| Revision as of 12:53, 12 October 2011 editCheMoBot (talk | contribs)Bots141,565 edits Updating {{chembox}} (no changed fields - added verified revid - updated 'DrugBank_Ref', 'UNII_Ref', 'ChEMBL_Ref', 'ChEBI_Ref', 'KEGG_Ref', 'ChEBI_Ref') per Chem/Drugbox validation (report [[Wikipedia_talk:WikiProject_Chemicals|er...← Previous edit | Revision as of 06:16, 23 March 2012 edit undo149.171.184.12 (talk)No edit summaryNext edit → | ||
| Line 43: | Line 43: | ||
| ==GM1 and the cholera toxin== | ==GM1 and the cholera toxin== | ||
| The bacteria ] produces a multimeric toxin called the ]. The A1 subunit of this toxin will gain entry to intestinal epithelial cells with the assistance of the B subunit via the GM1 ganglioside receptor. Once inside, the A1 subunit will ADP ribosylate the ] Alpha subunit which will prevent its ] activity. This will lock it in the active state and it will continuously stimulate adenylate cyclase. The sustained adenylate cyclase activity will lead to a sustained increase of ] which will cause electrolyte and water loss, causing ].{{Citation needed|date=September 2007}} | The bacteria ] produces a multimeric toxin called the ]. The secreted toxin attaches to the surface of the host mucosa cell by binding to GM1 gangliosides. GM1 consists of a sialc acid-containing oligosaccharide covalently attached to a ceramid lipid. The A1 subunit of this toxin will gain entry to intestinal epithelial cells with the assistance of the B subunit via the GM1 ganglioside receptor. Once inside, the A1 subunit will ADP ribosylate the ] Alpha subunit which will prevent its ] activity. This will lock it in the active state and it will continuously stimulate adenylate cyclase. The sustained adenylate cyclase activity will lead to a sustained increase of ] which will cause electrolyte and water loss, causing ].{{Citation needed|date=September 2007}} | ||
| Fortunately, the ] receptor is present in the small intestine. When the cholera patient is given a solution containing water, ] and ], the SGLT1 receptor will reabsorb sodium and glucose, while water will be passively absorbed with the sodium. This will replace the water and ] loss in the cholera induced diarrhea. | Fortunately, the ] receptor is present in the small intestine. When the cholera patient is given a solution containing water, ] and ], the SGLT1 receptor will reabsorb sodium and glucose, while water will be passively absorbed with the sodium. This will replace the water and ] loss in the cholera induced diarrhea. | ||
Revision as of 06:16, 23 March 2012
| This article provides insufficient context for those unfamiliar with the subject. Please help improve the article by providing more context for the reader. (October 2009) (Learn how and when to remove this message) |
 | |
| Names | |
|---|---|
| IUPAC name (2S,4S,5R,6R)-5-acetamido-2-oxyoxan-2-yl]oxy-2--2-(hydroxymethyl)oxan-3-yl]oxy-3-hydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-4-hydroxy-6-oxane-2-carboxylic acid | |
| Other names Monosialotetrahexosylganglioside | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| MeSH | G(M1)+Ganglioside |
| PubChem CID | |
InChI
| |
SMILES
| |
| Properties | |
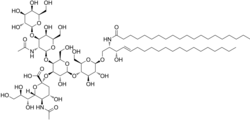
| Chemical formula | C77H139N3O31 |
| Molar mass | 1602.949 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
GM1 (monosialotetrahexosylganglioside) the "prototype" ganglioside, is a member of the ganglio series of gangliosides which contain one sialic acid residue. GM1 has important physiological properties and impacts neuronal plasticity and repair mechanisms, and the release of neurotrophins in the brain. Besides its function in the physiology of the brain, GM1 acts as the site of binding for both Cholera toxin and E. coli heat-labile enterotoxin (Traveller's diarrhea).
Antibodies to GM1 are increased in Guillain-Barré syndrome, dementia and lupus but their function is not clear. There is some evidence to suggest these antibodies are associated with diarrhea in Guillain-Barré syndrome.
GM1 and the cholera toxin
The bacteria Vibrio cholerae produces a multimeric toxin called the cholera toxin. The secreted toxin attaches to the surface of the host mucosa cell by binding to GM1 gangliosides. GM1 consists of a sialc acid-containing oligosaccharide covalently attached to a ceramid lipid. The A1 subunit of this toxin will gain entry to intestinal epithelial cells with the assistance of the B subunit via the GM1 ganglioside receptor. Once inside, the A1 subunit will ADP ribosylate the Gs Alpha subunit which will prevent its GTPase activity. This will lock it in the active state and it will continuously stimulate adenylate cyclase. The sustained adenylate cyclase activity will lead to a sustained increase of cAMP which will cause electrolyte and water loss, causing diarrhea.
Fortunately, the SGLT1 receptor is present in the small intestine. When the cholera patient is given a solution containing water, sodium and glucose, the SGLT1 receptor will reabsorb sodium and glucose, while water will be passively absorbed with the sodium. This will replace the water and electrolyte loss in the cholera induced diarrhea.
Additional images
References
- Mocchetti I (2005). "Exogenous gangliosides, neuronal plasticity and repair, and the neurotrophins". Cell. Mol. Life Sci. 62 (19–20): 2283–94. doi:10.1007/s00018-005-5188-y. PMID 16158191.
- Chen JC, Chang YS, Wu SL; et al. (2007). "Inhibition of Escherichia coli heat-labile enterotoxin-induced diarrhea by Chaenomeles speciosa". J Ethnopharmacol. 113 (2): 233–9. doi:10.1016/j.jep.2007.05.031. PMID 17624704.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Bansal AS, Abdul-Karim B, Malik RA; et al. (1994). "IgM ganglioside GM1 antibodies in patients with autoimmune disease or neuropathy, and controls". J. Clin. Pathol. 47 (4): 300–2. doi:10.1136/jcp.47.4.300. PMC 501930. PMID 8027366.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - Irie S, Saito T, Kanazawa N; et al. (1997). "Relationships between anti-ganglioside antibodies and clinical characteristics of Guillain-Barré syndrome". Intern. Med. 36 (9): 607–12. doi:10.2169/internalmedicine.36.607. PMID 9313102.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link)
| Glycoconjugates, lipids, and glycolipids: sphingolipids and glycosphingolipids, and metabolic intermediates | |
|---|---|
| Simple glycosphingolipids | |
| Globosides | |
| Gangliosides | |
| Other | |
| Autoantigens | |
|---|---|
| Dehydrogenase | |
| Transglutaminase | |
| Nucleoporins | |
| Other | |
This biochemistry article is a stub. You can help Misplaced Pages by expanding it. |
