| Revision as of 14:48, 12 June 2020 editFswitzer4 (talk | contribs)Extended confirmed users11,001 editsm Added FDA UNII← Previous edit | Latest revision as of 09:27, 25 June 2022 edit undoBrownHairedGirl (talk | contribs)Autopatrolled, Extended confirmed users, File movers, Pending changes reviewers, Rollbackers2,942,733 edits Cleanup 1 reference: convert <ref>URL {{webarchive}}</ref> to <ref>{{cite web}}</ref>. NB: title etc is missing, and will display error msgTag: AWB | ||
| Line 38: | Line 38: | ||
| }} | }} | ||
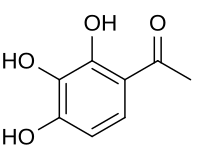
| '''Gallacetophenone''' is the ] derivative of ]. It can be synthesized from pyrogallol using ] and ].<ref>http://talkchem.com/synthetic-chemistry/gallacetophenone-synthesis-synthesis-of-gallacetophenone.html |
'''Gallacetophenone''' is the ] derivative of ]. It can be synthesized from pyrogallol using ] and ].<ref>{{cite web |url=http://talkchem.com/synthetic-chemistry/gallacetophenone-synthesis-synthesis-of-gallacetophenone.html |title= |website=talkchem.com |archive-url=https://web.archive.org/web/20100116013814/http://talkchem.com/synthetic-chemistry/gallacetophenone-synthesis-synthesis-of-gallacetophenone.html |archive-date=January 16, 2010}}</ref> | ||
| ==References== | ==References== | ||
| {{reflist}} | {{reflist}} | ||
| ] | ] | ||
| ] | ] | ||
| {{Ketone-stub}} | {{Ketone-stub}} | ||
Latest revision as of 09:27, 25 June 2022
 | |
| Names | |
|---|---|
| Preferred IUPAC name 1-(2,3,4-Trihydroxyphenyl)ethan-1-one | |
| Other names
1-(2,3,4-Trihydroxyphenyl)ethanone Alizarin Yellow C Galloacetophenone 2',3',4'-Trihydroxyacetophenone | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.007.665 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C8H8O4 |
| Molar mass | 168.148 g·mol |
| Melting point | 171 to 172 °C (340 to 342 °F; 444 to 445 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Gallacetophenone is the acetyl derivative of pyrogallol. It can be synthesized from pyrogallol using zinc chloride and acetic anhydride.
References
- talkchem.com https://web.archive.org/web/20100116013814/http://talkchem.com/synthetic-chemistry/gallacetophenone-synthesis-synthesis-of-gallacetophenone.html. Archived from the original on January 16, 2010.
{{cite web}}: Missing or empty|title=(help)
This article about a ketone is a stub. You can help Misplaced Pages by expanding it. |