| Revision as of 19:47, 19 August 2015 editJeremicus rex (talk | contribs)Extended confirmed users1,406 edits →In popular culture← Previous edit | Latest revision as of 02:45, 24 December 2024 edit undoPreimage (talk | contribs)Extended confirmed users1,364 editsm →Photochemistry: Add #Eder reaction anchor | ||
| (89 intermediate revisions by 61 users not shown) | |||
| Line 9: | Line 9: | ||
| | ImageSize1 = | | ImageSize1 = | ||
| | IUPACName = Dimercury dichloride | | IUPACName = Dimercury dichloride | ||
| | OtherNames = Mercurous chloride<br/>Calomel | | OtherNames = Mercury(I) chloride<br/>Mercurous chloride<br/>] | ||
| |Section1={{Chembox Identifiers | |Section1={{Chembox Identifiers | ||
| | CASNo = 10112-91-1 | | CASNo = 10112-91-1 | ||
| | CASNo_Ref = {{cascite|correct|CAS}} | | CASNo_Ref = {{cascite|correct|CAS}} | ||
| | Gmelin = 25976 | |||
| | UNII_Ref = {{fdacite|changed|FDA}} | |||
| | UNII = J2D46N657D | |||
| | PubChem = 24956 | | PubChem = 24956 | ||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| Line 25: | Line 28: | ||
| | RTECS = OV8750000 | | RTECS = OV8750000 | ||
| | EINECS = 233-307-5 | | EINECS = 233-307-5 | ||
| | UNNumber = 3077 |
| UNNumber = 3077 | ||
| }} | }} | ||
| |Section2={{Chembox Properties | |Section2={{Chembox Properties | ||
| Line 32: | Line 35: | ||
| | Appearance = White solid | | Appearance = White solid | ||
| | Density = 7.150 g/cm<sup>3</sup> | | Density = 7.150 g/cm<sup>3</sup> | ||
| | MeltingPtC = |
| MeltingPtC = 383 | ||
| | MeltingPt_notes = ( |
| MeltingPt_notes = (sublimes) | ||
| | BoilingPtC = 383 | |||
| | BoilingPt_notes = (sublimes) | |||
| | Solubility = 0.2 mg/100 mL | | Solubility = 0.2 mg/100 mL | ||
| | SolubleOther = insoluble in ], ] | | SolubleOther = insoluble in ], ] | ||
| | SolubilityProduct = 1.43{{e|−18}}<ref name="crc">{{cite book |author1=John Rumble |title=CRC Handbook of Chemistry and Physics |date=June 18, 2018 |publisher=CRC Press |isbn=978-1138561632 |pages=5–188|edition=99 |language=English}}</ref> | |||
| | RefractIndex = 1.973 | | RefractIndex = 1.973 | ||
| | MagSus = −26.0·10<sup>−6</sup> cm<sup>3</sup>/mol | |||
| }} | }} | ||
| | |
|Section5={{Chembox Hazards | ||
| | ExternalSDS = | | ExternalSDS = | ||
| | GHSPictograms = {{GHS07}}{{GHS09}} | |||
| | EUClass = Harmful ('''Xn''')<br/>Dangerous for the environment ('''N''') | |||
| | GHSSignalWord = Warning | |||
| | RPhrases = {{R22}}, {{R36/37/38}}, {{R50/53}} | |||
| | HPhrases = {{H-phrases|302|315|319|335|410}} | |||
| | SPhrases = {{S2}}, {{S13}}, {{S24/25}}, {{S46}}, {{S60}}, {{S61}} | |||
| | PPhrases = {{P-phrases|261|264|270|271|273|280|301+312|302+352|304+340|305+351+338|312|321|330|332+313|337+313|362|391|403+233|405|501}} | |||
| | NFPA-H = 3 | | NFPA-H = 3 | ||
| | NFPA-F = 0 | | NFPA-F = 0 | ||
| Line 50: | Line 54: | ||
| | FlashPt = Non-flammable | | FlashPt = Non-flammable | ||
| | LD50 = 210 mg/kg (rat, oral)<ref>{{IDLH|7439976|Mercury compounds (as Hg)}}</ref> | | LD50 = 210 mg/kg (rat, oral)<ref>{{IDLH|7439976|Mercury compounds (as Hg)}}</ref> | ||
| ⚫ | }} | ||
| |Section3={{Chembox Structure | |||
| | CrystalStruct = tetragonal | |||
| }} | }} | ||
| |Section4={{Chembox Thermochemistry | |Section4={{Chembox Thermochemistry | ||
| | DeltaHf = −265 kJ·mol<sup>−1</sup><ref name=b1>{{cite book| |
| DeltaHf = −265 kJ·mol<sup>−1</sup><ref name=b1>{{cite book |last=Zumdahl |first=Steven S. |title =Chemical Principles 6th Ed.| publisher = Houghton Mifflin Company| year = 2009| isbn = 978-0-618-94690-7|page=A22}}</ref> | ||
| | Entropy = 196 J·mol<sup>−1</sup>·K<sup>−1</sup><ref name=b1/> | | Entropy = 196 J·mol<sup>−1</sup>·K<sup>−1</sup><ref name=b1/> | ||
| }} | }} | ||
| |Section8={{Chembox Related | |Section8={{Chembox Related | ||
| | OtherAnions = ]<br/>]<br/>] | | OtherAnions = ]<br/>]<br/>] | ||
| | |
| OtherCompounds = ] | ||
| }} | }} | ||
| }} | }} | ||
| '''Mercury(I) chloride''' is the ] with the formula Hg<sub>2</sub>Cl<sub>2</sub>. Also known as |
'''Mercury(I) chloride''' is the ] with the formula Hg<sub>2</sub>Cl<sub>2</sub>. Also known as the ] ]<ref name=EB>{{cite EB1911|wstitle=Calomel}}</ref> (a rare mineral) or '''mercurous chloride''', this dense white or yellowish-white, odorless solid is the principal example of a ](I) compound. It is a component of ]s in ].<ref>{{Housecroft2nd|pages=696–697}}</ref><ref>{{cite book |last1=Skoog |first1=Douglas A. |first2=F. James |last2=Holler |first3=Timothy A. |last3=Nieman | title = Principles of Instrumental Analysis | edition = 5th | publisher = Saunders College Pub. | year = 1998 | pages = 253–271 | isbn = 978-0-03-002078-0}}</ref> | ||
| ==History== | ==History== | ||
| The name calomel is thought to come from the ] |
The name calomel is thought to come from the ] ''καλός'' "beautiful", and ''μέλας'' "black"; or ''καλός'' and ''μέλι'' "honey" from its sweet taste.<ref name=EB/> The "black" name (somewhat surprising for a white compound) is probably due to its characteristic ] reaction with ], which gives a spectacular black coloration due to the finely dispersed metallic ] formed. It is also referred to as the mineral ''horn quicksilver'' or ''horn mercury''.<ref name=EB/> | ||
| Calomel was taken internally and used as a laxative,<ref name=EB/> for example to treat ] in 1801, and disinfectant, as well as in the treatment of syphilis, until the early 20th century. Until fairly recently,{{when|date=February 2018}} it was also used as a horticultural fungicide, most notably as a root dip to help prevent the occurrence of ] amongst crops of the family ].<ref>Buczacki, S., ''Pests, Diseases and Disorders of Garden Plants'', Collins, 1998, pp 449-50. {{ISBN|0-00-220063-5}}</ref> | |||
| ⚫ | Mercury became a popular remedy for a variety of physical and mental ailments during the age of "] |
||
| ⚫ | Mercury became a popular remedy for a variety of physical and mental ailments during the age of "]". It was prescribed by doctors in America throughout the 18th century, and during the revolution, to make patients regurgitate and release their body from "impurities". ] was a well-known advocate of mercury in medicine and used calomel to treat sufferers of ] during its outbreak in ] in 1793. Calomel was given to patients as a ] or ] until they began to salivate and was often administered to patients in such great quantities that their hair and teeth fell out.<ref> | ||
| {{cite journal | {{cite journal | ||
| | title = Heavy Metal Medicine | | title = Heavy Metal Medicine | ||
| Line 75: | Line 85: | ||
| | issue = 1 | | issue = 1 | ||
| | date = January 2001 | | date = January 2001 | ||
| ⚫ | | url = http://pubs.acs.org/subscribe/journals/tcaw/10/i01/html/01chemch.html | access-date = 2009-02-02 | ||
| | publisher = ] | |||
| ⚫ | }}</ref> | ||
| ⚫ | | url = http://pubs.acs.org/subscribe/journals/tcaw/10/i01/html/01chemch.html | |
||
| }}</ref> Shortly after yellow fever struck Philadelphia, the disease broke out in Jamaica. A war of words erupted in the press concerning the best treatment for yellow fever; bleeding or calomel. Anecdotal evidence indicates calomel was more effective than bleeding.<ref> | |||
| {{cite book | |||
| | title = Recollections of a Georgia Loyalist | |||
| | last = Johnston| first = Elizabeth Lichtenstein | |||
| | pages = 82–83 | |||
| | publisher = The Bankside Press | |||
| | year = 1901 | |||
| ⚫ | }} | ||
| ⚫ | </ref> | ||
| == In popular culture == | |||
| Calomel is mentioned in ] operetta '']'' (1881), during a poem recitation in the first act, by the character Bunthorne, entitled "Oh, Hollow! Hollow! Hollow!": | |||
| What time the poet hath hymned<BR> | |||
| The writhing maid, lithe-limbed,<BR> | |||
| Quivering on amaranthine asphodel,<BR> | |||
| How can he paint her woes,<BR> | |||
| Knowing, as well he knows,<BR> | |||
| That all can be set right with '''calomel'''?<BR> | |||
| <BR> | |||
| When from the poet's plinth<BR> | |||
| The amorous colocynth<BR> | |||
| Yearns for the aloe, faint with rapturous thrills,<BR> | |||
| How can he hymn their throes<BR> | |||
| Knowing, as well he knows,<BR> | |||
| That they are only uncompounded pills?<BR> | |||
| <BR> | |||
| Is it, and can it be,<BR> | |||
| Nature hath this decree,<BR> | |||
| Nothing poetic in the world shall dwell?<BR> | |||
| Or that in all her works<BR> | |||
| Something poetic lurks,<BR> | |||
| Even in colocynth and '''calomel'''?<BR> | |||
| I cannot tell. | |||
| Calomel is also mentioned in a punning exchange between Wagstaff (Groucho Marx) and Baravelli (Chico Marx) in "]" (1932), as Wagstaff ]: | |||
| Wagstaff: I got it! "Haddock"!<BR> | |||
| Baravelli: 'At's a-funny, I got a "haddock" too.<BR> | |||
| Wagstaff: What do you take for a "haddock"?<BR> | |||
| Baravelli: Sometimes I take an aspirin, sometimes I take a calomel.<BR> | |||
| Wagstaff: You know, ].<BR> | |||
| Baravelli: You mean chocolate ]? I like-a that too, but you no guess it. | |||
| Yellow fever was also treated with calomel.<ref name="Johnston1901">{{cite book|last=Johnston|first=Elizabeth Lichtenstein|title=Recollections of a Georgia Loyalist...written in 1836|url=https://archive.org/details/recollectionsag00johngoog|page=|year=1901|publisher=Mansfield & Company|location=New York}} pp. 82-83.</ref> | |||
| Additionally Calomel is mentioned in "]" (1960) when Scout tries to explain an odd look on Jem's face: | |||
| ] brought calomel on their expedition. Researchers used that same mercury, found deep in ] pits, to retrace the locations of their respective locations and campsites.<ref>{{Cite news|url=https://io9.gizmodo.com/archaeologists-tracked-lewis-and-clark-by-following-the-1727887223?commerce_insets_disclosure=on|title=Archaeologists Tracked Lewis and Clark by Following Their Trail of Laxatives|last=Inglis-Arkell|first=Esther|work=io9|access-date=2018-11-09|language=en-US}}</ref> | |||
| When Jem returned, he found me still in Atticus’s lap, “Well, son?” said Atticus. <BR> | |||
| He set me on my feet, and I made a secret reconnaissance of Jem. He seemed to <BR> | |||
| be all in one piece, but he had a queer look on his face. Perhaps she had given him<BR> | |||
| a dose of calomel. | |||
| ==Properties== | ==Properties== | ||
| Mercury is unique among the group 12 metals for its ability to form the M–M bond so readily. Hg<sub>2</sub>Cl<sub>2</sub> is a linear molecule. The mineral calomel crystallizes in the ] system, with space group I4/m 2/m 2/m. The ] of the ] is shown below: | Mercury is unique among the group 12 metals for its ability to form the M–M bond so readily. Hg<sub>2</sub>Cl<sub>2</sub> is a linear molecule. The mineral calomel crystallizes in the ] system, with space group I4/m 2/m 2/m. The ] of the ] is shown below: | ||
| {| class="wikitable" style="margin:1em auto; text-align:center;" | |||
| <center> | |||
| ⚫ | | ]||] | ||
| {|align="center" class="wikitable" | |||
| ⚫ | | |
||
| |- | |- | ||
| | |
| unit cell||distorted octahedral coordination of Hg | ||
| |} | |} | ||
| </center> | |||
| The Hg–Hg bond length of 253 pm (Hg–Hg in the metal is 300 pm) and the Hg–Cl bond length in the linear Hg<sub>2</sub>Cl<sub>2</sub> unit is 243 pm.<ref name = "Wells">Wells A.F. (1984) ''Structural Inorganic Chemistry'' 5th edition Oxford Science Publications ISBN |
The Hg–Hg bond length of 253 pm (Hg–Hg in the metal is 300 pm) and the Hg–Cl bond length in the linear Hg<sub>2</sub>Cl<sub>2</sub> unit is 243 pm.<ref name = "Wells">Wells A.F. (1984) ''Structural Inorganic Chemistry'' 5th edition Oxford Science Publications {{ISBN|0-19-855370-6}}</ref> The overall coordination of each Hg atom is octahedral as, in addition to the two nearest neighbours, there are four other Cl atoms at 321 pm. Longer ] exist. | ||
| ==Preparation and reactions== | ==Preparation and reactions== | ||
| Mercurous chloride forms by the reaction of elemental mercury and mercuric chloride: | Mercurous chloride forms by the reaction of elemental mercury and mercuric chloride: | ||
| :Hg + HgCl<sub>2</sub> → Hg<sub>2</sub>Cl<sub>2</sub> | |||
| It can be prepared via ] reaction involving aqueous ] using various chloride sources including NaCl or HCl. | It can be prepared via ] involving aqueous ] using various chloride sources including NaCl or HCl. | ||
| : |
:2 HCl + Hg<sub>2</sub>(NO<sub>3</sub>)<sub>2</sub> → Hg<sub>2</sub>Cl<sub>2</sub> + 2 HNO<sub>3</sub> | ||
| ] causes Hg<sub>2</sub>Cl<sub>2</sub> to ]: | ] causes Hg<sub>2</sub>Cl<sub>2</sub> to ]: | ||
| :Hg<sub>2</sub>Cl<sub>2</sub> + 2 NH<sub>3</sub> → Hg + Hg(NH<sub>2</sub>)Cl + NH<sub>4</sub>Cl | |||
| ===Calomel electrode=== | ===Calomel electrode=== | ||
| Line 155: | Line 117: | ||
| ===Photochemistry=== | ===Photochemistry=== | ||
| Mercurous chloride decomposes into ] and elemental mercury upon exposure to UV light. | Mercurous chloride decomposes into ] and elemental mercury upon exposure to UV light. | ||
| :Hg<sub>2</sub>Cl<sub>2</sub> → HgCl<sub>2</sub> + Hg | |||
| The formation of Hg can be used to calculate the number of photons in the light beam, by the technique of ]. |
The formation of Hg can be used to calculate the number of photons in the light beam, by the technique of ]. | ||
| {{anchor|Eder reaction}}By utilizing a light reaction in the presence of ] and ], mercury(I) chloride, ] and ] are produced. | |||
| : |
:2 HgCl<sub>2</sub> + (NH<sub>4</sub>)<sub>2</sub>C<sub>2</sub>O<sub>4</sub> {{overset|Light|→}} Hg<sub>2</sub>Cl<sub>2(s)</sub> + 2 + 2 CO<sub>2</sub> | ||
| This particular reaction was discovered by J.M. Eder (hence the name '''Eder reaction''') in 1880 and reinvestigated by W. E. Rosevaere in 1929<ref>{{Cite journal | title = The X-Ray Photochemical Reaction between Potassium Oxalate and Mercuric Chloride |
This particular reaction was discovered by J. M. Eder (hence the name '''Eder reaction''') in 1880 and reinvestigated by W. E. Rosevaere in 1929.<ref>{{Cite journal |last=Roseveare |first=W. E. | title = The X-Ray Photochemical Reaction between Potassium Oxalate and Mercuric Chloride | journal = ] | year = 1930 | volume = 52 | issue = 7 | pages = 2612–2619 | doi = 10.1021/ja01370a005}}</ref> | ||
| ==Related mercury(I) compounds== | ==Related mercury(I) compounds== | ||
| ], Hg<sub>2</sub>Br<sub>2</sub>, is |
], Hg<sub>2</sub>Br<sub>2</sub>, is light yellow, whereas ], Hg<sub>2</sub>I<sub>2</sub>, is greenish in colour. Both are poorly soluble. ] is unstable in the absence of a strong acid. | ||
| ==Safety considerations== | ==Safety considerations== | ||
| {{main|Mercury poisoning}} | {{main|Mercury poisoning}} | ||
| Mercurous chloride is ], although due to its low solubility in water it is generally less dangerous than its ] counterpart. It was used in medicine as a ] and ] (laxative) in the ] from the late 1700s |
Mercurous chloride is ], although due to its low solubility in water it is generally less dangerous than its ] counterpart. It was used in medicine as a ] and ] (laxative) in the ] from the late 1700s through the 1860s. Calomel was also a common ingredient in ] powders in Britain up until 1954, causing widespread mercury poisoning in the form of ], which at the time had a mortality rate of 1 in 10.<ref>{{cite book | first = Walter | last = Sneader | title = Drug Discovery: A History | url = https://books.google.com/books?id=mYQxRY9umjcC&pg=PA46 | pages = 45–46 | publisher = ] | isbn = 978-0-471-89980-8 | year = 2005 | access-date = 2009-02-02}}</ref> These medicinal uses were later discontinued when the compound's toxicity was discovered. | ||
| It has also found uses in cosmetics as soaps and ] creams, but these preparations are now illegal to manufacture or import in many countries including the |
It has also found uses in cosmetics as soaps and ] creams, but these preparations are now illegal to manufacture or import in many countries including the US, Canada, Japan and the European Union.<ref>{{cite web |url=http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CONSLEG:1976L0768:20080424:en:PDF |title=Commission Directive 86/199/EEC, OJ L 149, p. 38 of 3.6.1986}}</ref> A study of workers involved in the production of these preparations showed that the sodium salt of ] (DMPS) was effective in lowering the ] of mercury and in decreasing the urinary mercury concentration to normal levels.<ref>{{cite journal |author1=D. Gonzalez-Ramirez |author2=M. Zuniga-Charles |author3=A. Narro-Juarez |author4=Y. Molina-Recio |author5=K. M. Hurlbut |author6=R. C. Dart |author7=H. V. Aposhian | title = DMPS (2,3-Dimercaptopropane-1-sulfonate, Dimaval) Decreases the Body Burden of Mercury in Humans Exposed to Mercurous Chloride | date=1 October 1998| journal = The Journal of Pharmacology and Experimental Therapeutics| volume = 287 | issue = 1 | pages = 8–12 | url = http://jpet.aspetjournals.org/cgi/content/abstract/287/1/8 | format = free full text | pmid = 9765315 }}</ref> | ||
| ==References== | ==References== | ||
| Line 176: | Line 140: | ||
| {{Commons category|Mercury(I) chloride}} | {{Commons category|Mercury(I) chloride}} | ||
| * | * | ||
| * | * | ||
| * | * | ||
| {{Mercury compounds}} | {{Mercury compounds}} | ||
| {{Chlorides}} | |||
| {{DEFAULTSORT:Mercury(I) Chloride}} | {{DEFAULTSORT:Mercury(I) Chloride}} | ||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ] | |||
| ] | ] | ||
| ] | ] | ||
| ] | |||
| ] | |||
Latest revision as of 02:45, 24 December 2024
 | |
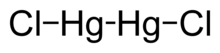 | |
 | |
| Names | |
|---|---|
| IUPAC name Dimercury dichloride | |
| Other names
Mercury(I) chloride Mercurous chloride Calomel | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.030.266 |
| EC Number |
|
| Gmelin Reference | 25976 |
| PubChem CID | |
| RTECS number |
|
| UNII | |
| UN number | 3077 |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | Hg2Cl2 |
| Molar mass | 472.09 g/mol |
| Appearance | White solid |
| Density | 7.150 g/cm |
| Melting point | 383 °C (721 °F; 656 K) (sublimes) |
| Solubility in water | 0.2 mg/100 mL |
| Solubility product (Ksp) | 1.43×10 |
| Solubility | insoluble in ethanol, ether |
| Magnetic susceptibility (χ) | −26.0·10 cm/mol |
| Refractive index (nD) | 1.973 |
| Structure | |
| Crystal structure | tetragonal |
| Thermochemistry | |
| Std molar entropy (S298) |
196 J·mol·K |
| Std enthalpy of formation (ΔfH298) |
−265 kJ·mol |
| Hazards | |
| GHS labelling: | |
| Pictograms |  
|
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335, H410 |
| Precautionary statements | P261, P264, P270, P271, P273, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P312, P321, P330, P332+P313, P337+P313, P362, P391, P403+P233, P405, P501 |
| NFPA 704 (fire diamond) |
 |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) | 210 mg/kg (rat, oral) |
| Safety data sheet (SDS) | ICSC 0984 |
| Related compounds | |
| Other anions | Mercury(I) fluoride Mercury(I) bromide Mercury(I) iodide |
| Related compounds | Mercury(II) chloride |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Mercury(I) chloride is the chemical compound with the formula Hg2Cl2. Also known as the mineral calomel (a rare mineral) or mercurous chloride, this dense white or yellowish-white, odorless solid is the principal example of a mercury(I) compound. It is a component of reference electrodes in electrochemistry.
History
The name calomel is thought to come from the Greek καλός "beautiful", and μέλας "black"; or καλός and μέλι "honey" from its sweet taste. The "black" name (somewhat surprising for a white compound) is probably due to its characteristic disproportionation reaction with ammonia, which gives a spectacular black coloration due to the finely dispersed metallic mercury formed. It is also referred to as the mineral horn quicksilver or horn mercury.
Calomel was taken internally and used as a laxative, for example to treat George III in 1801, and disinfectant, as well as in the treatment of syphilis, until the early 20th century. Until fairly recently, it was also used as a horticultural fungicide, most notably as a root dip to help prevent the occurrence of clubroot amongst crops of the family Brassicaceae.
Mercury became a popular remedy for a variety of physical and mental ailments during the age of "heroic medicine". It was prescribed by doctors in America throughout the 18th century, and during the revolution, to make patients regurgitate and release their body from "impurities". Benjamin Rush was a well-known advocate of mercury in medicine and used calomel to treat sufferers of yellow fever during its outbreak in Philadelphia in 1793. Calomel was given to patients as a purgative or cathartic until they began to salivate and was often administered to patients in such great quantities that their hair and teeth fell out.
Yellow fever was also treated with calomel.
Lewis and Clark brought calomel on their expedition. Researchers used that same mercury, found deep in latrine pits, to retrace the locations of their respective locations and campsites.
Properties
Mercury is unique among the group 12 metals for its ability to form the M–M bond so readily. Hg2Cl2 is a linear molecule. The mineral calomel crystallizes in the tetragonal system, with space group I4/m 2/m 2/m. The unit cell of the crystal structure is shown below:
 |

|
| unit cell | distorted octahedral coordination of Hg |
The Hg–Hg bond length of 253 pm (Hg–Hg in the metal is 300 pm) and the Hg–Cl bond length in the linear Hg2Cl2 unit is 243 pm. The overall coordination of each Hg atom is octahedral as, in addition to the two nearest neighbours, there are four other Cl atoms at 321 pm. Longer mercury polycations exist.
Preparation and reactions
Mercurous chloride forms by the reaction of elemental mercury and mercuric chloride:
- Hg + HgCl2 → Hg2Cl2
It can be prepared via metathesis reaction involving aqueous mercury(I) nitrate using various chloride sources including NaCl or HCl.
- 2 HCl + Hg2(NO3)2 → Hg2Cl2 + 2 HNO3
Ammonia causes Hg2Cl2 to disproportionate:
- Hg2Cl2 + 2 NH3 → Hg + Hg(NH2)Cl + NH4Cl
Calomel electrode
Main article: Saturated calomel electrodeMercurous chloride is employed extensively in electrochemistry, taking advantage of the ease of its oxidation and reduction reactions. The calomel electrode is a reference electrode, especially in older publications. Over the past 50 years, it has been superseded by the silver/silver chloride (Ag/AgCl) electrode. Although the mercury electrodes have been widely abandoned due to the dangerous nature of mercury, many chemists believe they are still more accurate and are not dangerous as long as they are handled properly. The differences in experimental potentials vary little from literature values. Other electrodes can vary by 70 to 100 millivolts.
Photochemistry
Mercurous chloride decomposes into mercury(II) chloride and elemental mercury upon exposure to UV light.
- Hg2Cl2 → HgCl2 + Hg
The formation of Hg can be used to calculate the number of photons in the light beam, by the technique of actinometry.
By utilizing a light reaction in the presence of mercury(II) chloride and ammonium oxalate, mercury(I) chloride, ammonium chloride and carbon dioxide are produced.
- 2 HgCl2 + (NH4)2C2O4 Light→ Hg2Cl2(s) + 2 + 2 CO2
This particular reaction was discovered by J. M. Eder (hence the name Eder reaction) in 1880 and reinvestigated by W. E. Rosevaere in 1929.
Related mercury(I) compounds
Mercury(I) bromide, Hg2Br2, is light yellow, whereas mercury(I) iodide, Hg2I2, is greenish in colour. Both are poorly soluble. Mercury(I) fluoride is unstable in the absence of a strong acid.
Safety considerations
Main article: Mercury poisoningMercurous chloride is toxic, although due to its low solubility in water it is generally less dangerous than its mercuric chloride counterpart. It was used in medicine as a diuretic and purgative (laxative) in the United States from the late 1700s through the 1860s. Calomel was also a common ingredient in teething powders in Britain up until 1954, causing widespread mercury poisoning in the form of pink disease, which at the time had a mortality rate of 1 in 10. These medicinal uses were later discontinued when the compound's toxicity was discovered.
It has also found uses in cosmetics as soaps and skin lightening creams, but these preparations are now illegal to manufacture or import in many countries including the US, Canada, Japan and the European Union. A study of workers involved in the production of these preparations showed that the sodium salt of 2,3-dimercapto-1-propanesulfonic acid (DMPS) was effective in lowering the body burden of mercury and in decreasing the urinary mercury concentration to normal levels.
References
- John Rumble (June 18, 2018). CRC Handbook of Chemistry and Physics (99 ed.). CRC Press. pp. 5–188. ISBN 978-1138561632.
- ^ Zumdahl, Steven S. (2009). Chemical Principles 6th Ed. Houghton Mifflin Company. p. A22. ISBN 978-0-618-94690-7.
- "Mercury compounds [except (organo) alkyls] (as Hg)". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ Chisholm, Hugh, ed. (1911). "Calomel" . Encyclopædia Britannica (11th ed.). Cambridge University Press.
- Housecroft, C. E.; Sharpe, A. G. (2004). Inorganic Chemistry (2nd ed.). Prentice Hall. pp. 696–697. ISBN 978-0-13-039913-7.
- Skoog, Douglas A.; Holler, F. James; Nieman, Timothy A. (1998). Principles of Instrumental Analysis (5th ed.). Saunders College Pub. pp. 253–271. ISBN 978-0-03-002078-0.
- Buczacki, S., Pests, Diseases and Disorders of Garden Plants, Collins, 1998, pp 449-50. ISBN 0-00-220063-5
- Koehler, Christopher S. W. (January 2001). "Heavy Metal Medicine". Today's Chemist at Work. 10 (1): 61–65. ISSN 1062-094X. Retrieved 2009-02-02.
- Johnston, Elizabeth Lichtenstein (1901). Recollections of a Georgia Loyalist...written in 1836. New York: Mansfield & Company. p. 82. pp. 82-83.
- Inglis-Arkell, Esther. "Archaeologists Tracked Lewis and Clark by Following Their Trail of Laxatives". io9. Retrieved 2018-11-09.
- Wells A.F. (1984) Structural Inorganic Chemistry 5th edition Oxford Science Publications ISBN 0-19-855370-6
- Roseveare, W. E. (1930). "The X-Ray Photochemical Reaction between Potassium Oxalate and Mercuric Chloride". J. Am. Chem. Soc. 52 (7): 2612–2619. doi:10.1021/ja01370a005.
- Sneader, Walter (2005). Drug Discovery: A History. John Wiley and Sons. pp. 45–46. ISBN 978-0-471-89980-8. Retrieved 2009-02-02.
- "Commission Directive 86/199/EEC, OJ L 149, p. 38 of 3.6.1986".
- D. Gonzalez-Ramirez; M. Zuniga-Charles; A. Narro-Juarez; Y. Molina-Recio; K. M. Hurlbut; R. C. Dart; H. V. Aposhian (1 October 1998). "DMPS (2,3-Dimercaptopropane-1-sulfonate, Dimaval) Decreases the Body Burden of Mercury in Humans Exposed to Mercurous Chloride" (free full text). The Journal of Pharmacology and Experimental Therapeutics. 287 (1): 8–12. PMID 9765315.
External links
- International Chemical Safety Card 0984
- National Pollutant Inventory - Mercury and compounds Fact Sheet
- NIOSH Pocket Guide to Chemical Hazards
| Mercury compounds | |||
|---|---|---|---|
| Mercury(I) | |||
| Mercury(II) |
| ||
| Mercury(IV) |
| ||
| Amalgams | |||
| Mercury cations | |||