of Offspring |publisher=ScienceDaily |date=10 June 2009 |accessdate=23 October 2011}}</ref> | |||
| In a 2010 study, mice were given BPA at doses thought to be equivalent to levels currently being experienced by humans. The research showed that BPA exposure affects the earliest stages of egg production in the ovaries of the developing mouse fetuses, thus suggesting that the next generation may suffer genetic defects in such biological processes as ] and ] replication. In addition, the research team noted that their study "revealed a striking down-regulation of mitotic/cell cycle genes, raising the possibility that BPA exposure immediately before meiotic entry might act to shorten the reproductive lifespan of the female" by reducing the total pool of fetal ].<ref name="pmid20739668">{{vcite journal | author = Lawson C, Gieske M, Murdoch B, Ye P, Li Y, Hassold T, Hunt PA | title = Gene expression in the fetal mouse ovary is altered by exposure to low doses of bisphenol A | journal = Biol. Reprod. | volume = 84 | issue = 1 | pages = 79–86 | year = 2011 | month = January | pmid = 20739668 | pmc = 3012563 | doi = 10.1095/biolreprod.110.084814 | laysummary = http://www.sciencedaily.com/releases/2010/08/100825093249.htm | laysource = ScienceDaily }}</ref> Another 2010 study with mice concluded that BPA exposure in utero leads to permanent DNA alterations in sensitivity to estrogen.<ref name="pmid20181937"/> Also in 2010, a rodent study found that by exposing fetal mice to BPA during pregnancy and examining gene expression and DNA in the uteruses of female fetuses, BPA exposure permanently affected the uterus by decreasing regulation of gene expression. The changes caused the mice to over-respond to estrogen throughout adulthood, long after the BPA exposure, thus suggesting that early exposure to BPA genetically "programmed" the uterus to be hyper-responsive to estrogen. Extreme estrogen sensitivity can lead to fertility problems, advanced puberty, altered mammary development and reproductive function, as well as a variety of hormone-related cancers. One of the authors concluded that BPA may be similar to ] that caused birth defects and cancers in young women whose mothers were given the drug during pregnancy.<ref name="pmid20181937">{{vcite journal | author = Bromer JG, Zhou Y, Taylor MB, Doherty L, Taylor HS | title = Bisphenol-A exposure in utero leads to epigenetic alterations in the developmental programming of uterine estrogen response | journal = FASEB J. | volume = 24 | issue = 7 | pages = 2273–80 | year = 2010 | month = July | pmid = 20181937 | pmc = 3230522 | doi = 10.1096/fj.09-140533 | laysummary = http://www.sciencedaily.com/releases/2010/02/100225101220.htm | laysource = ScienceDaily }}</ref> | |||
| A 2011 study using the rhesus monkey – a species that is very similar to humans in regard to pregnancy and fetal development – found that prenatal exposure to BPA causes changes in female primates' uterus development.<ref>{{vcite web |url=http://www.sciencedaily.com/releases/2011/06/110607121127.htm |title=Fetal exposure to BPA changes development of uterus in primates, study suggests |publisher=ScienceDaily |date=7 June 2011 |accessdate=1 February 2012}}</ref> A 2011 rodent study found that male rats exposed to BPA had lower sperm counts and testosterone levels than those of unexposed males.<ref>{{vcite web |url=http://www.sciencedaily.com/releases/2011/06/110606092740.htm |title=BPA lowers male fertility, mouse study finds |publisher=ScienceDaily |date=6 June 2011 |accessdate=1 February 2012}}</ref> A 2011 mice study found that male mice exposed to BPA became demasculinized and behaved more like females in their spatial navigational abilities. They were also less desirable to female mice.<ref name="pmid21709224">{{vcite journal | author = Jašarević E, Sieli PT, Twellman EE, Welsh TH, Schachtman TR, Roberts RM, Geary DC, Rosenfeld CS | title = Disruption of adult expression of sexually selected traits by developmental exposure to bisphenol A | journal = Proc. Natl. Acad. Sci. U.S.A. | volume = 108 | issue = 28 | pages = 11715–20 | year = 2011 | month = July | pmid = 21709224 | pmc = 3136312 | doi = 10.1073/pnas.1107958108 | laysummary = http://www.sciencedaily.com/releases/2011/06/110627151712.htm | laysource = ScienceDaily }}</ref> | |||
| ===General research=== | |||
| At an ] meeting in 2009, new research reported data from animals experimentally treated with BPA.<ref>{{vcite web | |||
| |url=http://www.sciencenews.org/view/generic/id/44577/title/More_troubling_news_about_BPA | |||
| |title=More Troubling News About BPA / Science News | |||
| |publisher=sciencenews.org | |||
| |accessdate=11 June 2009 | |||
| }}</ref> Studies presented at the group's annual meeting show BPA can affect the hearts of women, can permanently damage the DNA of mice, and appears to be entering the human body from a variety of unknown sources.<ref>{{vcite web | |||
| |url=http://www.reuters.com/article/2009/06/11/us-bisphenol-idUSTRE55A0JK20090611 | |||
| |title=Hormone experts worried about plastics, chemicals | |||
| |publisher=Reuters | |||
| |author=Maggie Fox | |||
| |accessdate=10 June 2009 | |||
| }}</ref> | |||
| A 2009 ''in vitro'' study on ] cells has found ] effects in exposure of BPA doses from 0.0002 to 0.2 µg/ml and concluded this finding "suggests that exposure of placental cells to low doses of BPA may cause detrimental effects, leading ''in vivo'' to adverse pregnancy outcomes such as ], intrauterine growth restriction, prematurity and pregnancy loss".<ref>{{vcite doi|10.1016/j.taap.2009.09.005}}</ref> | |||
| A 2009 study in rats concluded that BPA, at the reference safe limit for human exposure, was found to impact intestinal permeability and may represent a risk factor in female offspring for developing severe ] in adulthood.<ref>{{cite pmid|20018722}}</ref> | |||
| A 2010 study on mice has concluded that ] exposure to 10 µg/ml of BPA in drinking water enhances allergic sensitization and bronchial inflammation and responsiveness in an animal model of ],<ref>{{cite pmid|20123615}}</ref> and a 2011 study found that higher BPA concentrations in the urine of the pregnant women at 16 weeks were associated with wheezing, a symptom of asthma, in their babies.<ref>{{vcite web |url=http://www.sciencedaily.com/releases/2011/05/110501183817.htm |title=Chemical in plastic, BPA, exposure may be associated with wheezing in children |publisher=ScienceDaily |date=1 May 2011 |accessdate=1 February 2012}}</ref> | |||
| ====Studies on humans==== | |||
| =====Lang study and heart disease===== | |||
| The first large study of health effects on humans associated with bisphenol A exposure was published in September 2008 by Iain Lang and colleagues in the '']''.<ref name="Lang et al.">{{vcite journal |author=Lang IA, Galloway TS, Scarlett A, Henley WE, Depledge M, Wallace RB, Melzer D |title=Association of Urinary Bisphenol A Concentration With Medical Disorders and Laboratory Abnormalities in Adults |journal=] |volume=300 |issue=11 |pages=1303–10 |year=2008 |pmid= 18799442|doi=10.1001/jama.300.11.1303 |url=http://jama.ama-assn.org/cgi/content/full/300.11.1303}}</ref><ref>{{vcite web |url=http://www.newscientist.com/article/dn14739-plastic-bottle-chemical-linked-to-heart-disease.html |title=Plastic bottle chemical linked to heart disease |publisher=NewScientist |author=Alison Motluk |date=September 2008 |accessdate=2 February 2012}}</ref> The ] of almost 1,500 people assessed exposure to bisphenol A by looking at levels of the chemical in urine. The authors found that higher bisphenol A levels were ] associated with ], ], and abnormally high levels of certain liver enzymes. An editorial in the same issue concludes: | |||
| :"Based on this background information, the study by Lang et al,1 while preliminary with regard to these diseases in humans, should spur US regulatory agencies to follow the recent action taken by Canadian regulatory agencies, which have declared BPA a "toxic chemical" requiring aggressive action to limit human and environmental exposures.4 Alternatively, Congressional action could follow the precedent set with the recent passage of federal legislation designed to limit exposures to another family of compounds, phthalates, also used in plastic. Like BPA,5 phthalates are detectable in virtually everyone in the United States.6 This bill moves US policy closer to the European model, in which industry must provide data on the safety of a chemical before it can be used in products."<ref name="JAMAVS"/><ref>{{vcite doi | 10.1001/jama.300.11.1353}}</ref> | |||
| A later similar study performed by the same group of scientists, published in January 2010, confirmed, despite of lower concentrations of BPA in the second study sample, an associated increased risk for heart disease but not for diabetes or liver enzymes. Patients with the highest levels of BPA in their urine carried a 33% increased risk of coronary heart disease.<ref>{{vcite doi|10.1371/journal.pone.0008673}}</ref> | |||
| In 2012, David Melzer and colleagues also published a correlation between BPA levels in urine and heart disease. BPA exposure was higher in those with severe coronary artery stenoses compared to those with no vessel disease.<ref name="Melzer">{{vcite journal |author=Melzer D, Gates P, Osborn NJ, et al. |year=2012 |title=Urinary bisphenol a concentration and angiography-defined coronary artery stenosis |url=http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0043378 |journal=PLoS ONE 2012 |volume=7 }}</ref> | |||
| ====Brain tumors==== | |||
| A Chinese human study links BPA to noncancerous brain tumors. Those with higher urine BPA levels were about 1.6 times more likely to have meningioma compared to those with lower concentrations.<ref name="pmid22527848">{{cite journal | author = Duan B, Hu X, Zhao H, Qin J, Luo J | title = The relationship between urinary bisphenol A levels and meningioma in Chinese adults | journal = Int J Clin Oncol | volume = | issue = | pages = | year = 2012 | month = April | pmid = 22527848 | doi = 10.1007/s10147-012-0408-6 }}</ref> | |||
| =====Other studies===== | |||
| Studies have associated recurrent miscarriage with BPA serum concentrations,<ref>{{vcite doi|10.1093/humrep/deh888}}</ref> oxidative stress and inflammation in postmenopausal women with urinary concentrations,<ref>{{vcite doi|10.1016/j.envres.2009.04.014}}</ref> externalizing behaviors in two-year old children, especially among female children, with mother's urinary concentrations,<ref>{{vcite doi|10.1289/ehp.0900979}}</ref> altered hormone levels in men<ref>{{vcite doi|10.1021/es9028292}}</ref><ref>{{cite pmid|20494855}}</ref> and declining male sexual function<ref name="pmid20467048">{{vcite journal | author = Li DK, Zhou Z, Miao M, He Y, Qing D, Wu T, Wang J, Weng X, Ferber J, Herrinton LJ, Zhu Q, Gao E, Yuan W | title = Relationship between urine bisphenol-A level and declining male sexual function | journal = J. Androl. | volume = 31 | issue = 5 | pages = 500–6 | year = 2010 | pmid = 20467048 | doi = 10.2164/jandrol.110.010413 }}</ref> with urinary concentrations. The Canadian Health Measures Survey, 2007 to 2009 published in 2010 found that teenagers carry 30 percent more BPA in their bodies than older adults. The reason for this is not known.<ref>{{vcite news |title=Bisphenol A concentrations in the Canadian population, 2007 to 2009 |date=16 August 2010 |url=http://www.statcan.gc.ca/pub/82-625-x/2010002/article/11327-eng.htm |work=Statistics Canada |pages=82–625–XWE |accessdate=19 November 2010}}</ref> A 2010 study that analyzed BPA urinary concentrations has concluded that for people under 18 years of age BPA may negatively impact human immune function.<ref>{{cite pmid|21062687}}</ref> A study done in 2010 reported the daily excretion levels of BPA among European adults in a large-scale and high-quality population-based sample, and it was shown that higher BPA daily excretion was associated with an increase in serum total ] concentration in men.<ref>{{vcite web |url=http://ehp03.niehs.nih.gov/article/fetchArticle.action?articleURI=info%3Adoi%2F10.1289%2Fehp.1002367 |title=Daily Bisphenol A Excretion and Associations with Sex Hormone Concentrations: Results from the InCHIANTI Adult Population Study |work=Environmental Health Perspectives |accessdate=3 February 2012}}</ref> A 2011 study found higher BPA levels in women with ] compared to controls. Furthermore, researchers found a statistically significant positive association between male sex hormones and BPA in these women, suggesting a potential role of BPA in ovarian dysfunction.<ref>{{vcite web |url=http://www.sciencedaily.com/releases/2011/01/110113082720.htm |title=Bisphenol A may have role in ovarian dysfunction |doi=10.1210/jc.2010-1658 |publisher=ScienceDaily |date=13 January 2011 |accessdate=1 February 2012}}</ref> A 2010 study found that people over age 18 with higher levels of BPA exposure had higher CMV ] levels, which suggests their cell-mediated ] may not be functioning properly.<ref>{{vcite web |url=http://www.sciencedaily.com/releases/2010/11/101129101920.htm |title=Antibacterial Soaps: Being Too Clean Can Make People Sick, Study Suggests |publisher=ScienceDaily |date=29 November 2010 |accessdate=1 February 2012}}</ref> | |||
| =====Sexual difficulties===== | |||
| A 2009 study on Chinese workers in BPA factories found that workers were four times more likely to report ], reduced sexual desire and overall dissatisfaction with their sex life than workers with no heightened BPA exposure.<ref>{{vcite journal | |||
| |author=D. Li, Z. Zhou, D. Qing, Y. He, T. Wu, M. Miao, J. Wang, X. Weng, J.R. Ferber, L.J. Herrinton, Q. Zhu, E. Gao, H. Checkoway, and W. Yuan | |||
| |title=Occupational exposure to bisphenol-A (BPA) and the risk of Self-Reported Male Sexual Dysfunction | |||
| |journal=Human Reproduction | |||
| |volume= 25|issue= 2|pages= 519–27|year=2009 |pmid= 19906654|pmc= |doi=10.1093/humrep/dep381 | |||
| |url=}}</ref> BPA workers were also seven times more likely to have ejaculation difficulties. They were also more likely to report reduced sexual function within one year of beginning employment at the factory, and the higher the exposure, the more likely they were to have sexual difficulties.<ref>{{vcite web|url=http://www.ajc.com/health/content/shared-auto/healthnews/envm/632954.html |title=BPA Tied to Impotence in Men |publisher=Ajc.com |date=11 November 2009 |accessdate=23 October 2011}}</ref> | |||
| ====Historical studies==== | |||
| The first evidence of the ] of bisphenol A came from experiments on rats conducted in the 1930s,<ref name="W. Lawson, 1938 pp. 222"/><ref>{{vcite journal | author=Dodds E. C., Lawson Wilfrid | year = 1936 | title = Synthetic Œstrogenic Agents without the Phenanthrene Nucleus | url = | journal=Nature | volume = 137 | issue =3476 | page = 996 |bibcode=1936Natur.137..996D | doi=10.1038/137996a0}}</ref> but it was not until 1997 that adverse effects of low-dose exposure on laboratory animals were first reported.<ref name="C&ENews"/> | |||
| ===Low-dose exposure in animals=== | |||
| {| class="wikitable" | |||
| |- | |||
| ! Dose (µg/kg/day) | |||
| ! Effects (measured in studies of mice or rats,<br/>descriptions (in quotes) are from ])<ref name="globemittelstaedt">{{vcite news | last=Mittelstaedt | first=Martin | title='Inherently toxic' chemical faces its future | date=7 April 2007 | url=http://www.theglobeandmail.com/servlet/story/RTGAM.20070406.wbisphenolA0407/BNStory/National/ | accessdate=7 April 2007 |work=Globe and Mail |location=Canada | location=Toronto}}</ref><ref name="EWG-BPA">This table is adapted from: EWG, 2007. "Many studies confirm BPA's low-dose toxicity across a diverse range of toxic effects," Environmental Working Group Report: A Survey of Bisphenol A in U.S. Canned Foods. Retrieved 4 November 2007 at http://www.ewg.org/node/20941. All studies included in this table where judged by the CEHRH panel to be at least of moderate usefulness for assessing the risk of BPA to human reproduction.</ref> | |||
| ! Study Year | |||
| |- | |||
| | 0.025 | |||
| | "Permanent changes to genital tract" | |||
| | 2005<ref name="pmid15689538">{{vcite journal |author=Markey CM, Wadia PR, Rubin BS, Sonnenschein C, Soto AM |title=Long-term effects of fetal exposure to low doses of the xenoestrogen bisphenol-A in the female mouse genital tract |journal=] |volume=72 |issue=6 |pages=1344–51 |year=2005 |pmid=15689538 |doi=10.1095/biolreprod.104.036301 |url=http://www.biolreprod.org/cgi/pmidlookup?view=long&pmid=15689538 |accessdate=1 February 2012}}</ref> | |||
| |- | |||
| | 0.025 | |||
| | "Changes in breast tissue that predispose cells to hormones and carcinogens" | |||
| | 2005<ref name="pmid15919749">{{vcite journal |author=Muñoz-de-Toro M |title=Perinatal exposure to bisphenol-A alters peripubertal mammary gland development in mice |journal=] |volume=146 |issue=9 |pages=4138–47 |year=2005 |pmid=15919749 |doi=10.1210/en.2005-0340 |url=http://endo.endojournals.org/cgi/pmidlookup?view=long&pmid=15919749 |pmc=2834307 |author-separator=, |author2=Markey CM |author3=Wadia PR |display-authors=3 |last4=Luque |first4=EH |last5=Rubin |first5=BS |last6=Sonnenschein |first6=C |last7=Soto |first7=AM}}</ref> | |||
| |- | |||
| | 1 | |||
| | long-term adverse reproductive and carcinogenic effects | |||
| | 2009<ref name="pmid19590677"/> | |||
| |- | |||
| | 2 | |||
| | "increased prostate weight 30%" | |||
| | 1997<ref name="pmid9074884">{{vcite journal |author=Nagel SC, vom Saal FS, Thayer KA, Dhar MG, Boechler M, Welshons WV |title=Relative binding affinity-serum modified access (RBA-SMA) assay predicts the relative in vivo bioactivity of the xenoestrogens bisphenol A and octylphenol |journal=] |volume=105 |issue=1 |pages=70–6 |year=1997 |pmid=9074884 |doi=10.2307/3433065 |pmc=1469837 |jstor=3433065}}</ref> | |||
| |- | |||
| | 2<!-- abstract says says 2.0, EWG report says 2.4 --> | |||
| | "lower bodyweight, increase of ] in both genders, signs of early puberty and longer estrus." | |||
| | 2002<ref name="pmid11955942">{{vcite journal |author=Honma S, Suzuki A, Buchanan DL, Katsu Y, Watanabe H, Iguchi T |title=Low dose effect of in utero exposure to bisphenol A and diethylstilbestrol on female mouse reproduction |journal=Reproductive Toxicology | |||
| |volume=16 |issue=2 |pages=117–22 |year=2002 |pmid=11955942 |doi= 10.1016/S0890-6238(02)00006-0 |url=http://linkinghub.elsevier.com/retrieve/pii/S0890623802000060}}</ref> | |||
| |- | |||
| | 2.4 | |||
| | "Decline in testicular testosterone" | |||
| | 2004<ref name="pmid14605012">{{vcite journal |author=Akingbemi BT, Sottas CM, Koulova AI, Klinefelter GR, Hardy MP |title=Inhibition of testicular steroidogenesis by the xenoestrogen bisphenol A is associated with reduced pituitary luteinizing hormone secretion and decreased steroidogenic enzyme gene expression in rat Leydig cells |journal=] |volume=145 |issue=2 |pages=592–603 |year=2004 |pmid=14605012 |doi=10.1210/en.2003-1174 |url=http://endo.endojournals.org/cgi/pmidlookup?view=long&pmid=14605012}}</ref> | |||
| |- | |||
| | 2.5 | |||
| | "Breast cells predisposed to cancer" | |||
| | 2007<ref name="pmid17123778">{{vcite journal |author=Murray TJ, Maffini MV, Ucci AA, Sonnenschein C, Soto AM |title=Induction of mammary gland ductal hyperplasias and carcinoma in situ following fetal bisphenol A exposure |journal=Reproductive Toxicology |volume=23 |issue=3 |pages=383–90 |year=2007 |pmid=17123778 |doi=10.1016/j.reprotox.2006.10.002 |url=http://linkinghub.elsevier.com/retrieve/pii/S0890-6238(06)00263-2 |pmc=1987322}}</ref> | |||
| |- | |- | ||
| |]||] || 1478-61-1 || ] || ] | |||
| | 10 | |||
| | "Prostate cells more sensitive to hormones and cancer" | |||
| | 2006<ref name="pmid16740699">{{vcite journal |author=Ho SM, Tang WY, Belmonte de Frausto J, Prins GS |title=Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4 |journal=] |volume=66 |issue=11 |pages=5624–32 |year=2006 |pmid=16740699 |doi=10.1158/0008-5472.CAN-06-0516 |url=http://cancerres.aacrjournals.org/cgi/pmidlookup?view=long&pmid=16740699 |pmc=2276876}}</ref> | |||
| |- | |- | ||
| |]||] || 620-92-8 || ] || ] | |||
| | 10 | |||
| | "Decreased maternal behaviors" | |||
| | 2002<ref name="pmid12060838">{{vcite journal |author=Palanza PL, Howdeshell KL, Parmigiani S, vom Saal FS |title=Exposure to a low dose of bisphenol A during fetal life or in adulthood alters maternal behavior in mice |journal=] |volume=110 Suppl 3 |pages=415–22 |year=2002 |pmid=12060838 |url=http://ehpnet1.niehs.nih.gov/docs/2002/suppl-3/415-422palanza/abstract.html |pmc=1241192}}</ref> | |||
| |- | |- | ||
| |]||] || 80-09-1 || ] || ] | |||
| <!-- values in abstract (PMID 15079872) don't agree with LOAEL listed in EWG report| 30 | |||
| | "Hyperactivity" | |||
| | 2004 | |||
| |- --> | |||
| | 30 | |||
| | "Reversed the normal sex differences in brain structure and behavior" | |||
| | 2003<ref name="pmid12631470">{{vcite journal |author=Kubo K, Arai O, Omura M, Watanabe R, Ogata R, Aou S |title=Low dose effects of bisphenol A on sexual differentiation of the brain and behavior in rats |journal=] |volume=45 |issue=3 |pages=345–56 |year=2003 |pmid=12631470 |doi= 10.1016/S0168-0102(02)00251-1|url=http://linkinghub.elsevier.com/retrieve/pii/S0168010202002511}}</ref> | |||
| |- | |- | ||
| |]||] || 843-55-0 || ] || ] | |||
| | 50 | |||
| | Adverse neurological effects occur in ] | |||
| | 2008<ref name="pmid18768812"/> | |||
| |- | |- | ||
| |]||] || 5384-21-4 || ] || ] | |||
| | 50 | |||
| | Disrupts ovarian development | |||
| | 2009<ref name="pmid19535786"/> | |||
| |} | |} | ||
| ==Human safety== | |||
| <!-- controversial | |||
| ===Exposure=== | |||
| A study from 2008 concluded that blood levels of bisphenol A in neonatal mice are the same whether it is injected or ingested.<ref name="pmid18295446">{{vcite journal |author=Taylor JA, Welshons WV, Vom Saal FS |title=No effect of route of exposure (oral; subcutaneous injection) on plasma bisphenol A throughout 24h after administration in neonatal female mice |journal=Reprod. Toxicol. |volume=25 |issue=2 |pages=169–76 |year=2008 |month=February |pmid=18295446 |doi=10.1016/j.reprotox.2008.01.001 |url=http://linkinghub.elsevier.com/retrieve/pii/S0890-6238(08)00002-6 |accessdate=5 May 2008}}</ref>--> | |||
| ] lining of metal food, beverage cans and ]s.]] | |||
| The current U.S. human exposure limit set by the EPA is 50 µg/kg/day.<ref name="CASRN 80-05-7"/> | |||
| <!-- Ongoing discussion | |||
| In a 2010 commentary a group of scientists criticized a study designed to test low dose BPA exposure published in ''"Toxicological Sciences"''<ref>{{cite pmid|19864446}}</ref> and a later editorial by the same journal,<ref>{{cite pmid|20147444}}</ref> which claimed the rats used in the study were insensitive to estrogen and that had other problems like the use of polycabornate cages<ref name="Vom2010"/> while the authors disagreed.<ref>{{cite pmid|20207694}}</ref> | |||
| --> | |||
| As a result of the presence of BPA in plastics and other commonplace materials, most people are frequently exposed to trace levels of BPA.<ref>{{cite journal | vauthors = Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL | title = Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003-2004 | journal = Environmental Health Perspectives | volume = 116 | issue = 1 | pages = 39–44 | date = January 2008 | pmid = 18197297 | pmc = 2199288 | doi = 10.1289/ehp.10753 | bibcode = 2008EnvHP.116...39C }}</ref><ref>{{cite journal | vauthors = Thoene M, Rytel L, Nowicka N, Wojtkiewicz J | title = The state of bisphenol research in the lesser developed countries of the EU: a mini-review | journal = Toxicology Research | volume = 7 | issue = 3 | pages = 371–380 | date = May 2018 | pmid = 30090587 | pmc = 6062254 | doi = 10.1039/c8tx00064f }}</ref><ref>{{cite journal | vauthors = Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV | title = Human exposure to bisphenol A (BPA) | journal = Reproductive Toxicology | volume = 24 | issue = 2 | pages = 139–177 | date = August 2007 | pmid = 17825522 | doi = 10.1016/j.reprotox.2007.07.010 | bibcode = 2007RepTx..24..139V }}</ref> The primary source of human exposure is via food, as epoxy and PVC are used to line the inside of food cans to prevent corrosion of the metal by acidic foodstuffs. Polycarbonate drink containers are also a source of exposure, although most disposable drinks bottles are actually made of ], which contains no BPA. Among the non-food sources, exposure routes include through dust,<ref name="Tom2021"/> thermal paper,<ref name="paper1"/> clothing,<ref name="ir.rcees.ac.cn"/> dental materials,<ref>{{cite journal |last1=Van Landuyt |first1=K.L. |last2=Nawrot |first2=Tim |last3=Geebelen |first3=B. |last4=De Munck |first4=J. |last5=Snauwaert |first5=J. |last6=Yoshihara |first6=K. |last7=Scheers |first7=Hans |last8=Godderis |first8=Lode |last9=Hoet |first9=P. |last10=Van Meerbeek |first10=B. |title=How much do resin-based dental materials release? A meta-analytical approach |journal=Dental Materials |date=August 2011 |volume=27 |issue=8 |pages=723–747 |doi=10.1016/j.dental.2011.05.001|pmid=21664675 }}</ref> and medical devices.<ref name="Covaci"/> Although BPA exposure is common it does not accumulate within the body, with ] studies showing the ] of BPA in adult humans to be around two hours.<ref>{{cite journal | vauthors = Tsukioka T, Terasawa JI, Sato S, Hatayama Y, Makino T, Nakazawa H |title=Development of Analytical Method for Determining Trace Amounts of BPA in Urine Samples and Estimation of Exposure to BPA. |journal=Journal of Environmental Chemistry |date=2004 |volume=14 |issue=1 |pages=57–63 |doi=10.5985/jec.14.57|doi-access=free }}</ref><ref>{{cite journal | vauthors = Shin BS, Kim CH, Jun YS, Kim DH, Lee BM, Yoon CH, Park EH, Lee KC, Han SY, Park KL, Kim HS, Yoo SD| title = Physiologically based pharmacokinetics of bisphenol A | journal = Journal of Toxicology and Environmental Health. Part A | volume = 67 | issue = 23–24 | pages = 1971–1985 | date = December 2004 | pmid = 15513896 | doi = 10.1080/15287390490514615 | bibcode = 2004JTEHA..67.1971S | s2cid = 24467830 }}</ref> The body first converts it into more water-soluble compounds via ] or ], which are then removed from the body through the urine. This allows exposure to be easily determined by urine testing, facilitating convenient ] of populations.<ref name="auto">{{cite journal | vauthors = Huang RP, Liu ZH, Yin H, Dang Z, Wu PX, Zhu NW, Lin Z | title = Bisphenol A concentrations in human urine, human intakes across six continents, and annual trends of average intakes in adult and child populations worldwide: A thorough literature review | journal = The Science of the Total Environment | volume = 626 | pages = 971–981 | date = June 2018 | pmid = 29898562 | doi = 10.1016/j.scitotenv.2018.01.144 | s2cid = 49194096 | bibcode = 2018ScTEn.626..971H }}</ref><ref name=Covaci /><ref>{{cite journal |last1=Bousoumah |first1=Radia |last2=Leso |first2=Veruscka |last3=Iavicoli |first3=Ivo |last4=Huuskonen |first4=Pasi |last5=Viegas |first5=Susana |last6=Porras |first6=Simo P. |last7=Santonen |first7=Tiina |last8=Frery |first8=Nadine |last9=Robert |first9=Alain |last10=Ndaw |first10=Sophie |title=Biomonitoring of occupational exposure to bisphenol A, bisphenol S and bisphenol F: A systematic review |journal=Science of the Total Environment |date=August 2021 |volume=783 |pages=146905 |doi=10.1016/j.scitotenv.2021.146905|pmid=33865140 |bibcode=2021ScTEn.78346905B |s2cid=233290894 |doi-access=free|hdl=10400.21/13242 |hdl-access=free }}</ref> Food and drink containers made from Bisphenol A-containing plastics do not contaminate the content to cause any increased cancer risk.<ref name=cruk>{{cite web |publisher=] |url=https://www.cancerresearchuk.org/about-cancer/causes-of-cancer/cancer-myths/does-using-plastic-bottles-and-containers-cause-cancer |title=Does using plastic bottles and containers cause cancer? |date=23 December 2021}}</ref> | |||
| ===Xenoestrogen=== | |||
| There is evidence that bisphenol A functions as a ] by binding strongly to ] (ERR-γ).<ref name="matsushima"/> This ] (endogenous ligand unknown) behaves as a constitutive activator of transcription. BPA seems to bind strongly to ERR-γ (] = 5.5 nM), but not to the ] (ER).<ref name="matsushima"/> BPA binding to ERR-γ preserves its basal constitutive activity.<ref name="matsushima"/> It can also protect it from deactivation from the ] ].<ref name="matsushima">{{vcite journal | author=Matsushima A, Kakuta Y, Teramoto T, Koshiba T, Liu X, Okada H, Tokunaga T, Kawabata S, Kimura M, Shimohigashi Y | title = Structural evidence for endocrine disruptor bisphenol A binding to human nuclear receptor ERR gamma | journal=J. Biochem. | volume = 142 | issue = 4 | pages = 517–24 | year = 2007 | month = October | pmid = 17761695 | doi = 10.1093/jb/mvm158 | url = }}</ref> | |||
| ===Health effects and regulation=== | |||
| Different expression of ERR-γ in different parts of the body may account for variations in bisphenol A effects. For instance, ERR-γ has been found in high concentration in the ], explaining reports of high bisphenol accumulation in this tissue.<ref name="pmid19304792">{{vcite journal | author=Takeda Y, Liu X, Sumiyoshi M, Matsushima A, Shimohigashi M, Shimohigashi Y | title = Placenta expressing the greatest quantity of bisphenol A receptor ERR{gamma} among the human reproductive tissues: Predominant expression of type-1 ERRgamma isoform | journal=J. Biochem. | volume = 146 | issue = 1 | pages = 113–22 | year = 2009 | month = July | pmid = 19304792 | doi = 10.1093/jb/mvp049 | url = }}</ref> | |||
| {{main|Health effects of Bisphenol A}} | |||
| The health effects of BPA have been the subject of prolonged public and scientific debate,<ref name=WHO /><ref name=German2011>{{cite journal | vauthors = Hengstler JG, Foth H, Gebel T, Kramer PJ, Lilienblum W, Schweinfurth H, Völkel W, Wollin KM, Gundert-Remy U | title = Critical evaluation of key evidence on the human health hazards of exposure to bisphenol A | journal = Critical Reviews in Toxicology | volume = 41 | issue = 4 | pages = 263–291 | date = April 2011 | pmid = 21438738 | pmc = 3135059 | doi = 10.3109/10408444.2011.558487}}</ref><ref name=GLP>{{cite journal | vauthors = Myers JP, vom Saal FS, Akingbemi BT, Arizono K, Belcher S, Colborn T, Chahoud I, Crain DA, Farabollini F, Guillette LJ, Hassold T, Ho SM, Hunt PA, Iguchi T, Jobling S, Kanno J, Laufer H, Marcus M, McLachlan JA, Nadal A, Oehlmann J, Olea N, Palanza P, Parmigiani S, Rubin BS, Schoenfelder G, Sonnenschein C, Soto AM, Talsness CE, Taylor JA, Vandenberg LN, Vandenbergh JG, Vogel S, Watson CS, Welshons WV, Zoeller RT | display-authors = 6 | title = Why public health agencies cannot depend on good laboratory practices as a criterion for selecting data: the case of bisphenol A | journal = Environmental Health Perspectives | volume = 117 | issue = 3 | pages = 309–315 | date = March 2009 | pmid = 19337501 | pmc = 2661896 | doi = 10.1289/ehp.0800173 | bibcode = 2009EnvHP.117..309M }}</ref> with ] listing more than 18,000 scientific papers as of 2024.<ref>{{cite web |title=bisphenol a - Search Results - PubMed |url=https://pubmed.ncbi.nlm.nih.gov/?term=bisphenol+a |website=PubMed |access-date=26 January 2024 |language=en}}</ref> Concern is mostly related to its ]-like activity, although it can interact with other receptor systems as an ].<ref name="receptors">{{cite journal |last1=MacKay |first1=Harry |last2=Abizaid |first2=Alfonso |title=A plurality of molecular targets: The receptor ecosystem for bisphenol-A (BPA) |journal=Hormones and Behavior |date=May 2018 |volume=101 |pages=59–67 |doi=10.1016/j.yhbeh.2017.11.001|pmid=29104009 |s2cid=23088708 }}</ref> These interactions are all very weak, but exposure to BPA is effectively lifelong, leading to concern over possible cumulative effects. Studying this sort of long‑term, low‑dose interaction is difficult, and although there have been numerous studies, there are considerable discrepancies in their conclusions regarding the nature of the effects observed as well as the levels at which they occur.<ref name=WHO /> A common criticism is that industry-sponsored trials tend to show BPA as being safer than studies performed by academic or government laboratories,<ref name=GLP/><ref>{{cite journal |vauthors=vom Saal FS, Hughes C |title=An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment |journal=Environ. Health Perspect. |volume=113 |issue=8 |pages=926–33 |year=2005 |pmid=16079060 |pmc=1280330 |doi=10.1289/ehp.7713|bibcode=2005EnvHP.113..926V }}</ref> although this has also been explained in terms of industry studies being better designed.<ref name=German2011 /><ref>{{cite journal |last1=Teeguarden |first1=Justin G. |last2=Hanson-Drury |first2=Sesha |title=A systematic review of Bisphenol A "low dose" studies in the context of human exposure: A case for establishing standards for reporting "low-dose" effects of chemicals |journal=Food and Chemical Toxicology |date=December 2013 |volume=62 |pages=935–948 |doi=10.1016/j.fct.2013.07.007|pmid=23867546}}</ref> | |||
| In the 2010s public health agencies in the EU,<ref>{{cite web |title=Bisphenol A - ECHA |url=https://echa.europa.eu/hot-topics/bisphenol-a |website=echa.europa.eu |access-date=28 March 2022 |archive-date=8 June 2022 |archive-url=https://web.archive.org/web/20220608014918/https://echa.europa.eu/hot-topics/bisphenol-a |url-status=dead }}</ref><ref>{{cite book |title=EFSA explains the Safety of Bisphenol A: scientific opinion on bisphenol A (2015). |publisher=European Food Safety Authority |date=2015 |doi=10.2805/075460|author1=European Food Safety Authority |isbn=9789291996421 }}</ref><ref>{{cite journal |title=Scientific Opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs |journal=EFSA Journal |date=21 January 2015 |volume=13 |issue=1 |page=3978 |doi=10.2903/j.efsa.2015.3978|hdl=2164/12119 |hdl-access=free }}</ref> US,<ref>{{cite web | author = OCSPP US EPA |title=Risk Management for Bisphenol A (BPA) |url=https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/risk-management-bisphenol-bpa |website=www.epa.gov |access-date=28 March 2022 |language=en |date=21 September 2015}}</ref><ref>{{cite book | title = NTP Research Report on the Consortium Linking Academic and Regulatory Insights on Bisphenol A Toxicity (CLARITY-BPA): A Compendium of Published Findings | pages = 18 | date = October 2021 | pmid = 34910417 | doi = 10.22427/NTP-RR-18 | s2cid = 240266384 | author1 = CLARITY-BPA Research Program }}</ref> Canada,<ref>{{cite web |last=Health Canada |date=16 April 2013 |title=Bisphenol A (BPA) |url=https://www.canada.ca/en/health-canada/services/home-garden-safety/bisphenol-bpa.html |access-date=28 March 2022 |website=www.canada.ca (]) |publisher=Government of Canada}}</ref> Australia<ref>{{cite web |title=Bisphenol A (BPA) |url=https://www.foodstandards.gov.au/consumer/chemicals/bpa/Pages/default.aspx |access-date=28 March 2022 |website=Food Standards Australia New Zealand (]) |publisher=Department of Health (Australia) |archive-date=24 March 2022 |archive-url=https://web.archive.org/web/20220324044358/https://www.foodstandards.gov.au/consumer/chemicals/bpa/Pages/default.aspx |url-status=dead }}</ref> and Japan as well as the ]<ref name=WHO>{{cite book |title=Joint FAO/WHO expert meeting to review toxicological and health aspects of bisphenol A : final report, including report of stakeholder meeting on bisphenol A, 1-5 November 2010, Ottawa, Canada |date=2011 |publisher=World Health Organization |hdl=10665/44624 |isbn=978-92-4-156427-4 |url=https://apps.who.int/iris/handle/10665/44624 |access-date=23 March 2022 |language=en}}</ref> all reviewed the health risks of BPA, and found normal exposure to be below the level currently associated with risk. Regardless, due to the scientific uncertainty, many jurisdictions continued to take steps to reduce exposure on a precautionary basis. In particular, infants were considered to be at greater risk,<ref>{{cite book | vauthors = Aschberger K, Castello P, Hoekstra E |title=Bisphenol A and baby bottles : challenges and perspectives |publisher=The Publications Office of the European Union |date=2010 |doi=10.2788/97553 |isbn=9789279158698 |doi-access=free}}</ref> leading to bans on the use of BPA in ]s and related products by the US,<ref>{{cite web |title=Indirect Food Additives: Polymers |url=https://www.federalregister.gov/documents/2012/07/17/2012-17366/indirect-food-additives-polymers |website=Federal Register |date=17 July 2012 |publisher=U.S. Government Publishing Office}}{{Federal Register|77|41899}}</ref> Canada,<ref>{{cite web | author = Legislative Services Branch|title=Consolidated federal laws of canada, Canada Consumer Product Safety Act |url=https://laws-lois.justice.gc.ca/eng/acts/C-1.68/index.html |website=laws-lois.justice.gc.ca |date=1 July 2020}}</ref> and EU<ref>{{cite web |title=EUR-Lex - 32011L0008 - EN - EUR-Lex |url=https://eur-lex.europa.eu/eli/dir/2011/8/oj |website=EUR-Lex |publisher=European Union |language=en |quote=COMMISSION DIRECTIVE 2011/8/EU of 28 January 2011 amending Directive 2002/72/EC as regards the restriction of use of Bisphenol A in plastic infant feeding bottles}}</ref> amongst others. Bottle producers largely switched from polycarbonate to ] and there is some evidence that BPA exposure in infants has decreased as a result of this.<ref name="auto"/> The ] completed a re-evaluation into the risks of BPA in 2023, concluding that its ] should be greatly reduced.<ref>{{cite journal |last1=Lambré |first1=Claude |last2=Barat Baviera |first2=José Manuel |last3=Bolognesi |first3=Claudia |last4=Chesson |first4=Andrew |last5=Cocconcelli |first5=Pier Sandro |last6=Crebelli |first6=Riccardo |last7=Gott |first7=David Michael |last8=Grob |first8=Konrad |last9=Lampi |first9=Evgenia |last10=Mengelers |first10=Marcel |last11=Mortensen |first11=Alicja |last12=Rivière |first12=Gilles |last13=Silano (until December †) |first13=Vittorio |last14=Steffensen |first14=Inger-Lise |last15=Tlustos |first15=Christina |last16=Vernis |first16=Laurence |last17=Zorn |first17=Holger |last18=Batke |first18=Monika |last19=Bignami |first19=Margherita |last20=Corsini |first20=Emanuela |last21=FitzGerald |first21=Rex |last22=Gundert-Remy |first22=Ursula |last23=Halldorsson |first23=Thorhallur |last24=Hart |first24=Andrew |last25=Ntzani |first25=Evangelia |last26=Scanziani |first26=Eugenio |last27=Schroeder |first27=Henri |last28=Ulbrich |first28=Beate |last29=Waalkens-Berendsen |first29=Dina |last30=Woelfle |first30=Detlef |last31=Al Harraq |first31=Zainab |last32=Baert |first32=Katleen |last33=Carfì |first33=Maria |last34=Castoldi |first34=Anna F |last35=Croera |first35=Cristina |last36=Van Loveren |first36=Henk |title=Re-evaluation of the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs |journal=EFSA Journal |date=April 2023 |volume=21 |issue=4 |pages=e06857 |doi=10.2903/j.efsa.2023.6857|pmid=37089179 |pmc=10113887 |hdl=20.500.11815/4370 |hdl-access=free }}</ref> This lead to a European Union on 19 December 2024 to ban BPA in all the food contact materials, including plastic and coated packaging<ref>{{cite web |title=Regulation - EU - 2024/3190 - EN - EUR-Lex |url=https://eur-lex.europa.eu/eli/reg/2024/3190/oj/eng |website=eur-lex.europa.eu |access-date=14 January 2025 |language=en}}</ref>. The ban will come into force after an implementation period of up to three years. | |||
| ==Human exposure sources== | |||
| The major human exposure route to BPA is diet, including ingestion of contaminated food and water.<ref>Cichna-Markl, M. Methods (2012).</ref> There is limited evidence on inhalation exposure and the body of research on dermal absorption continues to grow. (see below) | |||
| {{rquote|right|The problem is, BPA is also a synthetic estrogen, and plastics with BPA can break down, especially when they're washed, heated or stressed, allowing the chemical to leach into food and water and then enter the human body. That happens to nearly all of us; the CDC has found BPA in the urine of 93% of surveyed Americans over the age of 6. If you don't have BPA in your body, you're not living in the modern world. |, ]<ref name="timeBPA1">{{vcite news|url=http://www.time.com/time/specials/packages/article/0,28804,1976909_1976908_1976938-2,00.html|title=The Perils of Plastic – Environmental Toxins – TIME|last=Walsh|first=Bryan|date=Thursday, 1 Apr. 2010|work=TIME|accessdate=2 July 2010}}</ref>}} | |||
| Bisphenol A has been known to be leached from the plastic lining of canned foods<ref>{{vcite web |url=http://www.ewg.org/reports/bisphenola |title=A Survey of Bisphenol A in U.S. Canned Foods |work=Environmental Working Group |date=5 March 2007 |accessdate=2 February 2012}}</ref> and polycarbonate plastics, especially those cleaned with harsh detergents or that contain acidic or high-temperature liquids. BPA is an ingredient in the internal coating of metal food and beverage cans used to protect the food from direct contact with the can. A recent ] study found that the majority of canned ]s it tested had low, but measurable levels of bisphenol A.<ref>{{vcite web|url=http://www.hc-sc.gc.ca/fn-an/securit/packag-emball/bpa/bpa_survey-enquete-can-eng.php|title=Survey of Bisphenol A in Canned Drink Products|last=Health Canada|accessdate=13 March 2009}}</ref> Furthermore, a study conducted by the University of Texas School of Public Health in 2010, found BPA in 63 of 105 samples of fresh and canned foods, foods sold in plastic packaging, and in cat and dog foods in cans and plastic packaging. This included fresh turkey, canned green beans, and canned infant formula.<ref>{{cite pmid|21038926}}</ref> While most human exposure is through diet, exposure can also occur through air and through skin absorption.<ref name="Melzer et al.">{{vcite journal |author=Lang IA Galloway TS, Scarlett A, Henley WE, Depledge M, Wallace, Robert B, Melzer, D |title=Association of Urinary Bisphenol A Concentration With Medical Disorders and Laboratory Abnormalities in Adults | journal = Journal of the American Medical Association | volume = 300 | issue = 11 | pages = 1303–10 | year = 2008 | pmid = 18799442 | doi = 10.1001/jama.300.11.1303 | url = http://jama.ama-assn.org/cgi/content/full/300.11.1303 }}</ref> | |||
| BPA exhibits very low ] (i.e. from a single large dose) as indicated by its ] of 4 g/kg (mouse). Reports indicate that it is a minor skin irritant as well, although less so than ].<ref name="Fiege" /> | |||
| A 2011 study published in ''Environmental Health Perspectives'', "Food Packaging and Bisphenol A and Bis(2-Ethyhexyl) Phthalate Exposure: Findings from a Dietary Intervention," selected 20 participants based on their self-reported use of canned and packaged foods to study BPA. Participants ate their usual diets, followed by three days of consuming foods that were not canned or packaged. The study's findings include: 1) evidence of BPA in participants’ urine decreased by 50% to 70% during the period of eating fresh foods; and 2), participants’ reports of their food practices suggested that consumption of canned foods and beverages and restaurant meals were the most likely sources of exposure to BPA in their usual diets. The researchers note that, even beyond these 20 participants, BPA exposure is widespread, with detectable levels in urine samples in more than an estimated 90% of the U.S. population.<ref>{{vcite web|url = http://journalistsresource.org/studies/society/health/food-packaging-diet-bpa-chemical/ |title = Food Packaging and Bisphenol A and Bis(2-Ethyhexyl) Phthalate Exposure: Findings from a Dietary Intervention|publisher = Journalist's Resource.org }}</ref> | |||
| === Pharmacology === | |||
| Free BPA is found in high concentration in ] and ], which would be expected to be more available for exposure than BPA bound into resin or plastic.<ref name="ReferenceA"/><ref>{{vcite web|url=http://www.sciencenews.org/view/generic/id/48084/title/Concerned_about_BPA_Check_your_receipts|title=Concerned About BPA: Check Your Receipts|last=Raloff|first=Janet|date=7 October 2009|publisher=Society for Science and the Public|accessdate=7 October 2009}}</ref><ref>{{vcite journal | author = Gehring M, Tennhardt L, Vogel D, Weltin D, Bilitewski B | title = Bisphenol A Contamination of Wastepaper, Cellulose and Recycled Paper Products | journal = Transactions on Ecology and the Environment | year = 2004 | volume = 78 | pages = 294–300 | url = http://rcswww.urz.tu-dresden.de/~gehring/deutsch/dt/vortr/040929ge.pdf | laysummary = http://rcswww.urz.tu-dresden.de/~gehring/deutsch/dt/poster/030331g2.pdf }}</ref> Popular uses of thermal paper include receipts, event and cinema tickets, labels, and airline tickets. A Swiss study found that 11 of 13 thermal printing papers contained {{nowrap|8 – 17 g/kg}} {{nowrap|Bisphenol A}} (BPA). Upon dry finger contact with a thermal paper receipt, roughly {{nowrap|1 μg}} BPA ({{nowrap|0.2 – 6 μg}}) was transferred to the forefinger and the middle finger. For wet or greasy fingers approximately {{nowrap|10 times}} more was transferred. Extraction of BPA from the fingers was possible up to {{nowrap|2 hours}} after exposure.<ref name="pmid20623271">{{vcite journal | author = Biedermann S, Tschudin P, Grob K | title = Transfer of bisphenol A from thermal printer paper to the skin | journal = Anal Bioanal Chem | volume = 398 | issue = 1 | pages = 571–6 | year = 2010 | month = September | pmid = 20623271 | doi = 10.1007/s00216-010-3936-9 }}</ref> Further, it has been demonstrated that thermal receipts placed in contact with ] in a wallet for 24 hours cause a dramatic increase in the concentration of BPA in paper currency, making paper money a secondary source of exposure.<ref name="pmid21744851">{{vcite journal | author = Liao C, Kannan K | title = High levels of bisphenol A in paper currencies from several countries, and implications for dermal exposure | journal = Environ. Sci. Technol. | volume = 45 | issue = 16 | pages = 6761–8 | year = 2011 | month = August | pmid = 21744851 | doi = 10.1021/es200977t }}</ref> Also, other paper products, such as toilet paper, newspapers and napkins, become contaminated with BPA during the recycling process.<ref name="BPA in paper">{{vcite journal | author = Liao C, Kannan K | title = Widespread occurrence of bisphenol A in paper and paper products: implications for human exposure | journal = Environ. Sci. Technol. | volume = 45 | issue = 21 | pages = 9372–9 | year = 2011 | month = November | pmid = 21939283 | doi = 10.1021/es202507f }}</ref> Free BPA can readily be transferred to skin, and residues on hands can be ingested.<ref name="epa-action-plan"/> Bodily intake through dermal absorption (99% of which comes from handling receipts) has been shown for the general population to be 0.219 ng/kg bw/day (occupationally exposed persons absorb higher amounts at 16.3 ng/kg bw/day)<ref name="BPA in paper"/> whereas aggregate intake (food/beverage/environment) for adults is estimated at 0.36–0.43 μg/kg bw/day (estimated intake for occupationally exposed adults is 0.043–100 μg/kg bw/day).<ref>{{vcite web|url=http://ntp.niehs.nih.gov/ntp/ohat/bisphenol/BPAFinalEPVF112607.pdf |title=NTP-CERHR EXPERT PANEL REPORT on the REPRODUCTIVE and DEVELOPMENTAL TOXICITY of BISPHENOL A |date=26 November 2007 |publisher=] |accessdate=2 March 2011}}</ref> | |||
| ], the major female sex hormone in humans (green) and BPA (purple). This displays the ] which allows BPA to mimic the effects of estradiol and other estrogens.]] | |||
| BPA has been found to interact with a diverse range of ]s, in both humans and animals.<ref name="receptors" /> It binds to both of the ] ]s (ERs), ] and ]. BPA is a ] (SERM), or ] of the ER, so it can serve as both an ] ] and ]. However, it is 1000- to 2000-fold less potent than ], the major female sex hormone in humans. At high concentrations, BPA also binds to and acts as an antagonist of the ] (AR). In addition to receptor binding, the compound has been found to affect ] ], including affecting ] and ] expression and interfering with ]-ligand binding.<ref>{{cite journal |last1=Akingbemi |first1=Benson T. |last2=Sottas |first2=Chantal M. |last3=Koulova |first3=Anna I. |last4=Klinefelter |first4=Gary R. |last5=Hardy |first5=Matthew P. |title=Inhibition of Testicular Steroidogenesis by the Xenoestrogen Bisphenol A Is Associated with Reduced Pituitary Luteinizing Hormone Secretion and Decreased Steroidogenic Enzyme Gene Expression in Rat Leydig Cells |journal=Endocrinology |date=1 February 2004 |volume=145 |issue=2 |pages=592–603 |doi=10.1210/en.2003-1174|pmid=14605012 |doi-access=free}}</ref> | |||
| Studies conducted by the ] found bisphenol A in the urine of 95% of adults sampled in 1988–1994<ref name="pmid15811827">{{vcite journal |author=Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Ekong J, Needham LL |title=Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population |journal=] |volume=113 |issue=4 |pages=391–5 |year=2005 |pmid=15811827 |doi= 10.1289/ehp.7534|url=http://ehpnet1.niehs.nih.gov/members/2004/7534/7534.html |pmc=1278476}}</ref> and in 93% of children and adults tested in 2003–04.<ref name="pmid18197297">{{vcite journal |author=Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL |title=Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004 |journal=] |volume=116 |issue=1 |pages=39–44 |year=2008 |pmid=18197297 |doi=10.1289/ehp.10753 |pmc=2199288}}</ref> While the EPA considers exposures up to 50 µg/kg/day to be safe, the most sensitive animal studies show effects at much lower doses.<ref name="globemittelstaedt"/><ref name="CASRN 80-05-7">{{vcite web |url=http://www.epa.gov/iris/subst/0356.htm |title=Integrated Risk Information System: Bisphenol A. (CASRN 80-05-7): Oral RfD Assessment: Bisphenol A |work=] |year=1988 |accessdate=2 February 2012}}</ref> | |||
| Bisphenol A's interacts with the ] (ERR-γ). This ] (endogenous ligand unknown) behaves as a constitutive activator of transcription. BPA seems to bind strongly to ERR-γ (] = 5.5 nM), but only weakly to the ER.<ref name="matsushima">{{cite journal | vauthors = Matsushima A, Kakuta Y, Teramoto T, Koshiba T, Liu X, Okada H, Tokunaga T, Kawabata S, Kimura M, Shimohigashi Y | title = Structural evidence for endocrine disruptor bisphenol A binding to human nuclear receptor ERR gamma | journal = Journal of Biochemistry | volume = 142 | issue = 4 | pages = 517–524 | date = October 2007 | pmid = 17761695 | doi = 10.1093/jb/mvm158 }}</ref> BPA binding to ERR-γ preserves its basal constitutive activity.<ref name="matsushima" /> It can also protect it from deactivation from the SERM ] (afimoxifene).<ref name="matsushima" /> This may be the mechanism by which BPA acts as a ].<ref name="matsushima" /> Different expression of ERR-γ in different parts of the body may account for variations in bisphenol A effects. BPA has also been found to act as an ] of the ] (GPR30).<ref name="ProssnitzBarton2014">{{cite journal | vauthors = Prossnitz ER, Barton M | title = Estrogen biology: new insights into GPER function and clinical opportunities | journal = Molecular and Cellular Endocrinology | volume = 389 | issue = 1–2 | pages = 71–83 | date = May 2014 | pmid = 24530924 | pmc = 4040308 | doi = 10.1016/j.mce.2014.02.002}}</ref> | |||
| In 2009, a study found that drinking from polycarbonate bottles increased urinary bisphenol A levels by two thirds, from 1.2 μg/g creatinine to 2 μg/g creatinine.<ref>{{vcite journal |author=Carwile JL, Luu HT, Bassett LS, Driscoll DA, Yuan C, Chang JY, Ye X, Calafat AM, Michels KB |title=Use of Polycarbonate Bottles and Urinary Bisphenol A Concentrations |journal=Environ. Health Perspect. |volume= 117|issue= 9|pages= 1368–72|year=2009 |pmc= 2737011|doi=10.1289/ehp.0900604 |url=http://www.ehponline.org/members/2009/0900604/0900604.pdf |pmid=19750099}}</ref> Consumer groups recommend that people wishing to lower their exposure to bisphenol A avoid ] and ] plastic containers (which shares ] 7 with many other plastics) unless the packaging indicates the plastic is bisphenol A-free.<ref>{{vcite web |url=http://www.loe.org/shows/segments.htm?programID=08-P13-00038&segmentID=4 |title=War of the Sciences |work=Living on Earth |author=Ashley Ahearn |date=19 September 2008 |accessdate=2 February 2012}}</ref> To avoid the possibility of BPA leaching into food or drink, the National Toxicology Panel recommends avoiding microwaving food in plastic containers, putting plastics in the dishwasher, or using harsh detergents.<ref>{{vcite web|url=http://www.npr.org/templates/story/story.php?storyId=94680753 |title=FDA Weighs Safety Of Bisphenol A |publisher=Npr.org |accessdate=23 October 2011}}</ref> | |||
| == Environmental safety == | |||
| In the U.S., consumption of soda, school lunches, and meals prepared outside the home was statistically significantly associated with higher urinary BPA.<ref>{{vcite doi|10.1038/jes.2010.9}}</ref> This cannot be correlated with polycarbonate plastic, which is far too expensive to be used in packaging of such products, so it remains to be seen where this BPA is coming from. | |||
| ===Distribution and degradation=== | |||
| BPA has been detectable in the natural environment since the 1990s and is now widely distributed.<ref name="auto1">{{cite journal |last1=Staples |first1=Charles A. |last2=Dome |first2=Philip B. |last3=Klecka |first3=Gary M. |last4=Oblock |first4=Sondra T. |last5=Harris |first5=Lynne R. |title=A review of the environmental fate, effects, and exposures of bisphenol A |journal=Chemosphere |date=April 1998 |volume=36 |issue=10 |pages=2149–2173 |doi=10.1016/S0045-6535(97)10133-3|pmid=9566294 |bibcode=1998Chmsp..36.2149S }}</ref> It is primarily a river pollutant,<ref name=":1">{{cite journal | vauthors = Corrales J, Kristofco LA, Steele WB, Yates BS, Breed CS, Williams ES, Brooks BW | title = Global Assessment of Bisphenol A in the Environment: Review and Analysis of Its Occurrence and Bioaccumulation | journal = Dose-Response | volume = 13 | issue = 3 | pages = 1559325815598308 | date = 2015-07-29 | pmid = 26674671 | pmc = 4674187 | doi = 10.1177/1559325815598308 }}</ref> but has also been observed in the marine environment,<ref name=Turkey>{{cite journal |last1=Ozhan |first1=Koray |last2=Kocaman |first2=Emel |title=Temporal and Spatial Distributions of Bisphenol A in Marine and Freshwaters in Turkey |journal=Archives of Environmental Contamination and Toxicology |date=February 2019 |volume=76 |issue=2 |pages=246–254 |doi=10.1007/s00244-018-00594-6|pmid=30610254 |bibcode=2019ArECT..76..246O |s2cid=58536418 }}</ref> in soils,<ref name=multi /> and lower levels can also be detected in air.<ref>{{cite journal |last1=Vasiljevic |first1=Tijana |last2=Harner |first2=Tom |title=Bisphenol A and its analogues in outdoor and indoor air: Properties, sources and global levels |journal=Science of the Total Environment |date=October 2021 |volume=789 |pages=148013 |doi=10.1016/j.scitotenv.2021.148013|pmid=34323825 |bibcode= 2021ScTEn.78948013V|doi-access=free }}</ref> The solubility of BPA in water is low (~300 g per ton of water)<ref name=water-solubility /> but this is still sufficient to make it a significant means of distribution into the environment.<ref name=multi>{{cite journal |last1=Cousins |first1=I.T. |last2=Staples |first2=C.A. |last3=Kleĉka |first3=G.M. |last4=Mackay |first4=D. |title=A Multimedia Assessment of the Environmental Fate of Bisphenol A |journal=Human and Ecological Risk Assessment|date=July 2002 |volume=8 |issue=5 |pages=1107–1135 |doi=10.1080/1080-700291905846|bibcode=2002HERA....8.1107C |s2cid=43509780 }}</ref> Many of the largest sources of BPA pollution are water-based, particularly wastewater from industrial facilities using BPA. | |||
| ] can be a major source of release when this includes ],<ref name=EU2008 /><ref>{{cite journal |last1=Fürhacker |first1=M |last2=Scharf |first2=S |last3=Weber |first3=H |title=Bisphenol A: emissions from point sources |journal=Chemosphere |date=September 2000 |volume=41 |issue=5 |pages=751–756 |doi=10.1016/S0045-6535(99)00466-X|pmid=10834378 |bibcode=2000Chmsp..41..751F }}</ref> ] from PVC items may also be a significant source,<ref name=":1"/> as can landfill ].<ref name="CEC">{{cite journal |last1=Qi |first1=Chengdu |last2=Huang |first2=Jun |last3=Wang |first3=Bin |last4=Deng |first4=Shubo |last5=Wang |first5=Yujue |last6=Yu |first6=Gang |title=Contaminants of emerging concern in landfill leachate in China: A review |journal=Emerging Contaminants |date=2018 |volume=4 |issue=1 |pages=1–10 |doi=10.1016/j.emcon.2018.06.001|doi-access=free}}</ref> | |||
| In all cases, ] can be highly effective at removing BPA, giving reductions of 91–98%.<ref name="pmid15765931">{{cite journal | vauthors = Drewes JE, Hemming J, Ladenburger SJ, Schauer J, Sonzogni W | title = An assessment of endocrine disrupting activity changes during wastewater treatment through the use of bioassays and chemical measurements | journal = Water Environment Research | volume = 77 | issue = 1 | pages = 12–23 | date = 2005 | pmid = 15765931 | doi = 10.2175/106143005x41573 | bibcode = 2005WaEnR..77...12D | s2cid = 12283834 }}</ref> Regardless, the remaining 2–9% of BPA will continue through to the environment, with low levels of BPA commonly observed in surface water and sediment in the U.S. and Europe.<ref>{{cite journal | vauthors = Klecka GM, Staples CA, Clark KE, Van der Hoeven N, Thomas DE, Hentges SG | title = Exposure analysis of bisphenol A in surface water systems in North America and Europe | journal = Environmental Science & Technology | volume = 43 | issue = 16 | pages = 6145–50 | date = August 2009 | pmid = 19746705 | doi = 10.1021/es900598e | bibcode = 2009EnST...43.6145K}}</ref> | |||
| BPA is also used to form epoxy resin coating of water pipes. In older buildings, such resin coatings are used to avoid replacement of deteriorating hot and cold water pipes.<ref>{{vcite web|url=http://www.aceduraflo.com/fixmypipes.html|title=Pipeline relining|accessdate=18 October 2010}}</ref> | |||
| Once in the environment BPA is aerobically biodegraded by a wide a variety of organisms.<ref name="auto1"/><ref>{{cite journal |last1=Kang |first1=J |last2=Katayama |first2=Y |last3=Kondo |first3=F |title=Biodegradation or metabolism of bisphenol A: From microorganisms to mammals |journal=Toxicology |date=16 January 2006 |volume=217 |issue=2–3 |pages=81–90 |doi=10.1016/j.tox.2005.10.001|pmid=16288945 |bibcode=2006Toxgy.217...81K }}</ref><ref>{{cite journal |last1=Zhang |first1=Chi |last2=Li |first2=Yi |last3=Wang |first3=Chao |last4=Niu |first4=Lihua |last5=Cai |first5=Wei |title=Occurrence of endocrine disrupting compounds in aqueous environment and their bacterial degradation: A review |journal=Critical Reviews in Environmental Science and Technology |date=2 January 2016 |volume=46 |issue=1 |pages=1–59 |doi=10.1080/10643389.2015.1061881|bibcode=2016CREST..46....1Z |s2cid=94353391}}</ref> Its ] in water has been estimated at between 4.5 and 15 days, degradation in the air is faster than this, while soil samples degrade more slowly.<ref name=multi /> BPA in sediment degrades most slowly of all, particularly where this is anaerobic. ] degradation has been reported, but is generally slower than biodegradation. Pathways include ], or reactions with minerals such as ] which may be present in soils and sediments.<ref>{{cite journal |last1=Im |first1=Jeongdae |last2=Löffler |first2=Frank E. |title=Fate of Bisphenol A in Terrestrial and Aquatic Environments |journal=Environmental Science & Technology |date=16 August 2016 |volume=50 |issue=16 |pages=8403–8416 |doi=10.1021/acs.est.6b00877|pmid=27401879 |bibcode=2016EnST...50.8403I |osti=1470902}}</ref> | |||
| ===Fetal and early-childhood exposures=== | |||
| ===Environmental effects=== | |||
| Children may be more susceptible to BPA exposure than adults. A recent study found higher urinary concentrations in young children than in adults under typical exposure scenarios.<ref>{{cite pmid|19440506}}</ref> In adults, BPA is eliminated from the body through a detoxification process in the liver. In infants and children, this pathway is not fully developed so they have a decreased ability to clear BPA from their systems. It is also estimated that from food consumption, infants and young children have higher BPA-exposure than adults.<ref name="pmid19931376">{{vcite journal | author = Beronius A, Rudén C, Håkansson H, Hanberg A | title = Risk to all or none? A comparative analysis of controversies in the health risk assessment of Bisphenol A | journal = Reprod. Toxicol. | volume = 29 | issue = 2 | pages = 132–46 | year = 2010 | month = April | pmid = 19931376 | doi = 10.1016/j.reprotox.2009.11.007 }}</ref> | |||
| BPA is an environmental ].<ref name="CEC" /> Despite its short half-life and non-] character, the continuous release of BPA into the environment causes continuous exposure to both plant<ref name=meta2>{{cite journal |last1=Xiao |first1=Changyun |last2=Wang |first2=Lihong |last3=Zhou |first3=Qing |last4=Huang |first4=Xiaohua |title=Hazards of bisphenol A (BPA) exposure: A systematic review of plant toxicology studies |journal=Journal of Hazardous Materials |date=February 2020 |volume=384 |pages=121488 |doi=10.1016/j.jhazmat.2019.121488|pmid=31699483 |bibcode=2020JHzM..38421488X |s2cid=207939269}}</ref> and animal life. Although many studies have been performed, these often focus on a limited range of ]s and can use BPA concentrations well beyond environmental levels.<ref name=meta3>{{cite journal |last1=Rubin |first1=Alexander M. |last2=Seebacher |first2=Frank |title=Bisphenols impact hormone levels in animals: A meta-analysis |journal=Science of the Total Environment |date=July 2022 |volume=828 |pages=154533 |doi=10.1016/j.scitotenv.2022.154533|pmid=35288143 |bibcode=2022ScTEn.82854533R |s2cid=247423338 }}</ref> As such, the precise effects of BPA on the growth, reproduction, and development of aquatic organism are not fully understood.<ref name=meta3 /> Regardless, the existing data shows the effects of BPA on wildlife to be generally negative.<ref name=meta1>{{cite journal |last1=Wu |first1=Nicholas C. |last2=Seebacher |first2=Frank |title=Effect of the plastic pollutant bisphenol A on the biology of aquatic organisms: A meta-analysis |journal=Global Change Biology |date=July 2020 |volume=26 |issue=7 |pages=3821–3833 |doi=10.1111/gcb.15127|pmid=32436328 |bibcode=2020GCBio..26.3821W |s2cid=218765595 }}</ref><ref name=RSC>{{cite journal |vauthors=Oehlmann J, Schulte-Oehlmann U, Kloas W, Jagnytsch O, Lutz I, Kusk KO, Wollenberger L, Santos EM, Paull GC, Van Look KJ, Tyler CR | title = A critical analysis of the biological impacts of plasticizers on wildlife | journal = Philosophical Transactions of the Royal Society B: Biological Sciences | volume = 364 | issue = 1526 | pages = 2047–62 | year = 2009 | pmid = 19528055 | doi = 10.1098/rstb.2008.0242 | pmc=2873012}}</ref> BPA appears able to affect development and reproduction in a wide range of wildlife,<ref name=RSC /><ref>{{Cite journal |last1=Wu |first1=Nicholas C. |last2=Rubin |first2=Alexander M. |last3=Seebacher |first3=Frank |date=2022-01-26 |title=Endocrine disruption from plastic pollution and warming interact to increase the energetic cost of growth in a fish |journal=Proceedings of the Royal Society B: Biological Sciences |language=en |volume=289 |issue=1967 |doi=10.1098/rspb.2021.2077 |issn=0962-8452 |pmc=8790379 |pmid=35078359}}</ref> with certain species being particularly sensitive, such as ]s and ]s.<ref name=meta1 /> | |||
| Studies have found that fetuses and young children exposed to BPA are at risk for secondary sexual developmental changes, brain and behavior changes and immune disorders.<ref name="Erler_Novak_2010"/> | |||
| Infants fed with liquid formula are among the most exposed, and those fed formula from polycarbonate bottles can consume up to 13 micrograms of bisphenol A per kg of body weight per day (μg/kg/day; see table below).<ref>{{vcite web | title = European Food Safety Authority Opinion | url =http://www.efsa.europa.eu/en/science/afc/afc_opinions/bisphenol_a.html | format = Abstract | accessdate =28 February 2007}}</ref> In the US and Canada, BPA has been found in infant liquid formula in concentrations varying from 0.48 to 11 ng/g.<ref name="infantformulaUS">{{cite pmid|20102208}}</ref><ref>{{cite pmid|18702469}}</ref> BPA has been rarely found in infant powder formula (only 1 of 14).<ref name="infantformulaUS"/> While breast milk is the optimal source of nutrition for infants, it is not always an option. The ] (HHS) states that "the benefit of a stable source of good nutrition from infant formula and food outweighs the potential risk of BPA exposure.".<ref>{{vcite web | title = Bisphenol A (BPA) Information for Parents | url=http://www.hhs.gov/safety/bpa/|publisher=]|accessdate=25 March 2011}}</ref> | |||
| A 2010 study of people in Austria, Switzerland, and Germany has suggested polycarbonate (PC) baby bottles as the most prominent role of exposure for infants, and canned food for adults and teenagers.<ref>{{vcite doi|10.1111/j.1539-6924.2009.01345.x}}</ref> In the United States, the growing concern over BPA exposure in infants in recent years has led the manufacturers of plastic baby bottles to stop using BPA in their bottles. However, babies may still be exposed if they are fed with old or hand-me-down bottles bought before the companies stopped using BPA. | |||
| One often overlooked source of exposure occurs when a pregnant woman is exposed, thereby exposing the fetus. Animal studies have shown that BPA can be found in both the placenta and the amniotic fluid of pregnant mice.<ref>{{cite pmid|12611660}}</ref> A small US study in 2009, funded by the ], detected an average of 2.8 ng/mL BPA in the blood of 9 out of the 10 ]s tested.<ref>{{vcite web|url=http://www.ewg.org/files/2009-Minority-Cord-Blood-Report.pdf|title=Cord Blood Contaminants in Minority Newborns|year=2009|publisher=]|accessdate=2 December 2009}}</ref> A study of 244 mothers indicated that exposure to BPA before birth could affect the behavior of girls' at age 3. Girls whose mother's urine contained high levels of BPA during pregnancy scored worse on tests of anxiety and hyperactivity. Although these girls still scored within a normal range, for every 10-fold increase in the BPA of the mother, the girls scored at least six points lower on the tests. Boys did not seem to be affected by their mother's BPA levels during pregnancy.<ref>{{vcite web|url=http://www.statesman.com/news/nation/study-links-bpa-with-girls-behavior-problems-1930237.html?cxtype=rss_news|title=Study links BPA with girls' behavior problems|year=2011|publisher=]|accessdate=24 October 2011}}</ref> After the baby is born, maternal exposure can continue to affect the infant through transfer of BPA to the infant via breast milk.<ref>{{cite pmid|15386523}}</ref><ref>{{cite pmid|16377264}}</ref> Because of these exposures that can occur both during and after pregnancy, mothers wishing to limit their child’s exposure to BPA should attempt to limit their own exposures during that time period. | |||
| While the majority of exposures have been shown to come through the diet, accidental ingestion can also be considered a source of exposure. One study conducted in Japan tested plastic baby books to look for possible leaching into saliva when babies chew on them.<ref>{{cite pmid|20508389}}</ref> While the results of this study have yet to be replicated, it gives reason to question whether exposure can also occur in infants through ingestion by chewing on certain books or toys. | |||
| {| class="wikitable" | |||
| |- | |||
| ! Population | |||
| ! Estimated daily bisphenol A intake, μg/kg/day.<br/>Table adapted from the National Toxicology Program Expert Panel Report. | |||
| |- | |||
| | Infant (0–6 months)<br/>formula-fed | |||
| | <center>1–24</center> | |||
| |- | |||
| | Infant (0–6 months)<br/>breast-fed | |||
| | <center>0.2–1 </center> | |||
| |- | |||
| | Infant (6–12 months) | |||
| | <center>1.65–13</center> | |||
| |- | |||
| | Child (1.5–6 years) | |||
| | <center>0.043–14.7</center> | |||
| |- | |||
| | Adult | |||
| | <center>0.008–1.5</center> | |||
| |} | |||
| ==Pharmacokinetics== | |||
| There is no agreement between scientists of a ] ] (PBPK) BPA model for humans. The effects of BPA on an organism depend on how much free BPA is available and for how long cells are exposed to it. ] in the liver, by conjugation with glucuronic acid to form the metabolite BPA-glucuronide (BPAG),<ref name="epa-action-plan"/> reduces the amount of free BPA, however BPAG can be deconjugated by ], an enzyme present in high concentration in placenta and other tissues.<ref name="Ginsberg"/><ref name="placenta">{{cite pmid|20382578}}</ref> Free BPA can also be inactivated by sulfation, a process that can also be reverted by ].<ref name="Ginsberg">{{vcite journal|last=Gary Ginsberg and Deborah C. Rice|date=November 2009|title=Does Rapid Metabolism Ensure Negligible Risk from Bisphenol A?|journal=Environmental Health Perspectives|volume=117|issue=11|pages=1639–1643|url=http://www.ehponline.org/members/2009/0901010/0901010.pdf|accessdate=16 November 2009|pmid=20049111|first1=G|last2=Rice|first2=DC|doi=10.1289/ehp.0901010|pmc=2801165}}</ref> | |||
| A 2009 research study found that some drugs, like ], ], ] and ] can, ''in vitro'', significantly inhibit BPA glucuronidation.<ref>{{cite pmid|19916736}}</ref> A 2010 study on rats embryos has found that ] may enhance developmental toxicity of BPA,<ref>{{cite pmid|20299547}}</ref> and another 2010 vitro study has shown that placenta ] may efflux BPA from placenta.<ref>{{cite pmid|20214975}}</ref> | |||
| A 2010 review of 80+ biomonitoring studies concluded that the general population is internally exposed to significant amounts of unconjugated BPA (in the ng/ml blood range).<ref>{{vcite doi|10.1289/ehp.0901716}}</ref> Using ] on 20 samples, BPA was detected in 100% of urine samples with a median of 1.25 ng/ml, and 10% of blood samples (] 0.5 ng/ml).<ref>{{vcite journal |author=Geens T, Neels H, Covaci A |title=Sensitive and selective method for the determination of bisphenol-A and triclosan in serum and urine as pentafluorobenzoate-derivatives using GC-ECNI/MS |journal=J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. |volume=877 |issue=31 |pages=4042–6 |year=2009 |month=December |pmid=19884050 |doi=10.1016/j.jchromb.2009.10.017 |url=}}</ref> In a 2011 study, researchers found that after a 5-day consumption of 1 serving of canned vegetarian soup, subjects exhibited a 1200% increase in urinary BPA concentrations compared to controls, suggesting that such a diet induces a peak of BPA elevation of unknown duration and of unknown biological safety.<ref>{{vcite journal| url=http://jama.ama-assn.org/content/306/20/2218.2.extract| title=Canned Soup Consumption and Urinary Bisphenol A: A Randomized Crossover Trial| author=Jenny L. Carwile, Xiaoyun Ye, Xiaoliu Zhou, Antonia M. Calafat, Karin B. Michels |journal=] |year=2011|volume=306|issue=20|pages=2218–2220 | doi= 10.1001/jama.2011.1721| pmid=22110104 }}</ref> | |||
| The best test methods for studying BPA effects are currently under discussion with scientists sharing different opinions.<ref>{{vcite journal |last= Borrell|first= Brendan |date= 21 April|year=2010|title= Toxicology: The big test for bisphenol A |journal=] |volume= 464|series= |issue= 7292|pages= 1122–1124 |pmid= 20414285|doi= 10.1038/4641122a |url=http://www.nature.com/news/2010/100421/full/4641122a.html|accessdate=21 April 2010 |quote= }}</ref> | |||
| ==Environmental risk== | |||
| In 2010 the EPA reported that over one million pounds of BPA are released into the environment annually.<ref name="Erler_Novak_2010"/> BPA can enter the environment either directly or through degradation of products, such as ocean-borne plastic trash.<ref>{{vcite web |title=Plastic Breaks Down in Ocean, After All – And Fast |first=Carolyn |last=Barry |work=] News |url=http://news.nationalgeographic.com/news/2009/08/090820-plastic-decomposes-oceans-seas.html |date=20 August 2009 |accessdate=1 February 2012}}</ref> Leaching of BPA from plastic and metal waste in landfills is one potential source of environmental contamination. A 2009 meta-analysis of BPA in the surface water system reported that BPA is found in surface water and sediment in the United States and Europe.<ref>Kle’cka, G., Staples, C., Clark, K., Anderhoeven, N., Thomas, D. and Hentges, S. Exposure Analysis of Bisphenol A in Surface Water Systems in North America and Europe. Environ. Sci. Technol. 2009, 43, 6145–6150.</ref> | |||
| In general, studies have shown that BPA can affect growth, reproduction and development in aquatic organisms. Among freshwater organisms, fish appear to be the most sensitive species. Evidence of endocrine-related effects in fish, aquatic invertebrates, amphibians and reptiles has been reported at environmentally relevant exposure levels lower than those required for acute toxicity. There is a widespread variation in reported values for endocrine-related effects, but many fall in the range of 1μg/L to 1 mg/L.<ref name="epa-action-plan"/> | |||
| As an environmental contaminant, BPA interferes with ] at the roots of ] plants associated with the ] ] '']''. Despite a ] in the soil of only 1–10 days, its ubiquity makes it an important ].<ref name="fox">{{vcite journal |author=Fox, J.E., J. Gulledge, E. Engelhaupt, M.E. Burrow & J.A. McLachlan |title=Pesticides reduce symbiotic efficiency of nitrogen-fixing rhizobia and host plants |journal=] |year=2007 |volume=104 |pages=10282–7 |doi=10.1073/pnas.0611710104 |pmid=17548832 |issue=24 |pmc=1885820|bibcode = 2007PNAS..10410282F }}</ref> According to ], "initial assessment shows that at low levels, bisphenol A can harm fish and organisms over time. Studies also indicate that it can currently be found in municipal wastewater."<ref>{{vcite web |url=http://www.chemicalsubstanceschimiques.gc.ca/fact-fait/bisphenol-a-eng.php |title=Bisphenol A Fact Sheet |publisher=Government of Canada |archiveurl=http://web.archive.org/web/20110423211655 http://www.chemicalsubstanceschimiques.gc.ca/fact-fait/bisphenol-a-eng.php |archivedate=23 April 2011 |accessdate=1 February 2012}}</ref> However, a study conducted in the United States in 2005 found that 91-98% of BPA may be removed from water during treatment at municipal water treatment plants.<ref>Drewes, J. E.; Hemming, J.; Ladenburger, S. J.; Schauer, J.; Sonzogni, W. An assessment of endocrine disrupting activity changes during wastewater treatment through the use of bioassays and chemical measurements. Water Environ. Res. 2005, 77, 12–23.</ref> | |||
| A 2009 review of the biological impacts of plasticizers on wildlife published by the ] with a focus on annelids (both aquatic and terrestrial), ], ], insects, fish and ]s concluded that BPA has been shown to affect reproduction in all studied animal groups, to impair development in crustaceans and amphibians and to induce genetic aberrations.<ref>{{vcite doi|10.1098/rstb.2008.0242}}</ref> | |||
| A large 2010 study of two rivers in Canada found that areas contaminated with hormone-like chemicals including bisphenol A showed females made up 85 per cent of the population of a certain fish, while females made up only 55 per cent in uncontaminated areas.<ref>{{vcite web |url=http://www.sciencedaily.com/releases/2010/07/100729122332.htm |title=Chemicals Are Likely Cause of Feminization of Fish Present in Two Rivers in Alberta, Canada, Researchers Find |publisher=ScienceDaily |date=29 July 2010 |accessdate=1 February 2012}}</ref> | |||
| ==Government and industry response== | |||
| ===World Health Organization=== | |||
| Arguing uncertainty of possible adverse health effects of low dose BPA exposure, especially on the nervous system and on behaviour, and also the differences of exposure of very young children, the WHO announced in November 2009 that it would organize an expert consultation in 2010 to assess BPA safety.<ref name="infosan"/> | |||
| The WHO expert panel recommended no new regulations limiting or banning the use of Bisphenol-A, stating that "initiation of public health measures would be premature."<ref>Brown, Eryn. , '']'', 11 November 2010. Retrieved on 7 February 2011.</ref> | |||
| ===Australia and New Zealand=== | |||
| The Australia and New Zealand Food Safety Authority (]) does not see any health risk with bisphenol A ]s if the manufacturer's instructions are followed. Levels of exposure are very low and do not pose a significant health risk. It added that "the move by overseas manufacturers to stop using BPA in baby bottles is a voluntary action and not the result of a specific action by regulators."<ref>{{vcite web |url=http://www.foodstandards.gov.au/newsroom/factsheets/factsheets2009/bisphenolabpaandfood4218.cfm |title=Bisphenol A (BPA) and food packaging |publisher=Food Standards Australia New Zealand |date=May 2009 |archiveurl=http://web.archive.org/web/20100109102336/http://www.foodstandards.gov.au/educationalmaterial/factsheets/factsheets2009/bisphenolabpaandfood4218.cfm |archivedate=9 January 2010 |accessdate=1 February 2012}}{{Dead link|date=May 2012}}</ref> It suggests the use of glass baby bottles if parents have any concerns.<ref>{{vcite web |url=http://www.nzfsa.govt.nz/consumers/chemicals-nutrients-additives-and-toxins/bisphenol-a.htm |title=Babies’ bottles and bisphenol A |date=May 2008 |publisher=] |archiveurl=http://web.archive.org/web/20100726062453/http://www.nzfsa.govt.nz/consumers/chemicals-nutrients-additives-and-toxins/bisphenol-a.htm |archivedate=26 July 2010 |accessdate=1 February 2012}}</ref> | |||
| ===Canada=== | |||
| In April 2008, ] concluded that, while adverse health effects were not expected, the margin of safety was too small for formula-fed infants<ref name="C&EN">{{vcite news |last= Morrissey |first=Susan R. |title=Banning Bisphenol A In Baby Bottles: Canada moves toward restricting the chemical; Congress proposes similar legislation |newspaper=Chemical and Engineering News |date=23 April 2008 |url=http://pubs.acs.org/cen/news/86/i17/8617news4.html}}</ref> and proposed classifying the chemical as "'toxic' to human health and the environment."<ref>{{vcite web |url=http://www.chemicalsubstanceschimiques.gc.ca/challenge-defi/batch-lot-2/bisphenol-a/index-eng.php |title=Bisphenol A |work=Health Canada |accessdate=2 February 2012}}</ref> | |||
| After the release of that assessment, Canadian Health Minister ] announced Canada's intent to ban the import, sale, and advertisement of polycarbonate baby bottles containing bisphenol A due to safety concerns, and investigate ways to reduce BPA contamination of baby formula packaged in metal cans. While the agency concluded that human exposures were less than levels believed unsafe, the margin of safety was not high enough for formula-fed infants.<ref name="HealthCanada"/><ref>The government will evaluate whether the ban will become law in October 2008. {{vcite news |title=Government of Canada Takes Action on Another Chemical of Concern: Bisphenol A |url=http://www.chemicalsubstanceschimiques.gc.ca/challenge-defi/bisphenol-a_e.html |archiveurl=http://web.archive.org/web/20080702234206/http://www.chemicalsubstanceschimiques.gc.ca/challenge-defi/bisphenol-a_e.html |archivedate=2 July 2008 |accessdate=2 February 2012}}</ref> Around the same time, ] announced that it was immediately ceasing sales in all its Canadian stores of food containers, water and baby bottles, sippy cups, and pacifiers containing bisphenol A, and that it would phase out baby bottles made with it in U.S. stores by early 2009.<ref>{{vcite web |url=http://www.marketwatch.com/story/wal-mart-to-pull-baby-bottles-made-with-chemical-bpa-washington-post?dist=msr_4 |title=Wal-Mart to pull baby bottles made with chemical BPA: Washington Post |work=Market Watch |date=18 April 2008 |accessdate=2 February 2012}}</ref> ] also announced it will stop using the chemical in its products,<ref>{{vcite web |url=http://www.nytimes.com/2008/04/18/business/18plastic.html?ref=business |title=Bottle Maker to Stop Using Plastic Linked to Health Concerns |work=New York Times |author=Ian Austen |date=18 April 2008 |accessdate=2 February 2012}}</ref> and ] said it too will cease selling baby bottles made from it.<ref>{{vcite news |title=Toys 'R' Us to phase out bisphenol A baby bottles |publisher=CBC News |date=22 April 2008 |url=http://www.cbc.ca/consumer/story/2008/04/22/bottles-bpa.html |accessdate=22 April 2008}}</ref> Subsequent news reports showed many retailers removing polycarbonate drinking products from their shelves.<ref>, Politics: Bisphenol A water-bottle removal expanding among Canadian retailers.{{dead link|date=October 2011}}</ref> | |||
| <!--outdated | |||
| In 2006, Canadian regulators selected bisphenol A as one of 200 substances deserving of thorough safety assessments, because preliminary studies found it "inherently toxic." They had not previously studied the chemical in depth, having accepted it under ]s when stricter regulations were passed in the 1980s.<ref name="globemittelstaedt"/>--> | |||
| The federal government proposed declaring {{nowrap|Bisphenol A}} a hazardous substance in October 2008 and has since placed it on its list of toxic substances. Health officials wrote in '']'' that "It is concluded that bisphenol A be considered as a substance that may be entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health."<ref>{{vcite news | title = Toxic status possible for bisphenol A in Canada | publisher=CBC News | date = 17 October 2008 | url = http://www.cbc.ca/health/story/2008/10/17/bpa-toxic.html | accessdate =17 October 2008}}</ref> The federal ministries of health and the ] announced they would seek to restrict imports, sales and advertising of ] baby bottles containing BPA.<ref>{{vcite news |title=Canada moves to ban bisphenol A in baby bottles |publisher=CBC News |date=18 October 2008 |url=http://www.cbc.ca/canada/story/2008/10/18/bpa-regulations.html |accessdate=20 October 2008}}</ref> | |||
| In its statement released on 18 October 2008, Health Canada noted that "bisphenol A exposure to newborns and infants is below levels that cause effects" and that the "general public need not be concerned".<ref>{{vcite web |url=http://www.hc-sc.gc.ca/ahc-asc/media/nr-cp/_2008/2008_167-eng.php |title=Government of Canada Protects Families With Bisphenol A Regulations |publisher=Health Canada |date=17 October 2008 |archiveurl=http://web.archive.org/web/20100226005022/http://www.hc-sc.gc.ca/ahc-asc/media/nr-cp/_2008/2008_167-eng.php |archivedate=26 February 2010 |accessdate=1 February 2012}}</ref> | |||
| ] listed bisphenol A as a "toxic substance" in September 2010.<ref name="gazette.gc.ca"/> | |||
| The new plastic Canadian currency has been measured as having the highest levels of BPA from several international currencies measured.<ref>{{vcite web |url=http://www.prosolia.com/applications_forensics.php |title=BPA in currencies |publisher=Prosolia}}</ref> | |||
| ===Europe=== | |||
| ====European Union==== | |||
| The updated 2008 European Union Risk Assessment Report on {{nowrap|bisphenol A}}, published in June 2008, by the European Commission and ] (EFSA), concluded that {{nowrap|bisphenol A}}-based products, such as ] plastic and ] resins, are safe for consumers and the environment when used as intended.<ref>]: United Kingdom. (2008). FINAL APPROVED VERSION AWAITING FOR PUBLICATION. .</ref> By October 2008, after the ] was published, the EFSA issued a statement concluding that the study provided no grounds to revise the current Tolerable Daily Intake (TDI) level for BPA of {{nowrap|0.05 mg/kg}} bodyweight.<ref>{{dead link|date=October 2011}}</ref> | |||
| A 2009 scientific study criticized the European risk assessment processes of endocrine disruptors, including BPA.<ref>{{cite pmid|19500631}}</ref> | |||
| On 22 December 2009, the EU Environment ministers released a statement expressing concerns over recent studies showing adverse effects of exposure to ]s.<ref>{{vcite web|url=http://www.consilium.europa.eu/uedocs/NewsWord/en/envir/112043.doc|title=Council conclusions on combination effects of chemicals|date=22 December 2009|publisher=Council of The European Union|accessdate=30 December 2009|location=Brussels}}</ref> | |||
| In September 2010, the European Food Safety Authority (EFSA) published its latest scientific opinion, based on a "comprehensive evaluation of recent toxicity data concluded that no new study could be identified, which would call for a revision of the current TDI".<ref name="European Food Safety Authority">{{vcite web|url=http://www.efsa.europa.eu/en/scdocs/scdoc/1829.htm|title=Scientific Opinion on Bisphenol A: evaluation of a study investigating its neurodevelopmental toxicity, review of recent scientific literature on its toxicity and advice on the Danish risk assessment of Bisphenol A|date=23 September 2010|publisher=European Food Safety Authority|accessdate=23 September 2010}}</ref> The Panel noted that some studies conducted on developing animals have suggested BPA-related effects of possible toxicological relevance, in particular biochemical changes in brain, immune-modulatory effects and enhanced susceptibility to breast tumours but considered that those studies had several shortcomings so the relevance of these findings for human health could not be assessed.<ref name="European Food Safety Authority"/> | |||
| On 25 November 2010, the ] said it will ban the manufacturing by {{nowrap|1 March 2011}} and ban the marketing and market placement of ] ]s containing the organic compound {{nowrap|bisphenol A}} (BPA) by {{nowrap|1 June 2011}}, according to ], ] in charge of health and consumer policy. This is backed by a majority of EU governments.<ref name="rban2011">{{vcite web |url=http://www.reuters.com/article/2010/11/25/us-eu-health-plastic-idUSTRE6AO3MS20101125 |title=EU to ban Bisphenol A in baby bottles in 2011 |work=Reuters |date=25 November 2010 |accessdate=2 February 2012}}</ref><ref name="pban2011">{{vcite web |url=http://www.physorg.com/news/2010-11-europe-baby-bottles-bisphenol-a.html |title=Europe bans baby bottles with Bisphenol-A |work=PhysOrg.com |date=25 November 2010 |accessdate=2 February 2012}}</ref> The ban was called an over-reaction by Richard Sharpe, of the ]'s Human Reproductive Sciences Unit, who said to be unaware of any convincing evidence justifying the measure and criticized it as being done on political, rather than scientific grounds.<ref>{{vcite news |title=EU bans bisphenol A chemical from babies' bottles |url= http://www.bbc.co.uk/news/world-europe-11843820 |agency=BBC |date=25 November 2010|quote=I do not know of any convincing evidence that bisphenol A exposure, in the amounts used in polycarbonate bottles, can cause any harm to babies as not only are the amounts so minuscule but they are rapidly broken down in the gut and liver. |work=BBC News}}</ref> | |||
| ====Denmark==== | |||
| In May 2009, the Danish parliament passed a resolution to ban the use of BPA in baby bottles, which has not been enacted by April 2010. In March 2010, a temporary ban was declared by the Health Minister.<ref>{{vcite web |url=http://www.dr.dk/Nyheder/Politik/2010/03/26/113304.htm |title=Danmark forbyder giftigt stof i sutteflasker |language=Danish |publisher=DR |date=26 March 2010 |accessdate=31 January 2012}}</ref> | |||
| ====Belgium==== | |||
| On March 2010, senator ] proposed legislation to ban BPA in ].<ref>{{vcite web |url=http://nieuws.be.msn.com/buitenland/artikel.aspx?cp-documentid=152421106 |title=Negen op tien zuigflessen zijn gevaarlijk |language=Dutch |publisher=Microsoft |date=3 May 2010 |accessdate=31 January 2012}}</ref> | |||
| ====France==== | |||
| On 5 February 2010, the French Food Safety Agency (AFSSA) questioned the previous assessments of the health risks of BPA, especially in regard to behavioral effects observed in rat pups following exposure in utero and during the first months of life.<ref>{{vcite web |url=http://www.afssa.fr/PM9100B6I0.htm|title=Bisphenol A: AFSSA recommends the development of new assessment methods|date=5 February 2010|accessdate=8 February 2010}}</ref><ref>{{vcite web |url=http://www.afssa.fr/cgi-bin/countdocs.cgi?Documents/MCDA2009sa0270EN.pdf |title=Opinion of the French Food Safety Agency on the critical analysis of the results of a study of the toxicity of bisphenol A on the development of the nervous system together with other recently-published data on its toxic effects |date=29 January 2010 |publisher=French Food Safety Agency |accessdate=8 February 2010}}</ref> In April 2010, the AFFSA suggested the adoption of better labels for food products containing BPA.<ref>{{vcite news |url=http://www.lefigaro.fr/flash-actu/2010/04/27/97001-20100427FILWWW00356-afssa-informations-sur-le-bisphenol-a.php |title=Afssa: informations sur le Bisphénol A |date=27 April 2010 |work=Le Figaro |language=French |language=french |accessdate=7 May 2010}}</ref> | |||
| On 24 March 2010, French Senate unanimously approved a proposition of law to ban BPA from baby bottles.<ref>{{vcite news|url=http://www.lemonde.fr/planete/article/2010/03/24/les-senateurs-votent-la-suspension-de-la-commercialisation-des-biberons-au-bisphenol-a_1324041_3244.html|title=Les sénateurs votent la suspension de la commercialisation des biberons au Bisphenol A|date=24 March 2010|work=Le Monde |language=French |accessdate=24 March 2010}}</ref> The National Assembly (Lower House) approved the text on 23 June 2010, which has been applicable law since 2 July 2010.<ref>{{vcite web|url=http://www.legifrance.gouv.fr/affichTexte.do?cidTexte=JORFTEXT000022414734&dateTexte= |title=Détail d'un texte |language={{fr icon}} |publisher=Legifrance.gouv.fr |accessdate=23 October 2011}}</ref> On 12 October 2011, the French National Assembly voted a law forbidding the use of Bisphenol A in products aimed at less than 3-year-old children for 2013, and 2014 for all food containers.<ref>{{vcite web|url=http://www.liberation.fr/depeches/01012365279-bisphenol-a-l-assemblee-vote-l-interdiction-dans-les-contenants-alimentaires |title=L'Assemblée unanime interdit les contenants alimentaires avec du bisphénol A – Libération |work=Libération |language=French |accessdate=23 October 2011}}</ref> | |||
| ====Germany==== | |||
| On 19 September 2008, the German Federal Institute for Risk Assessment (Bundesinstitut für Risikobewertung, BfR) stated that there was no reason to change the current risk assessment for bisphenol A on the basis of the Lang Study.<ref>{{vcite web |url=http://www.bfr.bund.de/cm/216/neue_studien_zu_bisphenol_a_stellen_die_bisherige_risikobewertung_nicht_in_frage.pdf |format=pdf |title=Neue Studien zu Bisphenol A stellen die bisherige Risikobewertung nicht in Frage |work=Bundesinstitut für Risikobewertung |language=German |date=19 September 2008 |accessdate=2 February 2012}}</ref> | |||
| In October 2009, the German environmental organization ] requested a ban on BPA for children's products, especially ],<ref>{{vcite web |url=http://www.bund.net/nc/bundnet/presse/pressemitteilungen/detail/zurueck/pressemitteilungen/artikel/schnuller-geben-hormonell-wirksames-bisphenol-a-ab-bund-fordert-verbot-der-chemikalie-fuer-babyarti |title=Schnuller geben hormonell wirksames Bisphenol A ab. BUND fordert Verbot der Chemikalie für Babyartikel |language=German |accessdate=15 October 2009}}</ref> and products that make contact with food.<ref>{{vcite web |url=http://www.derwesten.de/nachrichten/wp/2009/10/1/news-135346902/detail.html |title=Chemikalie in Schnullern entdeckt |date=1 October 2009 |language=German |accessdate=2 October 2009}}</ref> In response, some manufacturers voluntarily removed the problematic pacifiers from the market.<ref>{{vcite web |url=http://www.medizinauskunft.de/artikel/aktuell/2009/04_11_bisphenol_a.php |date=4 November 2009 |language=German |title=Bisphenol-A: Kehrtwende |work=MedizinAuskunft |archiveurl=http://web.archive.org/web/20110719060727/http://www.medizinauskunft.de/artikel/aktuell/2009/04_11_bisphenol_a.php |archivedate=19 July 2011 |accessdate=1 February 2012}}</ref> | |||
| ====Netherlands==== | |||
| On 6 November 2008, the Dutch Food and Consumer Product Safety Authority (VWA) stated in a newsletter that baby bottles made from polycarbonate plastic do not release measurable concentrations of bisphenol A and therefore are safe to use.<ref>{{dead link|date=October 2011}} {{nl icon}}</ref> | |||
| ====Switzerland==== | |||
| In February 2009, the Swiss Federal Office for Public Health, based on reports of other health agencies, stated that the intake of bisphenol A from food represents no risk to the consumer, including newborns and infants. However, in the same statement, it advised for proper use of polycarbonate baby bottles and listed alternatives.<ref>{{vcite web |url=http://www.bag.admin.ch/themen/lebensmittel/04861/06170/index.html?lang=de |title=Bisphenol A |publisher=Bundesamt für Gesundheit |accessdate=1 February 2012}}</ref> | |||
| ====Sweden==== | |||
| By May 2010, the Swedish Chemicals Agency asked for a BPA ban in baby bottles, but the Swedish Food Safety Authority prefers to await the expected European Food Safety Authority's updated review. The Minister of Environment said to wait for the EFSA review but not for too long.<ref>{{vcite web |url=http://www.dagenshandel.se/dh/DagensH.nsf/0/CEEB5E81FEE99BDFC1257720002F04A9 |title=Minister kör över Livsmedelsverket |language=Swedish |publisher=Dagens Handel |date=11 May 2010 |accessdate=1 February 2012}}</ref><ref> 10 May 2010. accessdate 13 May 2010.</ref> | |||
| From March 2011 it is prohibited to manufacture babybottles containing bisphenol A and from July 2011 they can not be bought in stores. On 12 April 2012, the Swedish government announced that Sweden will ban the endocrine disruptor Bisphenol A (BPA) in cans containing food for children under the age of three.<ref>{{cite web|title=Sweden acts on endocrine disruptor Bisphenol A|url=http://www.chemsec.org/news/news-2012/915-sweden-acts-on-endocrine-disruptor-bisphenol-a|publisher=ChemSec|accessdate=16 April 2012}}</ref> | |||
| ====United Kingdom==== | |||
| In December 2009, responding to a letter from a group of seven scientists that urged the UK Government to "adopt a standpoint consistent with the approach taken by other Governments who have ended the use of BPA in food contact products marketed at children",<ref>{{vcite news | last = Smith | first = Rebecca | title = Ban chemical linked to cancer in baby bottles: campaigners | newspaper=Telegraph | date = 1 December 2009 | accessdate=16 February 2010 | url = http://www.nomorebpa.org.uk/news/US_Acts_On_BPA_And_Baby%20_Bottles.php}}</ref> the UK Food Standards Agency reaffirmed, in January 2009, its view that "exposure of UK consumers to BPA from all sources, including ], was well below levels considered harmful".<ref>{{vcite web | url=http://www.nomorebpa.org.uk/news/US_Acts_On_BPA_And_Baby%20_Bottles.php | title=US Acts on BPA and Baby Bottles while UK Food Standards Agency dismisses concerns | date=18 January 2009 | publisher=Breast Cancer UK | accessdate=16 February 2010}}</ref> | |||
| ===Turkey=== | |||
| As of 10 June 2011, Turkey banned the use of BPA in baby bottles and other PC items produced for babies.<ref>{{vcite web|url=http://www.resmigazete.gov.tr/eskiler/2011/06/20110610-8.htm |title=Başbakanlık Mevzuatı Geliştirme ve Yayın Genel Müdürlüğü |publisher=Resmigazete.gov.tr |accessdate=23 October 2011}}</ref> | |||
| ===Japan=== | |||
| Between 1998 and 2003, the canning industry voluntarily replaced its BPA-containing epoxy resin can liners with BPA-free polyethylene terephthalate (PET) in many of its products. For other products, it switched to a different epoxy lining that yielded much less migration of BPA into food than the previously used resin. In addition, polycarbonate tableware for school lunches was replaced by BPA-free plastics. As a result of these changes, Japanese risk assessors have found that virtually no BPA is detectable in canned foods or drinks, and blood levels of BPA in the Japanese people have declined dramatically (50% in one study).<ref>{{vcite web |url=http://www.ewg.org/node/20938 |title=Bisphenol A: Toxic Plastics Chemical in Canned Food: Companies reduced BPA exposures in Japan |work=Environmental Working Group |accessdate=3 February 2012}}</ref> | |||
| ===United States=== | |||
| Currently 11 states have banned BPA from baby bottles and children's sippy cups. In October 2011, California became the latest to enact such legislation, when it passed the Toxin-Free Infants and Toddlers Act. The American Medical Association also recently announced its support of tighter restrictions on products containing BPA. | |||
| Organizations like the ] (ACC) have been lobbying to make BPA federally recognized as a safe product. The ACC is composed of companies that represent 85% of chemical manufacturing capacity in the United States.<ref>http://www.americanchemistry.com/Membership/Regular</ref> The ACC position states that BPA is safe for use in products. "Government and scientific bodies around the globe have extensively evaluated the weight of scientific evidence on bisphenol A (BPA) and have declared that BPA is safe as used, including in materials that come into contact with food, such as reusable foodstorage containers and linings in metal cans."<ref>About BPA: Weight of the Scientific Evidence Supports the Safety of BPA. http://plastics.americanchemistry.com/Product-Groups-and-Stats/PolycarbonateBPA-Global-Group/About-BPA-Weight-of-Scientific-Evidence-Supports-the-Safety-of-BPA.pdf Accessed online 28 March 2012.</ref> | |||
| Due to consumer pressure, by 2011 BPA was no longer being used in sippy cups and baby bottles. In October 2011, the ACC said that it had petitioned the FDA to make it clear to consumers that BPA is no longer present in those products. Steven Hentges of ACC's Polycarbonate/BPA Global Group said, "What we are trying to do is cut through the confusion and provide some clarity about sippy cups and baby bottles. We want that to be very clear, these products are not on the market. There is no need for parents or consumers to worry about them. They aren't there and they won't be in the future.",<ref>http://www.eenews.net/public/eenewspm/2011/10/07/1</ref> In July 2012, the FDA moved to ban BPA in baby bottles and sippy cups to satisfy a request by the ACC, stating that the measure was intended to "boost consumer confidence" and not due to safety concerns. (See 2012 below) | |||
| <!-- outdated and too extensive | |||
| ====April 2008==== | |||
| As of the release of NTP and Health Canada reports in April, 10 U.S. states, including California,<ref></ref> ],<ref name="WaPo">{{vcite news |last=Layton |first=Lyndsey |title=Studies on Chemical In Plastics Questioned |newspaper=The Washington Post |pages=A1 |year=2008 |date=27 April 2008 |url=http://www.washingtonpost.com/wp-dyn/content/article/2008/04/26/AR2008042602126.html |accessdate=3 February 2012}}</ref> and ],<ref>{{vcite news |title=Momentum Builds to End BPA in Plastics |newspaper=NewsInferno.com |date=22 April 2008 |url=http://www.newsinferno.com/legal-news/momentum-builds-to-end-bpa-in-plastics/2955 |accessdate=3 February 2012}}</ref> already had legislation pending that would affect the use of BPA. In the wake of these reports, ] ] (]–]) introduced legislation that would ban bisphenol A nationally from products for infants.<ref name="WaPo"/> In addition, the U.S. Congress is investigating the ], a chemical industry consulting firm, for its role in downplaying the health effects of bisphenol A and other chemicals,<ref>{{vcite web |url=http://cspinet.org/integrity/watch/200802111.html#5 |title=Congressional Probe Targets Consulting Group |work=] |date=11 February 2008 |accessdate=3 February 2012}}</ref> and the ] in the ] is investigating the use of BPA in baby products as well as the FDA's approval of the chemical. In asking the FDA to reassess its approval of bisphenol A, committee chairman ] (]–]) said "We would expect the FDA to make decisions based on the best available science...Yet the FDA relied on only two industry-funded studies, while other respected authorities used all available data to reach vastly different conclusions." The FDA maintained that bisphenol A is safe and did not recommend that people avoid using products made from it. The ] agreed, and its deputy director stressed that use of bisphenol A based plastics has many practical benefits and that "a ban could result in less effective protection of children from head, eye, or bodily injury."<ref name=C&ENews/> FDA then announced it would set up a task force to address these concerns, and in August it released a draft finding<ref>{{vcite web |url=http://www.fda.gov/ohrms/dockets/ac/08/briefing/2008-0038b1_01_02_FDA%20BPA%20Draft%20Assessment.pdf |format=pdf |title=Draft Assessment of Bisphenol A for Use in Food Contact Applications |work=US Food and Drug Administration |date=14 August 2008 |accessdate=2 February 2012}}</ref> concurring with its initial position that the chemical is safe. The agency will make its final decision after an advisory panel on the issues is convened in September.<ref>{{vcite news |url=http://www.washingtonpost.com/wp-dyn/content/article/2008/08/16/AR2008081600754.html |title=Health Highlights: Aug. 16, 2008 |date=16 August 2008|work=Washington Post |accessdate=17 August 2008}}</ref> | |||
| In response to these events, an ] (ACC)/BPA Global Group (an industry trade association) spokesman said, "The weight of scientific evidence, as assessed by Health Canada and other agencies around the world, provides reassurance that consumers can continue to safely use products made from bisphenol A."<ref>{{vcite web |url=http://www.washingtonpost.com/wp-dyn/content/story/2008/04/18/ST2008041803545.html |title=Canada Bans BPA From Baby Bottles |work=The Washington Post |author=Lyndsey Layton and Christopher Lee |date=19 April 2008 |accessdate=2 February 2012}}</ref> The ] also insisted that bisphenol A is safe, and argues that "Data purporting to demonstrate 'low' dose effects on the male reproductive system by BPA have not been successfully replicated and, therefore, are not credible to estimate human health risks and safety in light of the weight of a large body of evidence to the contrary."<ref>{{vcite news | title = Canada Could Ban Baby Bottles Containing Bisphenol A | publisher=Environment News Service | date = 22 April 2008 | url = http://www.ens-newswire.com/ens/apr2008/2008-04-22-05.asp | accessdate =24 April 2008 }}</ref> A spokesman for the ] industry said that without lining cans with bisphenol A based resins, '']'' and botulism poisoning would be "rampant."<ref name="C&ENews"/>--> | |||
| ====2008==== | |||
| In April the Federal BPA-Free Kids Act of 2008 (SB 2928) was introduced to the U.S. Senate. The bill sought to ban BPA in any product designed for use by children 7 years or younger. SB2928 would also require the Center for Disease Control to conduct a study about the health effects of BPA exposure. The bill was referred to the Committee on Commerce, Science, and Transportation and so far, no action has been taken.<ref name="Erler_Novak_2010"/> | |||
| In September, the National Toxicology Program finalized its report on bisphenol A, finding "some concern", midpoint of a five-level scale, that infants were at risk from exposure to the chemical.<ref name="NTP08"/> | |||
| At that time, the FDA reassured consumers that current limits were safe, but convened an outside panel of experts to review the issue. The ] was also released that month, and David Melzer, a co-author of the study, presented the results of the study before the FDA panel.<ref name="WaPoDos">{{vcite news|url=http://www.washingtonpost.com/wp-dyn/content/story/2008/09/16/ST2008091601215.html?sid=ST2008091601215&s_pos=list|title=Study Links Chemical BPA to Health Problems|last=Layton|first=Lindsey|date=16 September 2008|work=Washington Post |pages=A03|accessdate=17 September 2008}}</ref> | |||
| The editorial accompanying the Lang study's publication in ''JAMA'' criticized the FDA's assessment of bisphenol A: "A fundamental problem is that the current ADI for BPA is based on experiments conducted in the early 1980s using outdated methods (only very high doses were tested) and insensitive assays. More recent findings from independent scientists were rejected by the FDA, apparently because those investigators did not follow the outdated testing guidelines for environmental chemicals, whereas studies using the outdated, insensitive assays (predominantly involving studies funded by the chemical industry) are given more weight in arriving at the conclusion that BPA is not harmful at current exposure levels."<ref name="JAMAVS"/> | |||
| <!--irrelevant The ] similarly criticized the agency saying, "We're concerned that the FDA is basing its conclusion on two studies while downplaying the results of hundreds of other studies.... This appears to be a case of cherry-picking data with potentially high cost to human health."--><!-- unbalanced<ref name="WaPoDos"/> ], president of the ], also criticized the FDA, saying, in her testimony at the FDA's public meeting on the draft assessment of bisphenol A for use in food contact applications, that "At the very least, the FDA should require a prominent warning on products made with BPA".<ref>Szabo, Liz. "". ''USA Today''. 17 September 2008. Retrieved 29 October 2008</ref><ref> {{vcite news| title = Statement of Diana Zuckerman, PhD At the FDA Public Meeting on its Draft Assessment of Bisphenol A for Use in Food Contact Applications| url = http://www.center4research.org/BPA.html| accessdate =15 July 2009 |date=16 September 2008| author=]}}</ref> --><!--irrelevant In contrast, the American Chemistry Council, the manufacturing industry's lobby group, was skeptical of the latest study.<ref name="WaPoDos"/>--> | |||
| ====2009==== | |||
| Sunoco, a producer of gasoline and chemicals, is now refusing to sell the chemical to companies for use in food and water containers for children younger than 3, saying it can't be certain of the compound's safety. Sunoco plans to require its customers to guarantee that the chemical will not be used in children's food products.<ref>{{vcite news | author=Matthew Perrone |title=Sunoco restricts sales of chemical used in bottles |agency=The Seattle Times |date=12 March 2009 |url=http://seattletimes.nwsource.com/html/businesstechnology/2008848960_apbpasunoco.html |accessdate=3 February 2012}}</ref> | |||
| The six largest US companies that produce baby bottles decided to stop using bisphenol A in their products.<ref>{{vcite web |url=http://www.washingtonpost.com/wp-dyn/content/article/2009/03/05/AR2009030503285.html |title=No BPA For Baby Bottles In U.S. |work=The Washington Post |author=Lyndsey Layton |date=6 March 2009 |accessdate=2 February 2012}}</ref> | |||
| ], New York banned baby beverage containers made with bisphenol A.<ref>{{vcite web |url=http://www.northshoreoflongisland.com/Articles-i-2009-03-05-78665.112114-sub_County_bans_baby_bottle_plastic_with_BPA.html |title=County bans baby bottle plastic with BPA |work=northshoreoflongisland.com |author=Karen Forman |date=5 March 2009 |accessdate=2 February 2012}}</ref> | |||
| On 13 March, leaders from the House and Senate proposed legislation to ban bisphenol A.<ref>{{vcite web |url=http://www.washingtonpost.com/wp-dyn/content/article/2009/03/13/AR2009031303507.html |title=Bills Would Ban BPA From Food and Drink Containers |work=The Washington Post |date=14 March 2009 |accessdate=2 February 2012}}</ref> | |||
| In the same month, Rochelle Tyl, author of two studies used by FDA to assert BPA safety in August 2008, said those studies didn't claim that BPA is safe because they weren't designed to cover all aspects of the chemical's effects.<ref>{{vcite web |url=http://www.contracostatimes.com/nationandworld/ci_12123743 |title=Scientists reject FDA assertion of BPA's safety |author=Meg Kissinger and Susanne Rust |archiveurl=http://web.archive.org/web/20090416164151/http://www.contracostatimes.com/nationandworld/ci_12123743 |archivedate=16 April 2009 |work=Contra Costa Times |date=11 April 2009 |accessdate=2 February 2012}}</ref> | |||
| In May the first US jurisdictions to pass regulations limiting or banning BPA were ] and Chicago. Minnesota's regulation takes effect in 2010, "manufacturers of ... children's products containing BPA may not sell them in the state after 1 Jan. 2010. The ban extends to all retailers in the state a year later." The products impacted are known as sippy cups and baby bottles.<ref>{{vcite news|url=http://www.startribune.com/lifestyle/44586267.html|title=State bans chemical in baby bottles|last=Sternberg|first=Bob von|date=8 May 2009|work=Star Tribune|accessdate=11 July 2009}}</ref> The City of Chicago adopted a similar ban shortly thereafter. Coverage of Chicago's ban in the news showed a relentless opposition by the industry. A Chicago Tribune article noted an up-hill battle while passing legislation, " used FDA’s position on the issue when they tried to block the city’s measure."<ref>{{vcite news |url=http://archives.chicagotribune.com/2009/may/14/local/chi-chicago-bpa-baby-bottles-14may14|title=Chicago BPA ban: Chicago bans sale of baby bottles, sippy cups with dangerous chemical ... linked to diabetes, cancer and other illnesses |date=14 May 2009 |work=Chicago Tribune |archiveurl=http://web.archive.org/web/20100324090304/http://archives.chicagotribune.com/2009/may/14/local/chi-chicago-bpa-baby-bottles-14may14 |archivedate=24 March 2010 |accessdate=1 February 2012}}</ref> | |||
| In May 2009, the Washington Post accused the manufacturers of food and beverage containers and some of their biggest customers of trying to devise a public relations and lobbying strategy to block government BPA bans. Lyndsey Layton, from the Washington Post, criticized the FDA noting that, "Despite more than 100 published studies by government scientists and university laboratories that have raised health concerns about the chemical, the Food and Drug Administration has deemed it safe largely because of two studies, both funded by a chemical industry trade group".<ref>{{vcite news|url=http://www.washingtonpost.com/wp-dyn/content/article/2009/05/30/AR2009053002121.html |title=Strategy Being Devised To Protect Use of BPA |work=The Washington Post |date= 31 May 2009|accessdate=23 October 2011 |first=Lyndsey |last=Layton}}</ref> | |||
| In June 2009, the FDA announced the decision to reconsider the BPA safety levels.<ref>{{vcite news|last=Favole |first=Jared A. |url=http://online.wsj.com/article/SB124405286248681991.html |title=FDA to Revisit Decision on Safety of BPA |work=The Wall Street Journal |date=3 June 2009 |accessdate=23 October 2011}}</ref> | |||
| Grassroots political action led ] to become the first US state to ban bisphenol A from infant formula and baby food containers, as well from any reusable food or beverage container.<ref>{{vcite news|url=http://www.thehour.com/story/470418|title=Connecticut first state to ban BPA |last=Bodach|first=Jill|date=5 June 2009|work=The Hour|publisher=The Hour Publishing Co.|accessdate=9 September 2009}}</ref> A diverse coalition of over 50 Connecticut non-profits teamed up to support the measure, including health professionals, labor unions, faith communities, environmental organizations, public health, reproductive rights, and health affected groups. <ref>http://www.saferstates.com/2009/06/conn-ban.html</ref> | |||
| In July the California Environmental Protection Agency's Developmental and Reproductive Toxicant Identification Committee unanimously voted against placing Bisphenol A on the state's list of chemicals that are believed to cause reproductive harm. The panel, although concerned over the growing scientific research showing BPA's reproductive harm in animals, found that there was insufficient data of the effects in humans.<ref>{{vcite news|url=http://www.latimes.com/business/nationworld/wire/sns-ap-us-california-bpa,0,3478515.story|title=Calif. regulators will not list Bisphenol A under Prop. 65, call for more study|accessdate=19 July 2009 | deadurl=yes}} {{Dead link|date=August 2010|bot=RjwilmsiBot}}</ref> Critics point out that the same panel failed to add ] to the list until 2006, and only one chemical was added to the list in the last three years.<ref>{{vcite news|url=http://latimesblogs.latimes.com/greenspace/2009/07/bisphenol-a-california.html |title=California won't warn public about bisphenol A |publisher=Latimesblogs.latimes.com |date=17 July 2009 |accessdate=23 October 2011}}</ref> | |||
| On 3 August, Massachusetts' Department of Public Health advised mothers to take certain actions to prevent possible health impact in children. Mothers with children up to two years old were advised to limit exposure by avoiding products that might contain BPA, such as plastic drinking bottles and other plastic materials with ] of 7 or 3.<ref>{{vcite web|url=http://www.mass.gov/?pageID=eohhs2pressrelease&L=4&L0=Home&L1=Government&L2=Departments+and+Divisions&L3=Department+of+Public+Health&sid=Eeohhs2&b=pressrelease&f=090803_bpa_advisory&csid=Eeohhs2|title=Public Health Advisory Regarding Bisphenol A (BPA)|publisher=Commonwealth of Massachusetts|accessdate=4 August 2009}}</ref> | |||
| The '']'', as part of an ongoing investigative series into BPA and its effects, revealed plans by the ] to execute a major public relations blitz to promote BPA, including plans to attack and discredit those who report or comment negatively on the monomer and its effects.<ref>{{vcite web |url=http://www.jsonline.com/watchdog/watchdogreports/54195297.html |title=BPA industry fights back |author=Meg Kissinger and Susanne Rust |work=] |date=22 August 2009 |accessdate=2 February 2012}}</ref><ref>{{vcite web |url=http://www.jsonline.com/watchdog/watchdogreports/54195302.html |title='Watchdog' advocates for BPA |author=Susanne Rust and Meg Kissinger |work=Milwaukee Journal Sentinel |date=22 August 2009 |accessdate=2 February 2012}}</ref> | |||
| On 29 September, the ] announced that it is evaluating BPA, and another five chemicals, for action plan development.<ref>{{vcite web |url=http://www.epa.gov/oppt/existingchemicals/pubs/ecactionpln.html |title=Existing Chemical Action Plans |accessdate=1 October 2009}}</ref> | |||
| On 28 October, the ] announced $30,000,000 in ] grants to study the health effects of BPA. This money is expected to result in many ] publications.<ref>{{vcite journal |author=Sarewitz D |title=World view: A tale of two sciences |journal=Nature |volume=462 |issue=7273 |page=566 |year=2009 |month=December |pmid=19956236 |doi=10.1038/462566a}}</ref> | |||
| In November the '']'' magazine published an analysis of BPA content in some canned foods and beverages, where in specific cases the content of a single can of food could exceed the current FDA Cumulative Exposure Daily Intake.<ref name="consumerreports"/><ref>{{vcite web|url=http://www.foodmag.com.au/Article/Significant-bisphenol-A-levels-found-in-canned-food/504393.aspx|title=Significant bisphenol A levels found in canned food |date=4 November 2009|accessdate=4 November 2009}}</ref> | |||
| ====2010==== | |||
| On 15 January, the FDA expressed "some concern", the middle level in the scale of concerns, about the potential effects of BPA on the brain, behavior, and prostate gland in fetuses, infants, and young children, and announced it was taking reasonable steps to reduce human exposure to BPA in the food supply. However, the FDA is not recommending that families change the use of infant formula or foods, as it sees the benefit of a stable source of good nutrition as outweighing the potential risk from BPA exposure.<ref name="U.S. Food and Drug Administration"/> | |||
| On the same date, the U.S. Department of Health & Human Services released information to help parents to reduce children's BPA exposure.<ref>{{vcite web|url=http://www.hhs.gov/safety/bpa/|title=Bisphenol A (BPA) Information for Parents|date=15 January 2010|publisher=U.S. Department of Health & Human Services|accessdate=15 January 2010}}</ref> | |||
| In February, according to ''The Milwaukee Journal Sentinel'', which supports a BPA ban, after lobbyists for the chemical industry met with administration officials, the EPA delayed BPA regulation and did not include the chemical in an action plan released 30 December 2009.<ref>{{vcite news|url=http://www.jsonline.com/watchdog/watchdogreports/84321857.html|title=Regulator waffles on bisphenol A|last=Kissinger |first=Meg|date=14 February 2010|work=Journal Sentinel|accessdate=17 February 2010}}</ref><ref>{{vcite news|url=http://www.upi.com/Top_News/US/2010/02/14/EPA-delays-action-on-suspect-chemical/UPI-37321266190180/|title=EPA delays action on suspect chemical|date=14 February 2010|publisher=United Press International|accessdate=17 February 2010}}</ref> | |||
| Many US states are considering some sort of BPA ban.<ref>{{vcite news|url=http://content.usatoday.com/communities/greenhouse/post/2010/02/bans-sought-for-chemical-bpa-in-baby-toddler-products/1|title=Bans sought for chemical BPA in baby, toddler products|last=Koch|first=Wendy|date=16 February 2010|work=USA Today|accessdate=17 February 2010}}</ref> | |||
| On 29 March, the EPA declared BPA a "chemical of concern".<ref>{{vcite web |url=http://yosemite.epa.gov/opa/admpress.nsf/d0cf6618525a9efb85257359003fb69d/78110048d7f696d1852576f50054241a!OpenDocument |title=EPA to Scrutinize Environmental Impact of Bisphenol A |date=29 March 2010 |publisher=US Environmental Protection Agency |accessdate=12 April 2010}}</ref><ref name="actionplan">{{vcite web |url=http://www.epa.gov/oppt/existingchemicals/pubs/actionplans/bpa.html |title=Bisphenol A (BPA) Action Plan Summary |publisher=US Environmental Protection Agency |accessdate=12 April 2010}}</ref> | |||
| The 2008–2009 Annual Report of the President’s Cancer Panel declared: "because of the long latency period of many cancers, the available evidence argues for a precautionary approach to these diverse chemicals, which include (...) bisphenol A".<ref>{{vcite web |url=http://deainfo.nci.nih.gov/advisory/pcp/pcp08-09rpt/PCP_Report_08-09_508.pdf |title=Annual Report for 2008–2009 |last=Reuben |first=Suzanne H. |date=April 2010 |archiveurl=http://web.archive.org/web/20100602131450/http://deainfo.nci.nih.gov/advisory/pcp/pcp08-09rpt/PCP_Report_08-09_508.pdf? |archivedate=2 June 2010 |publisher=National Cancer Institute |accessdate=1 February 2012}}</ref> | |||
| Meanwhile, {{as of|lc=on|2011|4}}, ] has announced that it has found a BPA-free alternative can liner that apparently works even with tomatoes, a highly acidic product that has long baffled the industry in terms of finding a suitable substitute. General Mills says that with the next tomato harvest, it will begin using the BPA-free alternative in tomato products sold by its organic foods subsidiary ].<ref>{{vcite web|url=http://www.greenbiz.com/news/2010/04/19/general-mills-pull-bpa-organic-tomato-cans |title=General Mills to Pull BPA from Organic Tomato Cans, GreenerDesign, Accessed 08-09-2010 |publisher=Greenbiz.com |date=19 April 2010 |accessdate=23 October 2011}}</ref> Thus far, there has been no word on whether General Mills will use BPA-free alternatives on any of its other canned products. | |||
| ====2011==== | |||
| In 2011, 26 states proposed legislation that would ban certain uses of BPA. Many bills died in committee however, some are moving forward.<ref>Shader, Maggie. "California Joins 10 Other States in Banning BPA From Infant Feeding Containers." Consumerreports.org Consumer News. 6 October 2011.</ref> | |||
| In August 2010, the Maine Board of Environmental Protection voted unanimously to ban the sale of baby bottles and other reusable food and beverage containers made with bisphenol A as of January 2012.<ref>{{vcite web|author=1:18 am |url=http://new.bangordailynews.com/2010/06/18/politics/maine-board-weighs-bpa-bottle-ban/ |title=Maine board weighs BPA bottle ban – Maine Politics – Bangor Daily News |publisher=New.bangordailynews.com |date=18 June 2010 |accessdate=23 October 2011}}</ref> In February 2011, the newly elected governor of Maine, ], gained national attention when he spoke on a local TV news show saying he hoped to repeal the ban because, "There hasn't been any science that identifies that there is a problem" and added: "The only thing that I've heard is if you take a plastic bottle and put it in the microwave and you heat it up, it gives off a chemical similar to estrogen. So the worst case is some women may have little beards."<ref>{{vcite web |url=http://bangordailynews.com/2011/02/22/politics/gov-lepage-dismisses-dangers-of-bpa/ |title=LePage dismisses BPA dangers; ‘worst case is some women may have little beards’ |author=Kevin Miller |work=Health & Fitness – Bangor Daily News |date=22 February 2011 |accessdate=2 February 2012}}</ref><ref>{{vcite news| url=http://www.huffingtonpost.com/2011/02/23/maine-gov-paul-lepage-on-_n_827208.html |work=Huffington Post | title=Maine Gov. Paul LePage On BPA: 'Worst Case Is Some Women May Have Little Beards' | date=23 February 2011}}</ref> In April, the Maine legislature passed a bill to ban the use of BPA in baby bottles, sippy cups, and other reusable food and beverage containers, effective 1 January 2012. Governor LePage refused to sign the bill.<ref>{{vcite web |url=http://managementconsulted.com/core-content/dictionary/ |title=BPA Phase-Out Becomes Maine Law |publisher=Good Chemistry |date=25 April 2011 |accessdate=31 January 2012}}</ref> | |||
| A study by researchers at the Harvard School of Public Health indicates that BPA used in the lining of food cans is absorbed by the food and then ingested by consumers. The experiment involved 75 participants, half of whom ate a lunch of canned vegetable soup for five days, followed by five days of fresh soup; the other half did the same experiment in reverse order. "The analysis revealed that when participants ate the canned soup they experienced more than a 1,000 percent increase in their urinary concentrations of BPA, compared to when they dined on fresh soup."<ref> | |||
| {{vcite news|url=http://www.theglobeandmail.com/life/health/new-health/paul-taylor/bpa-being-absorbed-from-canned-food-study/article2248262/|title="BPA being absorbed from canned food" at theglobeandmail.com | location=Toronto |work=Globe and Mail |location=Canada |first=Paul|last=Taylor|date=24 November 2011}}</ref> | |||
| In October 2011 California banned BPA from baby bottles and toddlers' drinking cups. The ban will take effect 1 July 2013.<ref>{{cite news| url=http://latimesblogs.latimes.com/greenspace/2011/10/bpa-ban-signed-by-california-governor-jerry-brown.html | work=Los Angeles Times | title=Greenspace}}</ref> | |||
| In a July report the ] (AMA) declared that they believe that feeding products for babies and infants that contain BPA should be banned. In the report AMA said they would also like to see better federal oversight of BPA and the clear labeling of products containing it. It stressed the importance of the Food and Drug Administration to "actively incorporate current science into the regulation of food and beverage BPA-containing products."<ref>http://www.ama-assn.org/amednews/2011/07/04/prsg0704.htm</ref> | |||
| ====2012==== | |||
| In the spring of 2012, Colorado representative Daniel Kagan attempted to pass Colorado House Bill 12-1174, which would have prohibited the use of BPA in children’s products in the state of Colorado. The measure failed but may be re-introduced in January 2013.<ref>Katz, Danny. Director, Colorado Public Interest Research Group. Personal Interview. 13 March 2012.</ref> | |||
| The FDA recently concluded an assessment of scientific research on the effects of BPA. In the March 2012 Consumer Update, the FDA concluded that "the scientific evidence at this time does not suggest that the very low levels of human exposure to BPA through the diet are unsafe."<ref>FDA Continues to Study BPA: Consumer Updates. http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm297954. Accessed 4 April 2012.</ref> However, the FDA recognizes potential uncertainties in the overall interpretation of these studies including route of exposure used in the studies and the relevance of animal models to human health. The FDA is continuing to pursue additional research to resolve these uncertainties. | |||
| On 17 July, the FDA banned BPA from baby bottles and sippy cups. Since manufacturers have already stopped using the chemical in baby bottles and sippy cups, the decision was not over concerns about the safety of BPA but a response to a request by the ], the chemical industry’s main trade association, who believe that a ban would boost consumer confidence. <ref>http://www.nytimes.com/2012/07/18/science/fda-bans-bpa-from-baby-bottles-and-sippy-cups.html</ref> The ] called the ban "purely cosmetic". In a statement they said,“If the agency truly wants to prevent people from being exposed to this toxic chemical associated with a variety of serious and chronic conditions it should ban its use in cans of infant formula, food and beverages." The ] called the move inadequate saying, the FDA needs to ban BPA from all food packaging.<ref name="commondreams"/> In a statement a FDA spokesman said the agency's action was not based on safety concerns and that "the agency continues to support the safety of BPA for use in products that hold food."<ref name="huffpo"/> | |||
| == See also == | == See also == | ||
| ; Structurally related | |||
| * ] | |||
| * ] - used as a UV stabilizer in cosmetics and plastics | |||
| * ] (BADGE) | |||
| * ] - a proposed metabolite of BPA, which may show increased endocrine disrupting character | |||
| * ] (BPAF) | |||
| * ] - a metabolite of the synthetic insecticide ] | |||
| * ] (BPS) | |||
| ; Others | |||
| ==References== | |||
| * ] - next generation BPA replacement | |||
| {{Reflist|35em}} | |||
| * ] - used as a chain-length regulator in the production of polycarbonates and epoxy resins, it has also been studied as a potential endocrine disruptor | |||
| == |
== References == | ||
| {{ |
{{Reflist}} | ||
| * {{vcite journal|pmid=21295326|year=2011|last1=Kabiersch|first1=G|last2=Rajasärkkä|first2=J|last3=Ullrich|first3=R|last4=Tuomela|first4=M|last5=Hofrichter|first5=M|last6=Virta|first6=M|last7=Hatakka|first7=A|last8=Steffen|first8=K|title=Fate of bisphenol a during treatment with the litter-decomposing fungi Stropharia rugosoannulata and Stropharia coronilla|volume=83|issue=3|pages=226–32|doi=10.1016/j.chemosphere.2010.12.094|journal=Chemosphere}} | |||
| * | |||
| * | |||
| <!-- unbalanced, no other advocate website is listed * --> | |||
| <!-- old * --> | |||
| * {{vcite journal|last=Myers|first=John Peterson|date=March 2009|title=Why Public Health Agencies Cannot Depend on Good Laboratory Practices as a Criterion for Selecting Data: The Case of Bisphenol A|journal=Environmental Health Perspectives|volume=117|issue=3|pages=309–315|url=http://www.ehponline.org/members/2008/0800173/0800173.html|pmid=19337501|pmc=2661896|doi=10.1289/ehp.0800173|display-authors=1|last2=Welshons|first2=Wade V.|last3=Watson|first3=Cheryl S.|last4=Vogel|first4=Sarah|last5=Vandenbergh|first5=John G.|last6=Vandenberg|first6=Laura N.|last7=Taylor|first7=Julia A.|last8=Talsness|first8=Chris E.|last9=Soto|first9=Ana M.}} | |||
| * | |||
| * Attempt to regulate BPA in California defeated (from ''The Economist'') | |||
| <!-- old * News commentary on BPA Containing Products--> | |||
| <!-- * --> | |||
| * | |||
| * Layton, Lyndsey. 23 February 2010. Washington Post | |||
| * | |||
| * | |||
| {{HealthIssuesOfPlastics}} | |||
| {{Androgen receptor modulators}} | |||
| {{Estrogen receptor modulators}} | |||
| {{Estrogen-related receptor modulators}} | |||
| {{Authority control}} | |||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | ] | ||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 00:52, 19 January 2025
Chemicals used in plastics manufacturing
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name 4,4′-(Propane-2,2-diyl)diphenol | |
Other names
| |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.001.133 |
| EC Number |
|
| IUPHAR/BPS | |
| KEGG | |
| PubChem CID | |
| RTECS number |
|
| UNII | |
| UN number | 2430 |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C15H16O2 |
| Molar mass | 228.291 g·mol |
| Appearance | White solid |
| Odor | Phenolic, medical |
| Density | 1.217 g/cm |
| Melting point | 155 °C (311 °F; 428 K) |
| Boiling point | 250–252 °C (482–486 °F; 523–525 K) at 13 torrs (0.017 atm) |
| Solubility in water | 0.3 g/L (25 °C) |
| log P | 3.41 |
| Vapor pressure | 5×10 Pa (25 °C) |
| Hazards | |
| GHS labelling: | |
| Pictograms |    
|
| Signal word | Danger |
| Hazard statements | H317, H318, H335, H360, H411 |
| Precautionary statements | P201, P202, P261, P273, P302+P352, P304+P340, P305+P351+P338, P308+P313, P333+P313, P363, P403+P233 |
| NFPA 704 (fire diamond) |
 |
| Flash point | 227 °C (441 °F; 500 K) |
| Autoignition temperature |
510 °C (950 °F; 783 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Bisphenol A (BPA) is a chemical compound primarily used in the manufacturing of various plastics. It is a colourless solid which is soluble in most common organic solvents, but has very poor solubility in water. BPA is produced on an industrial scale by the condensation reaction of phenol and acetone. Global production in 2022 was estimated to be in the region of 10 million tonnes.
BPA's largest single application is as a co-monomer in the production of polycarbonates, which accounts for 65–70% of all BPA production. The manufacturing of epoxy resins and vinyl ester resins account for 25–30% of BPA use. The remaining 5% is used as a major component of several high-performance plastics, and as a minor additive in PVC, polyurethane, thermal paper, and several other materials. It is not a plasticizer, although it is often wrongly labelled as such.
The health effects of BPA have been the subject of prolonged public and scientific debate. BPA is a xenoestrogen, exhibiting hormone-like properties that mimic the effects of estrogen in the body. Although the effect is very weak, the pervasiveness of BPA-containing materials raises concerns, as exposure is effectively lifelong. Many BPA-containing materials are non-obvious but commonly encountered, and include coatings for the inside of food cans, clothing designs, shop receipts, and dental fillings. BPA has been investigated by public health agencies in many countries, as well as by the World Health Organization. While normal exposure is below the level currently associated with risk, several jurisdictions have taken steps to reduce exposure on a precautionary basis, in particular by banning BPA from baby bottles. There is some evidence that BPA exposure in infants has decreased as a result of this. BPA-free plastics have also been introduced, which are manufactured using alternative bisphenols such as bisphenol S and bisphenol F, but there is also controversy around whether these are actually safer.
History
Bisphenol A was first reported in 1891 by the Russian chemist Aleksandr Dianin.
In 1934, workers at I.G. Farbenindustrie reported the coupling of BPA and epichlorohydrin. Over the following decade, coatings and resins derived from similar materials were described by workers at the companies of DeTrey Freres in Switzerland and DeVoe and Raynolds in the US. This early work underpinned the development of epoxy resins, which in turn motivated production of BPA. The utilization of BPA further expanded with discoveries at Bayer and General Electric on polycarbonate plastics. These plastics first appeared in 1958, being produced by Mobay, General Electric, and Bayer.
The British biochemist Edward Charles Dodds tested BPA as an artificial estrogen in the early 1930s. Subsequent work found that it bound to estrogen receptors tens of thousands of times more weakly than estradiol, the major natural female sex hormone. Dodds eventually developed a structurally similar compound, diethylstilbestrol (DES), which was used as a synthetic estrogen drug in women and animals until it was banned due to its risk of causing cancer; the ban on use of DES in humans came in 1971 and in animals, in 1979. BPA was never used as a drug.
Production
The synthesis of BPA still follows Dianin's general method, with the fundamentals changing little in 130 years. The condensation of acetone (hence the suffix 'A' in the name) with two equivalents of phenol is catalyzed by a strong acid, such as concentrated hydrochloric acid, sulfuric acid, or a solid acid resin such as the sulfonic acid form of polystyrene sulfonate. An excess of phenol is used to ensure full condensation and to limit the formation of byproducts, such as Dianin's compound. BPA is fairly cheap to produce, as the synthesis benefits from a high atom economy and large amounts of both starting materials are available from the cumene process. As the only by-product is water, it may be considered an industrial example of green chemistry. Global production in 2022 was estimated to be in the region of 10 million tonnes.
Usually, the addition of acetone takes place at the para position on both phenols, however minor amounts of the ortho-para (up to 3%) and ortho-ortho isomers are also produced, along with several other minor by‑products. These are not always removed and are known impurities in commercial samples of BPA.
Properties
BPA has a fairly high melting point but can be easily dissolved in a broad range of organic solvents including toluene, ethanol and ethyl acetate. It may be purified by recrystallisation from acetic acid with water. Crystals form in the monoclinic space group P 21/n (where n indicates the glide plane); within this individual molecules of BPA are arraigned with a 91.5° torsion angle between the phenol rings. Spectroscopic data is available from AIST.
Uses and applications

Main uses
Polycarbonates
Main article: PolycarbonateAbout 65–70% of all bisphenol A is used to make polycarbonate plastics, which can consist of nearly 90% BPA by mass. Polymerisation is achieved by a reaction with phosgene, conducted under biphasic conditions; the hydrochloric acid is scavenged with aqueous base. This process converts the individual molecules of BPA into large polymer chains, effectively trapping them.
Epoxy and vinyl ester resins
About 25–30% of all BPA is used in the manufacture of epoxy resins and vinyl ester resins. For epoxy resin, it is first converted to its diglycidyl ether (usually abbreviated BADGE or DGEBA). This is achieved by a reaction with epichlorohydrin under basic conditions.
Some of this is further reacted with methacrylic acid to form bis-GMA, which is used to make vinyl ester resins. Alternatively, and to a much lesser extent, BPA may be ethoxylated and then converted to its diacrylate and dimethacrylate derivatives (bis-EMA, or EBPADMA). These may be incorporated at low levels in vinyl ester resins to change their physical properties and see common use in dental composites and sealants.
Minor uses
The remaining 5% of BPA is used in a wide range of applications, many of which involve plastic. BPA is a main component of several high-performance plastics, the production of these is low compared to other plastics but still equals several thousand tons a year. Comparatively minor amounts of BPA are also used as additives or modifiers in some commodity plastics. These materials are much more common but their BPA content will be low.
Plastics
- As a major component
- Polycyanurates can be produced from BPA by way of its dicyanate ester (BADCy). This is formed by a reaction between BPA and cyanogen bromide. Examples include BT-Epoxy, which is one of a number of resins used in the production of printed circuit boards.
- Polyetherimides such as Ultem can be produced from BPA via a nitro-displacement of appropriate bisnitroimides. These thermoplastic polyimide plastics have exceptional resistance to mechanical, thermal and chemical damage. They are used in medical devices and other high performance instrumentation.
- Polybenzoxazines may be produced from a number of biphenols, including BPA.
- Polysulfones can be produced from BPA and bis(4-chlorophenyl) sulfone forming poly(bisphenol-A sulfone) (PSF). It is used as a high performance alternative to polycarbonate.
- Bisphenol-A formaldehyde resins are a subset of phenol formaldehyde resins. They are used in the production of high-pressure laminates
- As a minor component
- Polyurethane can incorporate BPA and its derivatives as hard segment chain extenders, particularly in memory foams.
- PVC can contain BPA and its derivatives through multiple routes. BPA is sometimes used as an antioxidant in phthalates, which are extensively used as plasticizers for PVC. BPA has also been used as an antioxidant to protect sensitive PVC heat stabilizers. Historically 5–10% by weight of BPA was included in barium-cadmium types, although these have largely been phased out due health concerns surrounding the cadmium. BPA diglycidyl ether (BADGE) is used as an acid scavenger, particularly in PVC dispersions, such as organosols or plastisols, which are used as coatings for the inside of food cans, as well as embossed clothes designs produced using heat transfer vinyl or screen printing machines.
- BPA is used to form a number of flame retardants used in plastics. Bromination of BPA forms tetrabromobisphenol A (TBBPA), which is mainly used as a reactive component of polymers, meaning that it is incorporated into the polymer backbone. It is used to prepare fire-resistant polycarbonates by replacing some bisphenol A. A lower grade of TBBPA is used to prepare epoxy resins, used in printed circuit boards. TBBPA is also converted to tetrabromobisphenol-A-bis(2,3,-dibromopropyl ether) (TBBPA-BDBPE) which can be used as a flame retardant in polypropylene. TBBPA-BDBPE is not chemically bonded to the polymer and can leach out into the environment. The use of these compounds is diminishing due to restrictions on brominated flame retardants. The reaction of BPA with phosphorus oxychloride and phenol forms bisphenol-A bis(diphenyl phosphate) (BADP), which is used as a liquid flame retarder in some high performance polymer blends such as polycarbonate/ABS mixtures.
Other applications
- BPA is used as an antioxidant in several fields, particularly in brake fluids.
- BPA is used as a developing agent in thermal paper (shop receipts). Recycled paper products can also contain BPA, although this can depend strongly on how it is recycled. Deinking can remove 95% of BPA, with the pulp produced used to make newsprint, toilet paper and facial tissues. If deinking is not performed then the BPA remains in the fibers, paper recycled this way is usually made into corrugated fiberboard.
- Ethoxylated BPA finds minor use as a 'levelling agent' in tin electroplating.
- Several drug candidates have also been developed from bisphenol A, including ralaniten, ralaniten acetate, and EPI-001.
BPA substitutes
See also: BisphenolConcerns about the health effects of BPA have led some manufacturers replacing it with other bisphenols, such as bisphenol S and bisphenol F. These are produced in a similar manner to BPA, by replacing acetone with other ketones, which undergo analogous condensation reactions. Thus, in bisphenol F, the F signifies formaldehyde. Health concerns have also been raised about these substitutes. Alternative polymers, such as tritan copolyester have been developed to give the same properties as polycarbonate (durable, clear) without using BPA or its analogues.
| Structural formula | Name | CAS | Reactants | |
|---|---|---|---|---|
 |
Bisphenol AF | 1478-61-1 | Phenol | Hexafluoroacetone |
| Bisphenol F | 620-92-8 | Phenol | Formaldehyde | |
 |
Bisphenol S | 80-09-1 | Phenol | Sulfur trioxide |
 |
Bisphenol Z | 843-55-0 | Phenol | Cyclohexanone |
 |
Tetramethyl bisphenol F | 5384-21-4 | 2,6-xylenol | Formaldehyde |
Human safety
Exposure

As a result of the presence of BPA in plastics and other commonplace materials, most people are frequently exposed to trace levels of BPA. The primary source of human exposure is via food, as epoxy and PVC are used to line the inside of food cans to prevent corrosion of the metal by acidic foodstuffs. Polycarbonate drink containers are also a source of exposure, although most disposable drinks bottles are actually made of PET, which contains no BPA. Among the non-food sources, exposure routes include through dust, thermal paper, clothing, dental materials, and medical devices. Although BPA exposure is common it does not accumulate within the body, with toxicokinetic studies showing the biological half-life of BPA in adult humans to be around two hours. The body first converts it into more water-soluble compounds via glucuronidation or sulfation, which are then removed from the body through the urine. This allows exposure to be easily determined by urine testing, facilitating convenient biomonitoring of populations. Food and drink containers made from Bisphenol A-containing plastics do not contaminate the content to cause any increased cancer risk.
Health effects and regulation
Main article: Health effects of Bisphenol AThe health effects of BPA have been the subject of prolonged public and scientific debate, with PubMed listing more than 18,000 scientific papers as of 2024. Concern is mostly related to its estrogen-like activity, although it can interact with other receptor systems as an endocrine-disrupting chemical. These interactions are all very weak, but exposure to BPA is effectively lifelong, leading to concern over possible cumulative effects. Studying this sort of long‑term, low‑dose interaction is difficult, and although there have been numerous studies, there are considerable discrepancies in their conclusions regarding the nature of the effects observed as well as the levels at which they occur. A common criticism is that industry-sponsored trials tend to show BPA as being safer than studies performed by academic or government laboratories, although this has also been explained in terms of industry studies being better designed.
In the 2010s public health agencies in the EU, US, Canada, Australia and Japan as well as the WHO all reviewed the health risks of BPA, and found normal exposure to be below the level currently associated with risk. Regardless, due to the scientific uncertainty, many jurisdictions continued to take steps to reduce exposure on a precautionary basis. In particular, infants were considered to be at greater risk, leading to bans on the use of BPA in baby bottles and related products by the US, Canada, and EU amongst others. Bottle producers largely switched from polycarbonate to polypropylene and there is some evidence that BPA exposure in infants has decreased as a result of this. The European Food Safety Authority completed a re-evaluation into the risks of BPA in 2023, concluding that its tolerable daily intake should be greatly reduced. This lead to a European Union on 19 December 2024 to ban BPA in all the food contact materials, including plastic and coated packaging. The ban will come into force after an implementation period of up to three years.
BPA exhibits very low acute toxicity (i.e. from a single large dose) as indicated by its LD50 of 4 g/kg (mouse). Reports indicate that it is a minor skin irritant as well, although less so than phenol.
Pharmacology

BPA has been found to interact with a diverse range of hormone receptors, in both humans and animals. It binds to both of the nuclear estrogen receptors (ERs), ERα and ERβ. BPA is a selective estrogen receptor modulator (SERM), or partial agonist of the ER, so it can serve as both an estrogen agonist and antagonist. However, it is 1000- to 2000-fold less potent than estradiol, the major female sex hormone in humans. At high concentrations, BPA also binds to and acts as an antagonist of the androgen receptor (AR). In addition to receptor binding, the compound has been found to affect Leydig cell steroidogenesis, including affecting 17α-hydroxylase/17,20 lyase and aromatase expression and interfering with LH receptor-ligand binding.
Bisphenol A's interacts with the estrogen-related receptor γ (ERR-γ). This orphan receptor (endogenous ligand unknown) behaves as a constitutive activator of transcription. BPA seems to bind strongly to ERR-γ (dissociation constant = 5.5 nM), but only weakly to the ER. BPA binding to ERR-γ preserves its basal constitutive activity. It can also protect it from deactivation from the SERM 4-hydroxytamoxifen (afimoxifene). This may be the mechanism by which BPA acts as a xenoestrogen. Different expression of ERR-γ in different parts of the body may account for variations in bisphenol A effects. BPA has also been found to act as an agonist of the GPER (GPR30).
Environmental safety
Distribution and degradation
BPA has been detectable in the natural environment since the 1990s and is now widely distributed. It is primarily a river pollutant, but has also been observed in the marine environment, in soils, and lower levels can also be detected in air. The solubility of BPA in water is low (~300 g per ton of water) but this is still sufficient to make it a significant means of distribution into the environment. Many of the largest sources of BPA pollution are water-based, particularly wastewater from industrial facilities using BPA. Paper recycling can be a major source of release when this includes thermal paper, leaching from PVC items may also be a significant source, as can landfill leachate.
In all cases, wastewater treatment can be highly effective at removing BPA, giving reductions of 91–98%. Regardless, the remaining 2–9% of BPA will continue through to the environment, with low levels of BPA commonly observed in surface water and sediment in the U.S. and Europe.
Once in the environment BPA is aerobically biodegraded by a wide a variety of organisms. Its half life in water has been estimated at between 4.5 and 15 days, degradation in the air is faster than this, while soil samples degrade more slowly. BPA in sediment degrades most slowly of all, particularly where this is anaerobic. Abiotic degradation has been reported, but is generally slower than biodegradation. Pathways include photo-oxidation, or reactions with minerals such as goethite which may be present in soils and sediments.
Environmental effects
BPA is an environmental contaminant of emerging concern. Despite its short half-life and non-bioaccumulating character, the continuous release of BPA into the environment causes continuous exposure to both plant and animal life. Although many studies have been performed, these often focus on a limited range of model organisms and can use BPA concentrations well beyond environmental levels. As such, the precise effects of BPA on the growth, reproduction, and development of aquatic organism are not fully understood. Regardless, the existing data shows the effects of BPA on wildlife to be generally negative. BPA appears able to affect development and reproduction in a wide range of wildlife, with certain species being particularly sensitive, such as invertebrates and amphibians.
See also
- Structurally related
- 4,4'-Dihydroxybenzophenone - used as a UV stabilizer in cosmetics and plastics
- Dinitrobisphenol A - a proposed metabolite of BPA, which may show increased endocrine disrupting character
- HPTE - a metabolite of the synthetic insecticide methoxychlor
- Others
- 2,2,4,4-Tetramethyl-1,3-cyclobutanediol - next generation BPA replacement
- 4-tert-Butylphenol - used as a chain-length regulator in the production of polycarbonates and epoxy resins, it has also been studied as a potential endocrine disruptor
References
- Lim CF, Tanski JM (3 August 2007). "Structural Analysis of Bisphenol-A and its Methylene, Sulfur, and Oxygen Bridged Bisphenol Analogs". Journal of Chemical Crystallography. 37 (9): 587–595. Bibcode:2007JCCry..37..587L. doi:10.1007/s10870-007-9207-8. S2CID 97284173.
- ^ Shareef A, Angove MJ, Wells JD, et al. (11 May 2006). "Aqueous Solubilities of Estrone, 17β-Estradiol, 17α-Ethynylestradiol, and Bisphenol A". Journal of Chemical & Engineering Data. 51 (3): 879–881. doi:10.1021/je050318c.
- Robinson BJ, Hui JP, Soo EC, et al. (2009). "Estrogenic Compounds in Seawater and Sediment from Halifax Harbour, Nova Scotia, Canada". Environmental Toxicology and Chemistry. 28 (1): 18–25. Bibcode:2009EnvTC..28...18R. doi:10.1897/08-203.1. PMID 18702564. S2CID 13528747.
- "Chemical Fact Sheet – Cas #80057 CASRN 80-05-7". speclab.com. 1 April 2012. Archived from the original on 12 February 2012. Retrieved 14 June 2012.
- ^ Mitrofanova SE, Bakirova IN, Zenitova LA, et al. (September 2009). "Polyurethane varnish materials based on diphenylolpropane". Russian Journal of Applied Chemistry. 82 (9): 1630–1635. doi:10.1134/S1070427209090225. S2CID 98036316.
- ^ Sigma-Aldrich Co., Bisphenol A.
- ^ Fiege H, Voges HW, Hamamoto T, et al. (2000). "Phenol Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a19_313. ISBN 978-3527306732.
- ^ Abraham A, Chakraborty P (June 2020). "A review on sources and health impacts of bisphenol A". Reviews on Environmental Health. 35 (2): 201–210. doi:10.1515/reveh-2019-0034. PMID 31743105. S2CID 208186123.
- ^ European Commission. Joint Research Centre. Institute for Health Consumer Protection (2010). Updated European Union risk assessment report : 4,4'-isopropylidenediphenol (bisphenol-A) : environment addendum of February 2008. Publications Office. p. 6. doi:10.2788/40195. ISBN 9789279175428.
- ^ Vasiljevic T, Harner T (May 2021). "Bisphenol A and its analogues in outdoor and indoor air: Properties, sources and global levels". The Science of the Total Environment. 789: 148013. Bibcode:2021ScTEn.78948013V. doi:10.1016/j.scitotenv.2021.148013. PMID 34323825.
- Cadogan DF, Howick CJ (2000). "Plasticizers". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a20_439. ISBN 3527306730.
- ^ Joint FAO/WHO expert meeting to review toxicological and health aspects of bisphenol A : final report, including report of stakeholder meeting on bisphenol A, 1-5 November 2010, Ottawa, Canada. World Health Organization. 2011. hdl:10665/44624. ISBN 978-92-4-156427-4. Retrieved 23 March 2022.
- ^ Hengstler JG, Foth H, Gebel T, et al. (April 2011). "Critical evaluation of key evidence on the human health hazards of exposure to bisphenol A". Critical Reviews in Toxicology. 41 (4): 263–291. doi:10.3109/10408444.2011.558487. PMC 3135059. PMID 21438738.
- ^ Myers JP, vom Saal FS, Akingbemi BT, et al. (March 2009). "Why public health agencies cannot depend on good laboratory practices as a criterion for selecting data: the case of bisphenol A". Environmental Health Perspectives. 117 (3): 309–315. Bibcode:2009EnvHP.117..309M. doi:10.1289/ehp.0800173. PMC 2661896. PMID 19337501.
{{cite journal}}: CS1 maint: overridden setting (link) - Egan M (2013). "Sarah A. Vogel. Is It Safe? BPA and the Struggle to Define the Safety of Chemicals". Isis. 105 (1). Berkeley: University of California Press: 254. doi:10.1086/676809. ISSN 0021-1753.
- ^ Blair RM (1 March 2000). "The Estrogen Receptor Relative Binding Affinities of 188 Natural and Xenochemicals: Structural Diversity of Ligands". Toxicological Sciences. 54 (1): 138–153. doi:10.1093/toxsci/54.1.138. PMID 10746941.
- ^ Geens T, Aerts D, Berthot C, et al. (October 2012). "A review of dietary and non-dietary exposure to bisphenol-A" (PDF). Food and Chemical Toxicology. 50 (10): 3725–3740. doi:10.1016/j.fct.2012.07.059. PMID 22889897.
- Noonan GO, Ackerman LK, Begley TH (July 2011). "Concentration of bisphenol A in highly consumed canned foods on the U.S. market". Journal of Agricultural and Food Chemistry. 59 (13): 7178–7185. Bibcode:2011JAFC...59.7178N. doi:10.1021/jf201076f. PMID 21598963.
- ^ Xue J, Liu W, Kannan K (May 2017). "Bisphenols, Benzophenones, and Bisphenol A Diglycidyl Ethers in Textiles and Infant Clothing". Environmental Science & Technology. 51 (9): 5279–5286. Bibcode:2017EnST...51.5279X. doi:10.1021/acs.est.7b00701. PMID 28368574. Archived from the original on 29 December 2022. Retrieved 12 April 2022.
- ^ Björnsdotter MK, de Boer J, Ballesteros-Gómez A (September 2017). "Bisphenol A and replacements in thermal paper: A review". Chemosphere. 182: 691–706. Bibcode:2017Chmsp.182..691B. doi:10.1016/j.chemosphere.2017.05.070. hdl:1871.1/0c9480c5-48ce-4955-8d53-39b8b246802f. PMID 28528315.
- Ahovuo-Saloranta A, Forss H, Walsh T, et al. (July 2017). "Pit and fissure sealants for preventing dental decay in permanent teeth". The Cochrane Database of Systematic Reviews. 2017 (7): CD001830. doi:10.1002/14651858.CD001830.pub5. PMC 6483295. PMID 28759120.
- ^ Huang RP, Liu ZH, Yin H, et al. (June 2018). "Bisphenol A concentrations in human urine, human intakes across six continents, and annual trends of average intakes in adult and child populations worldwide: A thorough literature review". The Science of the Total Environment. 626: 971–981. Bibcode:2018ScTEn.626..971H. doi:10.1016/j.scitotenv.2018.01.144. PMID 29898562. S2CID 49194096.
- Thoene M, Dzika E, Gonkowski S, et al. (February 2020). "Bisphenol S in Food Causes Hormonal and Obesogenic Effects Comparable to or Worse than Bisphenol A: A Literature Review". Nutrients. 12 (2): 532. doi:10.3390/nu12020532. PMC 7071457. PMID 32092919.
- ^ Chen D, Kannan K, Tan H, et al. (7 June 2016). "Bisphenol Analogues Other Than BPA: Environmental Occurrence, Human Exposure, and Toxicity—A Review". Environmental Science & Technology. 50 (11): 5438–5453. Bibcode:2016EnST...50.5438C. doi:10.1021/acs.est.5b05387. PMID 27143250.
- Eladak S, Grisin T, Moison D, et al. (2015). "A new chapter in the bisphenol A story: bisphenol S and bisphenol F are not safe alternatives to this compound". Fertility and Sterility. 103 (1): 11–21. doi:10.1016/j.fertnstert.2014.11.005. PMID 25475787.
- See:
- А. Дианина (1891) "О продуктахъ конденсацiи кетоновъ съ фенолами" (On condensation products of ketones with phenols), Журнал Русского физико-химического общества (Journal of the Russian Physical Chemistry Society), 23 : 488-517, 523–546, 601–611; see especially pages 491-493 ("Диметилдифенолметань" (dimethyldiphenolmethane)).
- Reprinted in condensed form in: A. Dianin (1892) "Condensationsproducte aus Ketonen und Phenolen" (Condensation products of ketones and phenols), Berichte der Deutschen chemischen Gesellschaft zu Berlin, 25, part 3 : 334-337. doi:10.1002/cber.18920250333
- Pham HQ, Marks MJ (2012). "Epoxy Resins". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a09_547.pub2. ISBN 978-3527306732.
- Serini V (2000). "Polycarbonates". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a21_207. ISBN 978-3-527-30673-2.
- ^ Vogel SA (November 2009). "The politics of plastics: the making and unmaking of bisphenol a "safety"". American Journal of Public Health. 99 (Suppl 3): S559 – S566. doi:10.2105/AJPH.2008.159228. PMC 2774166. PMID 19890158.
- Dodds EC, Lawson W (1936). "Synthetic Œstrogenic Agents without the Phenanthrene Nucleus". Nature. 137 (3476): 996. Bibcode:1936Natur.137..996D. doi:10.1038/137996a0. S2CID 4171635.
- Dodds EC, Lawson W (1938). "Molecular Structure in Relation to Oestrogenic Activity. Compounds without a Phenanthrene Nucleus". Proceedings of the Royal Society of London B: Biological Sciences. 125 (839): 222–232. Bibcode:1938RSPSB.125..222D. doi:10.1098/rspb.1938.0023.
- Kwon JH, Katz LE, Liljestrand HM (October 2007). "Modeling binding equilibrium in a competitive estrogen receptor binding assay". Chemosphere. 69 (7): 1025–1031. Bibcode:2007Chmsp..69.1025K. doi:10.1016/j.chemosphere.2007.04.047. PMID 17559906.
- Uglea CV, Negulescu II (1991). Synthesis and Characterization of Oligomers. CRC Press. p. 103. ISBN 978-0-8493-4954-6.
- De Angelis A, Ingallina P, Perego C (March 2004). "Solid Acid Catalysts for Industrial Condensations of Ketones and Aldehydes with Aromatics". Industrial & Engineering Chemistry Research. 43 (5): 1169–1178. doi:10.1021/ie030429+.
- ^ Terasaki M, Nomachi M, Edmonds JS, et al. (May 2004). "Impurities in industrial grade 4,4'-isopropylidene diphenol (bisphenol A): possible implications for estrogenic activity". Chemosphere. 55 (6): 927–931. Bibcode:2004Chmsp..55..927T. doi:10.1016/j.chemosphere.2003.11.063. PMID 15041297.
- Pahigian JM, Zuo Y (September 2018). "Occurrence, endocrine-related bioeffects and fate of bisphenol A chemical degradation intermediates and impurities: A review". Chemosphere. 207: 469–480. Bibcode:2018Chmsp.207..469P. doi:10.1016/j.chemosphere.2018.05.117. PMID 29807346. S2CID 44172964.
- Haynes WM (2017). CRC handbook of chemistry and physics : a ready-reference book of chemical and physical data (2016-2017, 97th ed.). Boca Raton, Florida: CRC Press, Inc. pp. 3–56. ISBN 9781498754293.
- Perrin DD, Armarego WL (1988). Purification of laboratory chemicals. Butterworth-Heinemann. p. 208. ISBN 9780080347141.
- "2,2-bis(4-Hydroxyphenyl)propane". www.ccdc.cam.ac.uk. The Cambridge Crystallographic Data Centre. Retrieved 29 June 2022.
- Okada K (July 1996). "X-ray crystal structure analyses and atomic charges of color former and developer. I. Color developers". Journal of Molecular Structure. 380 (3): 223–233. Bibcode:1996JMoSt.380..223O. doi:10.1016/0022-2860(95)09168-8.
- Wolak JE, Knutson J, Martin JD, et al. (1 December 2003). "Dynamic Disorder and Conformer Exchange in the Crystalline Monomer of Polycarbonate". The Journal of Physical Chemistry B. 107 (48): 13293–13299. doi:10.1021/jp036527q.
- "4,4'-isopropylidenediphenol". sdbs.db.aist.go.jp. Spectral Database for Organic Compounds (SDBS). Retrieved 8 August 2024.
- Serini V (2000). "Polycarbonates". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a21_207. ISBN 978-3-527-30673-2.
- Ng F, Couture G, Philippe C, et al. (January 2017). "Bio-Based Aromatic Epoxy Monomers for Thermoset Materials". Molecules. 22 (1): 149. doi:10.3390/molecules22010149. PMC 6155700. PMID 28106795.
- Kroschwitz JI (1998). Kirk-Othmer Encyclopedia of Chemical Technology. Vol. 5 (5 ed.). Wiley. p. 8. ISBN 978-0-471-52695-7.
- Gonçalves F, Kawano Y, Pfeifer C, et al. (August 2009). "Influence of BisGMA, TEGDMA, and BisEMA contents on viscosity, conversion, and flexural strength of experimental resins and composites". European Journal of Oral Sciences. 117 (4): 442–446. doi:10.1111/j.1600-0722.2009.00636.x. PMID 19627357.
- Sideridou I, Tserki V, Papanastasiou G (April 2002). "Effect of chemical structure on degree of conversion in light-cured dimethacrylate-based dental resins". Biomaterials. 23 (8): 1819–1829. doi:10.1016/S0142-9612(01)00308-8. PMID 11950052.
- Sideridou ID, Achilias DS (July 2005). "Elution study of unreacted Bis-GMA, TEGDMA, UDMA, and Bis-EMA from light-cured dental resins and resin composites using HPLC". Journal of Biomedical Materials Research Part B: Applied Biomaterials. 74B (1): 617–626. doi:10.1002/jbm.b.30252. PMID 15889433.
- ^ Geens T, Goeyens L, Covaci A (September 2011). "Are potential sources for human exposure to bisphenol-A overlooked?". International Journal of Hygiene and Environmental Health. 214 (5): 339–347. Bibcode:2011IJHEH.214..339G. doi:10.1016/j.ijheh.2011.04.005. PMID 21570349.
- Hamerton I (1994). Chemistry and technology of cyanate ester resins (1st ed.). London: Blackie Academic & Professional. ISBN 978-0-7514-0044-1.
- Takekoshi T, Kochanowski JE, Manello JS, et al. (June 1985). "Polyetherimides. I. Preparation of dianhydrides containing aromatic ether groups". Journal of Polymer Science: Polymer Chemistry Edition. 23 (6): 1759–1769. Bibcode:1985JPoSA..23.1759T. doi:10.1002/pol.1985.170230616.
- Lau KS (2014). "10 - High-Performance Polyimides and High Temperature Resistant Polymers". Handbook of thermoset plastics (3rd ed.). San Diego: William Andrew. pp. 319–323. ISBN 978-1-4557-3107-7.
- Vijayakumar CT, Shamim Rishwana S, Surender R, et al. (2 January 2014). "Structurally diverse benzoxazines: synthesis, polymerization, and thermal stability". Designed Monomers and Polymers. 17 (1): 47–57. doi:10.1080/15685551.2013.797216. S2CID 94255723.
- Ghosh NN, Kiskan B, Yagci Y (November 2007). "Polybenzoxazines—New high performance thermosetting resins: Synthesis and properties". Progress in Polymer Science. 32 (11): 1344–1391. doi:10.1016/j.progpolymsci.2007.07.002.
- Kirk-Othmer Encyclopedia of Chemical Technology. Wiley. 26 January 2001. doi:10.1002/0471238961.0118151323080920.a01. ISBN 978-0-471-48494-3.
- Laza JM, Veloso A, Vilas JL (10 January 2021). "Tailoring new bisphenol a ethoxylated shape memory polyurethanes". Journal of Applied Polymer Science. 138 (2): 49660. doi:10.1002/app.49660. S2CID 224955435.
- Król P (2008). Linear polyurethanes : synthesis methods, chemical structures, properties and applications. Leiden: VSP. pp. 11–14. ISBN 9789004161245.
- "European Union Summary Risk Assessment Report - Bis (2-ethylhexyl) Phthalate (DEHP)". Joint Research Centre (JRC) Publications Repository. European Commission. 16 July 2008. ISSN 1018-5593. Retrieved 24 November 2021.[REDACTED]
- Shah AC, Poledna DJ (September 2003). "Review of PVC dispersion and blending resin products". Journal of Vinyl and Additive Technology. 9 (3): 146–154. doi:10.1002/vnl.10076. S2CID 98016356.
- Shah AC, Poledna DJ (September 2002). "Review of specialty PVC resins". Journal of Vinyl and Additive Technology. 8 (3): 214–221. doi:10.1002/vnl.10365. S2CID 97146596.
- Dagani MJ, Barda HJ, Benya TJ, et al. "Bromine Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a04_405. ISBN 978-3-527-30673-2.
- Gauthier LT, Laurich B, Hebert CE, et al. (20 August 2019). "Tetrabromobisphenol-A-Bis(dibromopropyl ether) Flame Retardant in Eggs, Regurgitates, and Feces of Herring Gulls from Multiple North American Great Lakes Locations". Environmental Science & Technology. 53 (16): 9564–9571. Bibcode:2019EnST...53.9564G. doi:10.1021/acs.est.9b02472. PMID 31364365. S2CID 198998658.
- Pawlowski KH, Schartel B (November 2007). "Flame retardancy mechanisms of triphenyl phosphate, resorcinol bis(diphenyl phosphate) and bisphenol A bis(diphenyl phosphate) in polycarbonate/acrylonitrile–butadiene–styrene blends". Polymer International. 56 (11): 1404–1414. doi:10.1002/pi.2290.
- Lamprea K, Bressy A, Mirande-Bret C, et al. (August 2018). "Alkylphenol and bisphenol A contamination of urban runoff: an evaluation of the emission potentials of various construction materials and automotive supplies" (PDF). Environmental Science and Pollution Research International. 25 (22): 21887–21900. Bibcode:2018ESPR...2521887L. doi:10.1007/s11356-018-2272-z. PMID 29796891. S2CID 44140721.
- Liao C, Kannan K (November 2011). "Widespread occurrence of bisphenol A in paper and paper products: implications for human exposure". Environmental Science & Technology. 45 (21): 9372–9379. Bibcode:2011EnST...45.9372L. doi:10.1021/es202507f. PMID 21939283.
- Rochester JR, Bolden AL (July 2015). "Bisphenol S and F: A Systematic Review and Comparison of the Hormonal Activity of Bisphenol A Substitutes". Environmental Health Perspectives. 123 (7): 643–650. Bibcode:2015EnvHP.123..643R. doi:10.1289/ehp.1408989. PMC 4492270. PMID 25775505.
- Calafat AM, Ye X, Wong LY, et al. (January 2008). "Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003-2004". Environmental Health Perspectives. 116 (1): 39–44. Bibcode:2008EnvHP.116...39C. doi:10.1289/ehp.10753. PMC 2199288. PMID 18197297.
- Thoene M, Rytel L, Nowicka N, et al. (May 2018). "The state of bisphenol research in the lesser developed countries of the EU: a mini-review". Toxicology Research. 7 (3): 371–380. doi:10.1039/c8tx00064f. PMC 6062254. PMID 30090587.
- Vandenberg LN, Hauser R, Marcus M, et al. (August 2007). "Human exposure to bisphenol A (BPA)". Reproductive Toxicology. 24 (2): 139–177. Bibcode:2007RepTx..24..139V. doi:10.1016/j.reprotox.2007.07.010. PMID 17825522.
- Van Landuyt K, Nawrot T, Geebelen B, et al. (August 2011). "How much do resin-based dental materials release? A meta-analytical approach". Dental Materials. 27 (8): 723–747. doi:10.1016/j.dental.2011.05.001. PMID 21664675.
- Tsukioka T, Terasawa JI, Sato S, et al. (2004). "Development of Analytical Method for Determining Trace Amounts of BPA in Urine Samples and Estimation of Exposure to BPA". Journal of Environmental Chemistry. 14 (1): 57–63. doi:10.5985/jec.14.57.
- Shin BS, Kim CH, Jun YS, et al. (December 2004). "Physiologically based pharmacokinetics of bisphenol A". Journal of Toxicology and Environmental Health. Part A. 67 (23–24): 1971–1985. Bibcode:2004JTEHA..67.1971S. doi:10.1080/15287390490514615. PMID 15513896. S2CID 24467830.
- Bousoumah R, Leso V, Iavicoli I, et al. (August 2021). "Biomonitoring of occupational exposure to bisphenol A, bisphenol S and bisphenol F: A systematic review". Science of the Total Environment. 783: 146905. Bibcode:2021ScTEn.78346905B. doi:10.1016/j.scitotenv.2021.146905. hdl:10400.21/13242. PMID 33865140. S2CID 233290894.
- "Does using plastic bottles and containers cause cancer?". Cancer Research UK. 23 December 2021.
- "bisphenol a - Search Results - PubMed". PubMed. Retrieved 26 January 2024.
- ^ MacKay H, Abizaid A (May 2018). "A plurality of molecular targets: The receptor ecosystem for bisphenol-A (BPA)". Hormones and Behavior. 101: 59–67. doi:10.1016/j.yhbeh.2017.11.001. PMID 29104009. S2CID 23088708.
- vom Saal FS, Hughes C (2005). "An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment". Environ. Health Perspect. 113 (8): 926–33. Bibcode:2005EnvHP.113..926V. doi:10.1289/ehp.7713. PMC 1280330. PMID 16079060.
- Teeguarden JG, Hanson-Drury S (December 2013). "A systematic review of Bisphenol A "low dose" studies in the context of human exposure: A case for establishing standards for reporting "low-dose" effects of chemicals". Food and Chemical Toxicology. 62: 935–948. doi:10.1016/j.fct.2013.07.007. PMID 23867546.
- "Bisphenol A - ECHA". echa.europa.eu. Archived from the original on 8 June 2022. Retrieved 28 March 2022.
- European Food Safety Authority (2015). EFSA explains the Safety of Bisphenol A: scientific opinion on bisphenol A (2015). European Food Safety Authority. doi:10.2805/075460. ISBN 9789291996421.
- "Scientific Opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs". EFSA Journal. 13 (1): 3978. 21 January 2015. doi:10.2903/j.efsa.2015.3978. hdl:2164/12119.
- OCSPP US EPA (21 September 2015). "Risk Management for Bisphenol A (BPA)". www.epa.gov. Retrieved 28 March 2022.
- CLARITY-BPA Research Program (October 2021). NTP Research Report on the Consortium Linking Academic and Regulatory Insights on Bisphenol A Toxicity (CLARITY-BPA): A Compendium of Published Findings. p. 18. doi:10.22427/NTP-RR-18. PMID 34910417. S2CID 240266384.
- Health Canada (16 April 2013). "Bisphenol A (BPA)". www.canada.ca (Health Canada). Government of Canada. Retrieved 28 March 2022.
- "Bisphenol A (BPA)". Food Standards Australia New Zealand (FSANZ). Department of Health (Australia). Archived from the original on 24 March 2022. Retrieved 28 March 2022.
- Aschberger K, Castello P, Hoekstra E (2010). Bisphenol A and baby bottles : challenges and perspectives. The Publications Office of the European Union. doi:10.2788/97553. ISBN 9789279158698.
- "Indirect Food Additives: Polymers". Federal Register. U.S. Government Publishing Office. 17 July 2012.77 FR 41899
- Legislative Services Branch (1 July 2020). "Consolidated federal laws of canada, Canada Consumer Product Safety Act". laws-lois.justice.gc.ca.
- "EUR-Lex - 32011L0008 - EN - EUR-Lex". EUR-Lex. European Union.
COMMISSION DIRECTIVE 2011/8/EU of 28 January 2011 amending Directive 2002/72/EC as regards the restriction of use of Bisphenol A in plastic infant feeding bottles
- Lambré C, Barat Baviera JM, Bolognesi C, et al. (April 2023). "Re-evaluation of the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs". EFSA Journal. 21 (4): e06857. doi:10.2903/j.efsa.2023.6857. hdl:20.500.11815/4370. PMC 10113887. PMID 37089179.
- "Regulation - EU - 2024/3190 - EN - EUR-Lex". eur-lex.europa.eu. Retrieved 14 January 2025.
- Akingbemi BT, Sottas CM, Koulova AI, et al. (1 February 2004). "Inhibition of Testicular Steroidogenesis by the Xenoestrogen Bisphenol A Is Associated with Reduced Pituitary Luteinizing Hormone Secretion and Decreased Steroidogenic Enzyme Gene Expression in Rat Leydig Cells". Endocrinology. 145 (2): 592–603. doi:10.1210/en.2003-1174. PMID 14605012.
- ^ Matsushima A, Kakuta Y, Teramoto T, et al. (October 2007). "Structural evidence for endocrine disruptor bisphenol A binding to human nuclear receptor ERR gamma". Journal of Biochemistry. 142 (4): 517–524. doi:10.1093/jb/mvm158. PMID 17761695.
- Prossnitz ER, Barton M (May 2014). "Estrogen biology: new insights into GPER function and clinical opportunities". Molecular and Cellular Endocrinology. 389 (1–2): 71–83. doi:10.1016/j.mce.2014.02.002. PMC 4040308. PMID 24530924.
- ^ Staples CA, Dome PB, Klecka GM, et al. (April 1998). "A review of the environmental fate, effects, and exposures of bisphenol A". Chemosphere. 36 (10): 2149–2173. Bibcode:1998Chmsp..36.2149S. doi:10.1016/S0045-6535(97)10133-3. PMID 9566294.
- ^ Corrales J, Kristofco LA, Steele WB, et al. (29 July 2015). "Global Assessment of Bisphenol A in the Environment: Review and Analysis of Its Occurrence and Bioaccumulation". Dose-Response. 13 (3): 1559325815598308. doi:10.1177/1559325815598308. PMC 4674187. PMID 26674671.
- Ozhan K, Kocaman E (February 2019). "Temporal and Spatial Distributions of Bisphenol A in Marine and Freshwaters in Turkey". Archives of Environmental Contamination and Toxicology. 76 (2): 246–254. Bibcode:2019ArECT..76..246O. doi:10.1007/s00244-018-00594-6. PMID 30610254. S2CID 58536418.
- ^ Cousins I, Staples C, Kleĉka G, et al. (July 2002). "A Multimedia Assessment of the Environmental Fate of Bisphenol A". Human and Ecological Risk Assessment. 8 (5): 1107–1135. Bibcode:2002HERA....8.1107C. doi:10.1080/1080-700291905846. S2CID 43509780.
- Vasiljevic T, Harner T (October 2021). "Bisphenol A and its analogues in outdoor and indoor air: Properties, sources and global levels". Science of the Total Environment. 789: 148013. Bibcode:2021ScTEn.78948013V. doi:10.1016/j.scitotenv.2021.148013. PMID 34323825.
- Fürhacker M, Scharf S, Weber H (September 2000). "Bisphenol A: emissions from point sources". Chemosphere. 41 (5): 751–756. Bibcode:2000Chmsp..41..751F. doi:10.1016/S0045-6535(99)00466-X. PMID 10834378.
- ^ Qi C, Huang J, Wang B, et al. (2018). "Contaminants of emerging concern in landfill leachate in China: A review". Emerging Contaminants. 4 (1): 1–10. doi:10.1016/j.emcon.2018.06.001.
- Drewes JE, Hemming J, Ladenburger SJ, et al. (2005). "An assessment of endocrine disrupting activity changes during wastewater treatment through the use of bioassays and chemical measurements". Water Environment Research. 77 (1): 12–23. Bibcode:2005WaEnR..77...12D. doi:10.2175/106143005x41573. PMID 15765931. S2CID 12283834.
- Klecka GM, Staples CA, Clark KE, et al. (August 2009). "Exposure analysis of bisphenol A in surface water systems in North America and Europe". Environmental Science & Technology. 43 (16): 6145–50. Bibcode:2009EnST...43.6145K. doi:10.1021/es900598e. PMID 19746705.
- Kang J, Katayama Y, Kondo F (16 January 2006). "Biodegradation or metabolism of bisphenol A: From microorganisms to mammals". Toxicology. 217 (2–3): 81–90. Bibcode:2006Toxgy.217...81K. doi:10.1016/j.tox.2005.10.001. PMID 16288945.
- Zhang C, Li Y, Wang C, et al. (2 January 2016). "Occurrence of endocrine disrupting compounds in aqueous environment and their bacterial degradation: A review". Critical Reviews in Environmental Science and Technology. 46 (1): 1–59. Bibcode:2016CREST..46....1Z. doi:10.1080/10643389.2015.1061881. S2CID 94353391.
- Im J, Löffler FE (16 August 2016). "Fate of Bisphenol A in Terrestrial and Aquatic Environments". Environmental Science & Technology. 50 (16): 8403–8416. Bibcode:2016EnST...50.8403I. doi:10.1021/acs.est.6b00877. OSTI 1470902. PMID 27401879.
- Xiao C, Wang L, Zhou Q, et al. (February 2020). "Hazards of bisphenol A (BPA) exposure: A systematic review of plant toxicology studies". Journal of Hazardous Materials. 384: 121488. Bibcode:2020JHzM..38421488X. doi:10.1016/j.jhazmat.2019.121488. PMID 31699483. S2CID 207939269.
- ^ Rubin AM, Seebacher F (July 2022). "Bisphenols impact hormone levels in animals: A meta-analysis". Science of the Total Environment. 828: 154533. Bibcode:2022ScTEn.82854533R. doi:10.1016/j.scitotenv.2022.154533. PMID 35288143. S2CID 247423338.
- ^ Wu NC, Seebacher F (July 2020). "Effect of the plastic pollutant bisphenol A on the biology of aquatic organisms: A meta-analysis". Global Change Biology. 26 (7): 3821–3833. Bibcode:2020GCBio..26.3821W. doi:10.1111/gcb.15127. PMID 32436328. S2CID 218765595.
- ^ Oehlmann J, Schulte-Oehlmann U, Kloas W, et al. (2009). "A critical analysis of the biological impacts of plasticizers on wildlife". Philosophical Transactions of the Royal Society B: Biological Sciences. 364 (1526): 2047–62. doi:10.1098/rstb.2008.0242. PMC 2873012. PMID 19528055.
- Wu NC, Rubin AM, Seebacher F (26 January 2022). "Endocrine disruption from plastic pollution and warming interact to increase the energetic cost of growth in a fish". Proceedings of the Royal Society B: Biological Sciences. 289 (1967). doi:10.1098/rspb.2021.2077. ISSN 0962-8452. PMC 8790379. PMID 35078359.
| Estrogen receptor modulators | |||||||
|---|---|---|---|---|---|---|---|
| ERTooltip Estrogen receptor |
| ||||||
| GPERTooltip G protein-coupled estrogen receptor |
| ||||||
| Estrogen-related receptor modulators | |
|---|---|
| ERRαTooltip Estrogen-related receptor alpha |
|
| ERRβTooltip Estrogen-related receptor beta |
|
| ERRγTooltip Estrogen-related receptor gamma |
|
| |