| Revision as of 19:12, 9 January 2012 editBeetstra (talk | contribs)Edit filter managers, Administrators172,084 edits Saving copy of the {{chembox}} taken from revid 466844283 of page Sulfurous_acid for the Chem/Drugbox validation project (updated: '').← Previous edit | Revision as of 19:13, 9 January 2012 edit undoBeetstra (talk | contribs)Edit filter managers, Administrators172,084 edits Saving copy of the {{chembox}} taken from revid 469384763 of page Sulfur_trioxide for the Chem/Drugbox validation project (updated: 'ChEBI').Next edit → | ||
| Line 1: | Line 1: | ||
| {{ambox | text = This page contains a copy of the infobox ({{tl|chembox}}) taken from revid of page ] with values updated to verified values.}} | {{ambox | text = This page contains a copy of the infobox ({{tl|chembox}}) taken from revid of page ] with values updated to verified values.}} | ||
| {{ |
{{Chembox | ||
| | Verifiedfields = changed | | Verifiedfields = changed | ||
| | Watchedfields = changed | |||
| | verifiedrevid = |
| verifiedrevid = 413343925 | ||
| | Name = Sulfurous acid | |||
| | ImageFileL1 = Sulfur-trioxide-2D-dimensions.svg | |||
| | ImageSizeL1 = 135px | |||
| ⚫ | | |
||
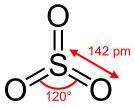
| | ImageNameL1 = Structure and dimensions of sulfur trioxide | |||
| | ImageFile = Sulfurous-acid-2D-pyramidal.png | |||
| | ImageFileR1 = Sulfur-trioxide-3D-vdW.png | |||
| | ImageSize = 150px | |||
| | |
| ImageSizeR1 = 120px | ||
| | ImageNameR1 = Space-filling model of sulfur trioxide | |||
| | ImageFile1 = Sulfurous-acid-3D-balls.png | |||
| | PIN = Sulfur trioxide | |||
| | ImageSize1 = 200px | |||
| | SystematicName = Sulfonylideneoxidane | |||
| | ImageName1 = Ball-and-stick model of sulfurous acid | |||
| | OtherNames = Sulfan{{Verify source|date=September 2010}}<br /> | |||
| | IUPACName = Sulfurous acid | |||
| Sulfuric anhydride | |||
| | Section1 = {{Chembox Identifiers | | Section1 = {{Chembox Identifiers | ||
| | InChI1 = 1/O3S/c1-4(2)3 | |||
| | InChIKey1 = AKEJUJNQAAGONA-UHFFFAOYAX | |||
| ⚫ | | CASNo = 7446-11-9 | ||
| ⚫ | | CASNo_Ref = {{cascite|correct|CAS}} | ||
| | CASNo1 = 3162-58-1 | |||
| | CASNo1_Comment = <br />(monokis(trimethylammoniate)) | |||
| | PubChem = 24682 | |||
| | PubChem_Ref = {{Pubchemcite}} | |||
| | PubChem1 = 22235242 | |||
| | PubChem1_Comment = <br>(hemihydrate) | |||
| | PubChem1_Ref = {{Pubchemcite}} | |||
| | PubChem2 = 22235243 | |||
| | PubChem2_Comment = (monoammoniate) | |||
| | PubChem2_Ref = {{Pubchemcite}} | |||
| | PubChem3 = 23035042 | |||
| | PubChem3_Comment = (monohydrate) | |||
| | PubChem3_Ref = {{Pubchemcite}} | |||
| | PubChem4 = 19427588 | |||
| | PubChem4_Comment = (monoformamate) | |||
| | PubChem4_Ref = {{Pubchemcite}} | |||
| | PubChem5 = 222852 | |||
| | PubChem5_Comment = <br>(monokis(trimethylammoniate)) | |||
| | PubChem5_Ref = {{Pubchemcite}} | |||
| ⚫ | | ChemSpiderID = 23080 | ||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | EINECS = 231-197-3 | |||
| ⚫ | | ChemSpiderID = |
||
| | |
| UNNumber = UN 1829 | ||
| | |
| ChEBI_Ref = {{ebicite|correct|EBI}} | ||
| | |
| ChEBI = 29384 | ||
| | RTECS = WT4830000 | |||
| | KEGG_Ref = {{keggcite|correct|kegg}} | |||
| ⚫ | | StdInChI_Ref = {{stdinchicite|changed|chemspider}} | ||
| | KEGG = C00094 | |||
| | |
| StdInChI = 1S/O3S/c1-4(2)3 | ||
| ⚫ | | StdInChIKey_Ref = {{stdinchicite|changed|chemspider}} | ||
| | InChIKey = LSNNMFCWUKXFEE-UHFFFAOYAJ | |||
| ⚫ | | StdInChIKey = AKEJUJNQAAGONA-UHFFFAOYSA-N | ||
| | ChEBI_Ref = {{ebicite|changed|EBI}} | |||
| | |
| SMILES = O=S(=O)=O | ||
| | |
| InChI = 1S/O3S/c1-4(2)3 | ||
| | InChIKey = AKEJUJNQAAGONA-UHFFFAOYSA-N | |||
| | ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| | |
| Gmelin = 1448}} | ||
| ⚫ | | StdInChI_Ref = {{stdinchicite| |
||
| | StdInChI = 1S/H2O3S/c1-4(2)3/h(H2,1,2,3) | |||
| ⚫ | | StdInChIKey_Ref = {{stdinchicite| |
||
| ⚫ | | StdInChIKey = |
||
| ⚫ | | CASNo = |
||
| | CASNo_Ref = {{cascite|correct|CAS}} | |||
| ⚫ | }} | ||
| | Section2 = {{Chembox Properties | | Section2 = {{Chembox Properties | ||
| | Formula = |
| Formula = SO<sub>3</sub> | ||
| | MolarMass = |
| MolarMass = 80.066 g/mol<!--2009Weight--> | ||
| | |
| Density = 1.92 g/cm<sup>3</sup>, liquid | ||
| | MeltingPt = 16.9 °C, 290.1 K, 62.4 °F | |||
| }} | |||
| | BoilingPtC = 45 | |||
| ⚫ | | Solubility = ] to ] | ||
| ⚫ | }} | ||
| | Section4 = {{Chembox Thermochemistry | |||
| | DeltaHf = −397.77 kJ/mol | |||
| | Entropy = 256.77 J K<sup>−1</sup> mol<sup>−1</sup>}} | |||
| | Section7 = {{Chembox Hazards | | Section7 = {{Chembox Hazards | ||
| | ExternalMSDS = | | ExternalMSDS = {{Dead link|date=May 2010}} | ||
| | EUIndex = 016- |
| EUIndex = 016-019-00-2 | ||
| | EUClass = |
| EUClass = ] ('''ox''') | ||
| | RPhrases = {{ |
| RPhrases = {{R14}}, {{R35}}, {{R37}} | ||
| | SPhrases = {{S1/2 |
| SPhrases = {{S1/2}}, {{S26}}, {{S30}}, {{S45}} | ||
| | NFPA-H = 3 | |||
| | NFPA-F = 0 | |||
| | NFPA-R = 3 | |||
| | NFPA-O = OX | |||
| | FlashPt = Non-flammable | | FlashPt = Non-flammable | ||
| | LD50 = | |||
| | LC50 = rat, 4hr 375 mg/m3 | |||
| | PEL = | |||
| }} | }} | ||
| | Section8 = {{Chembox Related | | Section8 = {{Chembox Related | ||
| | OtherAnions = | |||
| ⚫ | | |
||
| | OtherCations = ]<br />] | |||
| | OtherFunctn = ]<br />] | |||
| | Function = ] ]s | |||
| | OtherCpds = ] | |||
| }} | }} | ||
| }} | }} | ||
Revision as of 19:13, 9 January 2012
| This page contains a copy of the infobox ({{chembox}}) taken from revid 469384763 of page Sulfur_trioxide with values updated to verified values. |
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name Sulfur trioxide | |||
| Systematic IUPAC name Sulfonylideneoxidane | |||
| Other names
Sulfan Sulfuric anhydride | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChEBI | |||
| ChemSpider | |||
| EC Number |
| ||
| Gmelin Reference | 1448 | ||
| PubChem CID | |||
| RTECS number |
| ||
| UN number | UN 1829 | ||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | SO3 | ||
| Molar mass | 80.066 g/mol | ||
| Density | 1.92 g/cm, liquid | ||
| Melting point | 16.9 °C, 290.1 K, 62.4 °F | ||
| Boiling point | 45 °C (113 °F; 318 K) | ||
| Solubility in water | hydrolyses to Sulfuric Acid | ||
| Thermochemistry | |||
| Std molar entropy (S298) |
256.77 J K mol | ||
| Std enthalpy of formation (ΔfH298) |
−397.77 kJ/mol | ||
| Hazards | |||
| NFPA 704 (fire diamond) |
 | ||
| Flash point | Non-flammable | ||
| Lethal dose or concentration (LD, LC): | |||
| LC50 (median concentration) | rat, 4hr 375 mg/m3 | ||
| Related compounds | |||
| Other cations | Selenium trioxide Tellurium trioxide | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Chemical compound