| Revision as of 10:21, 29 January 2013 editYobot (talk | contribs)Bots4,733,870 editsm →External links: clean up / fixed sortkey (checkwiki error #37 and #6) using AWB (8873)← Previous edit | Revision as of 08:17, 21 July 2013 edit undoVaccinationist (talk | contribs)Extended confirmed users4,736 editsmNo edit summaryNext edit → | ||
| Line 1: | Line 1: | ||
| {{Drugbox | {{Drugbox | ||
| | verifiedrevid = 396294114 | | verifiedrevid = 396294114 | ||
| | image = Amlodipine. |
| image = Amlodipine.svg | ||
| | |
| width = 185 | ||
| | image2 = Atorvastatin.svg | |||
| | width2 = 250 | |||
| <!--Combo data--> | <!--Combo data--> | ||
| Line 41: | Line 43: | ||
| | StdInChIKey = PEAJXAZCGDWDGS-CNZCJKERSA-N | | StdInChIKey = PEAJXAZCGDWDGS-CNZCJKERSA-N | ||
| }} | }} | ||
| The drug combination '''atorvastatin/amlodipine''' (trade names '''Caduet''' in the ] and ], and '''Envacar''' elsewhere) is a medication approved by the ] (FDA) for the treatment of ] and ]. It is a ] drug containing the ] ] and the ] ] being marketed by the ] ].<ref>{{cite web|url=http://www.fda.gov/Safety/MedWatch/SafetyInformation/ucm202933.htm|format=PDF|title=CADUET (amlodipine besylate/atorvastatin calcium) Tablets|month=February | year=2010|work=NDA 21-540/S-009|publisher=]|accessdate=2010-09-25}}</ref> | The drug combination '''atorvastatin/amlodipine''' (trade names '''Caduet''' in the ], ] and ], and '''Envacar''' elsewhere) is a medication approved by the ] (FDA) for the treatment of ] and ]. It is a ] drug containing the ] ] and the ] ] being marketed by the ] ].<ref>{{cite web|url=http://www.fda.gov/Safety/MedWatch/SafetyInformation/ucm202933.htm|format=PDF|title=CADUET (amlodipine besylate/atorvastatin calcium) Tablets|month=February | year=2010|work=NDA 21-540/S-009|publisher=]|accessdate=2010-09-25}}</ref> | ||
| == References == | == References == | ||
| Line 53: | Line 55: | ||
| ] | ] | ||
| ] | ] | ||
| {{cardiovascular-drug-stub}} | {{cardiovascular-drug-stub}} | ||
Revision as of 08:17, 21 July 2013
Pharmaceutical compound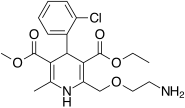 | |
 | |
| Combination of | |
|---|---|
| Amlodipine | Calcium channel blocker |
| Atorvastatin | Statin |
| Clinical data | |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
The drug combination atorvastatin/amlodipine (trade names Caduet in the US, Australia and Russia, and Envacar elsewhere) is a medication approved by the Food and Drug Administration (FDA) for the treatment of high cholesterol and high blood pressure. It is a fixed-dose combination drug containing the calcium channel blocker amlodipine and the statin atorvastatin being marketed by the pharmaceutical company Pfizer.
References
- "CADUET (amlodipine besylate/atorvastatin calcium) Tablets" (PDF). NDA 21-540/S-009. United States Food and Drug Administration. 2010. Retrieved 2010-09-25.
{{cite web}}: Unknown parameter|month=ignored (help)
External links
- Manufacturer's site (caduet.com)
This drug article relating to the cardiovascular system is a stub. You can help Misplaced Pages by expanding it. |