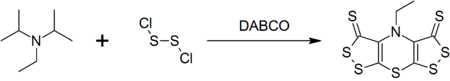| Revision as of 07:32, 25 March 2012 editPlasmic Physics (talk | contribs)Extended confirmed users, Rollbackers19,174 editsNo edit summary← Previous edit | Revision as of 08:20, 25 March 2012 edit undoPlasmic Physics (talk | contribs)Extended confirmed users, Rollbackers19,174 editsNo edit summaryNext edit → | ||
| Line 31: | Line 31: | ||
| | ExactMass = 129.151749613 g mol<sup>−1</sup> | | ExactMass = 129.151749613 g mol<sup>−1</sup> | ||
| | Appearance = Colourless, transparent liquid | | Appearance = Colourless, transparent liquid | ||
| | Odor = Ichtyal, ammoniacal | |||
| | Density = 742 mg mL<sup>−1</sup> | | Density = 742 mg mL<sup>−1</sup> | ||
| | MeltingPtCL = -273.15 | | MeltingPtCL = -273.15 | ||
Revision as of 08:20, 25 March 2012
 | |
| Names | |
|---|---|
| Other names Ethyldiisopropylamine | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.027.629 |
| EC Number |
|
| MeSH | N,N-diisopropylethylamine |
| PubChem CID | |
| UN number | 2733 |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C8H19N |
| Molar mass | 129.247 g·mol |
| Appearance | Colourless, transparent liquid |
| Odor | Ichtyal, ammoniacal |
| Density | 742 mg mL |
| Boiling point | 126.6 °C; 259.8 °F; 399.7 K |
| Vapor pressure | 4.1 kPa (at 37.70 °C) |
| Refractive index (nD) | 1.414 |
| Hazards | |
| GHS labelling: | |
| Pictograms |   
|
| Signal word | Danger |
| Hazard statements | H225, H301, H314, H412 |
| Precautionary statements | P210, P273, P280, P301+P310, P305+P351+P338, P310 |
| Flash point | 10 °C |
| Explosive limits | 0.7–6.3% |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) | 200–500 mg kg (oral, rat) |
| Related compounds | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
N,N-Diisopropylethylamine, or Hünig's base, DIPEA or DIEA, is an organic compound and an amine. It is used in organic chemistry as a base. Because the nitrogen atom is shielded by the two isopropyl groups and an ethyl group only a proton is small enough to easily fit. Just like 2,2,6,6-tetramethylpiperidine, this compound is a good base but a poor nucleophile, which makes it a useful organic reagent. Hünig's base is named after the German chemist Siegfried Hünig.
Preparation
Hünig's base is commercially available. It is traditionally prepared by the reaction of diisopropylamine with diethyl sulfate.
Reactions
Non-nucleophilic base
Hünig's base was investigated for its use as a selective reagent in the alkylation of secondary amines to tertiary amines by alkyl halides. This organic reaction is often hampered by a quaternization reaction to the quaternary ammonium salt but this side-reaction is absent when Hünig's base is present.
Synthesis of scorpionine
Hünig's base forms a complex heterocyclic compound called scorpionine by a reaction with disulfur dichloride catalyzed by DABCO in a remarkable one-pot synthesis.
References
- Hünig, S. (1958). "Spezifische Protonenacceptoren als Hilfsbasen bei Alkylierungs- und Dehydrohalogenierungsreaktionen". Chemische Berichte. 91 (2): 380–392. doi:10.1002/cber.19580910223.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - An efficient and operationally convenient general synthesis of tertiary amines by direct alkylation of secondary amines with alkyl halides in the presence of Huenig’s base Jason L. Moore, Stephen M. Taylor, and Vadim A. Soloshonok Arkivoc (EJ-1549C) pp 287-292 2005 Online Article.
- From Hünig's Base to Bis(dithiolo)-thiazines in One Pot: The Fast Route to Highly Sulfurated Heterocycles W. Rees, Carlos F. Marcos,Cecilia Polo, Tomás Torroba,Oleg A. Rakitin Angewandte Chemie International Edition Volume 36, Issue 3 , Pages 281 - 283 2003 Abstract.