| Revision as of 13:36, 4 July 2024 editMichael D. Turnbull (talk | contribs)Extended confirmed users13,874 edits Tidy up per MOS:CHEM← Previous edit | Revision as of 13:40, 4 July 2024 edit undoMichael D. Turnbull (talk | contribs)Extended confirmed users13,874 edits Add 1947 cite to same methodNext edit → | ||
| Line 47: | Line 47: | ||
| ==Synthesis== | ==Synthesis== | ||
| :] | :] | ||
| The ] group of ] (1) is reduced by palladium-catalysed ] to an ], which cyclises to give a mixture of the ] ester (2) and its corresponding acid (3). The mixture is ] using ] to convert all of the ester to the acid, and this material is cyclised to give rolziracetam using ].<ref>{{cite patent |country=US |number=4663464 |inventor=Marvin S. Hoekstra |title=Process for the preparation of dihydro-1H-pyrrolizine-3,5-(2H,6H)-dione |status=patent |gdate=1987-05-05 |fdate=1986-03-21 |pridate=1986-03-21 |assign1=Warner Lambert Co LLC}}</ref> | The ] group of ] (1) is reduced by palladium-catalysed ] to an ], which cyclises to give a mixture of the ] ester (2) and its corresponding acid (3). The mixture is ] using ] to convert all of the ester to the acid, and this material is cyclised to give rolziracetam using ].<ref>{{cite journal |doi=10.1021/ja01195a067 |title=The Synthesis of Pyrrolizidines<sup>1</sup> |date=1947 |last1=Leonard |first1=Nelson J. |last2=Hruda |first2=Lillian Ruth |last3=Long |first3=Frank W. |journal=Journal of the American Chemical Society |volume=69 |issue=3 |pages=690–692 |pmid=20289459 }}</ref><ref>{{cite patent |country=US |number=4663464 |inventor=Marvin S. Hoekstra |title=Process for the preparation of dihydro-1H-pyrrolizine-3,5-(2H,6H)-dione |status=patent |gdate=1987-05-05 |fdate=1986-03-21 |pridate=1986-03-21 |assign1=Warner Lambert Co LLC}}</ref> | ||
| ==See also== | ==See also== | ||
Revision as of 13:40, 4 July 2024
Chemical compound Pharmaceutical compound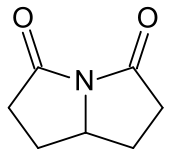 | |
 | |
| Clinical data | |
|---|---|
| Other names | 2,6,7,8-tetrahydro-1H-pyrrolizine-3,5-dione, CI 911 & Lukes-Šorm dilactam. |
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C7H9N2O2 |
| Molar mass | 153.161 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (what is this?) (verify) | |
Rolziracetam is a nootropic drug of the racetam family.
Rolziracetam was found to improve performance on a delayed-response task in aged rhesus monkeys. It has a wide margin of safety in animals and has been evaluated for use in cognitively impaired human subjects.
Synthesis
The nitro group of dimethyl 4-nitropimelate (1) is reduced by palladium-catalysed hydrogenation to an amino group, which cyclises to give a mixture of the lactam ester (2) and its corresponding acid (3). The mixture is hydrolysed using sodium hydroxide to convert all of the ester to the acid, and this material is cyclised to give rolziracetam using acetic anhydride.
See also
References
- Butler DE, Leonard JD, Caprathe BW, L'Italien YJ, Pavia MR, Hershenson FM, Poschel PH, Marriott JG (March 1987). "Amnesia-reversal activity of a series of cyclic imides". Journal of Medicinal Chemistry. 30 (3): 498–503. doi:10.1021/jm00386a010. PMID 3820221.
- Leonard, Nelson J.; Hruda, Lillian Ruth; Long, Frank W. (1947). "The Synthesis of Pyrrolizidines". Journal of the American Chemical Society. 69 (3): 690–692. doi:10.1021/ja01195a067. PMID 20289459.
- US patent 4663464, Marvin S. Hoekstra, "Process for the preparation of dihydro-1H-pyrrolizine-3,5-(2H,6H)-dione", issued 1987-05-05, assigned to Warner Lambert Co LLC
| Racetams | |
|---|---|
| Racetams |
|
| Phenylpiracetams |
|
| Racetam-like |
|
This drug article relating to the nervous system is a stub. You can help Misplaced Pages by expanding it. |
