| Revision as of 02:31, 11 September 2011 editSquids and Chips (talk | contribs)Extended confirmed users, Pending changes reviewers37,148 editsm WPCleaner (v1.09) Repaired link to disambiguation page - (You can help) - Convex← Previous edit | Revision as of 02:41, 11 September 2011 edit undoCheMoBot (talk | contribs)Bots141,565 edits Updating {{chembox}} (no changed fields - added verified revid - updated 'DrugBank_Ref', 'UNII_Ref', 'ChEMBL_Ref') per Chem/Drugbox validation (report errors or bugs)Next edit → | ||
| Line 1: | Line 1: | ||
| {{chembox | {{chembox | ||
| | verifiedrevid = |
| verifiedrevid = 447509288 | ||
| | ImageFile = Corannulene.svg | | ImageFile = Corannulene.svg | ||
| | ImageSize = 150px | | ImageSize = 150px | ||
Revision as of 02:41, 11 September 2011
 | |
 | |
| Names | |
|---|---|
| IUPAC name Dibenzofluoranthene | |
| Other names circulene | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C20H10 |
| Molar mass | 250.29 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Corannulene is a polycyclic aromatic hydrocarbon with chemical formula C20H10. The molecule consists of a cyclopentane ring fused with 5 benzene rings, so another name for it is circulene. It is of scientific interest because it is a geodesic polyarene and can be considered a fragment of buckminsterfullerene. Due to this connection and also its bowl shape, corannulene is also known as a buckybowl. Corannulene exhibits a bowl-to-bowl inversion with an inversion barrier of 10.2 kcal/mol (42.7 kJ/mol) at −64 °C.
Synthesis
Several synthetic routes exist to corannulene. Flash vacuum pyrolysis techniques generally have lower chemical yields than solution-chemistry syntheses, but offer routes to more derivatives. Corannulane was first isolated in 1966 by multistep organic synthesis. A flash vacuum pyrolysis method followed in 1991. One synthesis based on solution chemistry consists of a nucleophilic displacement–elimination reaction of an octabromide with potassium hydroxide:
The bromine substituents are removed with an excess of n-butyllithium.
Much effort is directed at functionalization of the corannulene ring with novel functional groups such as ethynyl groups, ether groups, thioether groups, platinum function groups, aryl groups, phenalenyl fused and indeno extensions.
Aromaticity
The observed aromaticity for this compound is explained with a so-called annulene-within-an-annulene model. According to this model corannulene is made up of an aromatic 6 electron cyclopentadienyl anion surrounded by an aromatic 14 electron annulenyl cation. This model was suggested by Barth and Lawton in the first synthesis of corannulene in 1966. They also suggested the trivial name 'corannulene', which is derived from the annulene-within-an-annulene model: core + annulene.
However, later theoretical calculations have disputed the validity of this approximation.
Corannulene anions
Corannulene can be reduced up to a tetraanion in a series of one-electron reductions. This has been performed with alkali metals, electrochemically and with bases. The corannulene dianion is antiaromatic and tetraanion is again aromatic. With lithium as reducing agent two tetraanions form a supramolecular dimer with two bowls stacked into each other with 4 lithium ions in between and 2 pairs above and below the stack.. This self-assembly motif was applied in the organization of fullerenes. Penta-substituted fullerenes (with methyl or phenyl groups) charged with five electrons form supramolecular dimers with a complementary corannulene tetraanion bowl, 'stitched' by interstitial lithium cations. In a related system 5 lithium ions are sandwiched between two corannulene bowls
In one cyclopentacorannulene a concave - concave aggregate is observed by NMR spectroscopy with 2 C–Li–C bonds connecting the tetraanions.
Metals tend to bind to the convex face of the annulene. Concave binding has been reported for a cesium / crown ether system
Corannulene radicals
Corannulene-based free radicals have also been synthesised and studied. A semiquinone radical anion obtained by reduction of the diketone by a sodium mirror (see below) has been reported stable in the solid state
In this radical anion spin density is delocalized with a significant contribution from the central cyclopentadienyl radical.
Corannulene carbocations
Corannulene can react with electrophiles to form a corannulene carbocation. Reaction with chloromethane and aluminium chloride results in the formation of an AlCl4 salt with a methyl group situated at the center with the cationic center at the rim. X-ray diffraction analysis shows the that the new carbon-carbon bond is elongated (157 pm)
Bicorannulenyl
Bicorannulenyl is the corannulene dimer, in which two corannulene units are connected through a single bond. The molecule's stereochemistry consists of two chiral elements: the asymmetry of a singly substituted corannulenyl, and the helical twist about the central bond. In the neutral state, bicorannulenyl exists as 12 conformers, which intercovert through multiple bowl-inversions and bond-rotations. When bicorannulenyl is reduced to a dianion with potassium metal, the central bond assumes significant double-bond character. This is due to its orbital structure, which has a LUMO orbital localized on the central bond. When bicorannulenyl is reduced to an octaanion with lithium metal, it self-assembles into supramolecular oligomers. This is based on the "charged polyarene stacking" self-assembly motif.
Applications
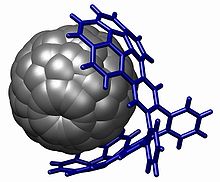
The corannulene group is used in host-guest chemistry with interactions based on pi stacking , notably with fullerenes (the buckycatcher) but also with nitrobenzene
With long aliphatic side chains corannulenes are reported forming a thermotropic hexagonal columnar liquid crystalline mesophase. Corannulenes have also been used as the core group in a dendrimer or as coordinating ligand to metals. Corannulenes with ethynyl groups are investigated for their potential use as blue emitters.
In space
Efforts to detect corannulene in space have thus far failed.
See also
References
- Template:Cite DOI
- ^ Template:Cite DOI
- ^ Template:Cite DOI
- Template:Cite DOI
- Template:Cite DOI
- Template:Cite DOI
- ^ Template:Cite DOI
- Template:Cite DOI
- Template:Cite DOI
- Template:Cite DOI
- ^ Template:Cite DOI
- Template:Cite DOI
- Template:Cite DOI
- Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1016/0166-1280(94)03961-J, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1016/0166-1280(94)03961-Jinstead. - Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1021/jp8038779, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1021/jp8038779instead. - Template:Cite DOI
- Template:Cite DOI
- A Main Group Metal Sandwich: Five Lithium Cations Jammed Between Two Corannulene Tetraanion Decks Zabula, et al. Science 19 August 2011: 1008-1011. doi:10.1126/science.1208686
- Template:Cite DOI
- Spisak, S. N., Zabula, A. V., Filatov, A. S., Rogachev, A. Y. and Petrukhina, M. A. (2011), Selective Endo and Exo Binding of Alkali Metals to Corannulene. Angewandte Chemie International Edition, 50: 8090–8094. doi:10.1002/anie.201103028
- The First Bowl-Shaped Stable Neutral Radical with a Corannulene System: Synthesis and Characterization of the Electronic Structure Yasushi Morita, Shinsuke Nishida, Tadahiro Kobayashi, Kozo Fukui, Kazunobu Sato, Daisuke Shiomi, Takeji Takui, Kazuhiro Nakasuji Organic Letters 2004 6 (9), 1397-1400 doi:10.1021/ol0497786
- Template:Cite DOI
- Template:Cite DOI
- Ueda, A., Ogasawara, K., Nishida, S., Ise, T., Yoshino, T., Nakazawa, S., Sato, K., Takui, T., Nakasuji, K. and Morita, Y. (2010), A Bowl-Shaped ortho-Semiquinone Radical Anion: Quantitative Evaluation of the Dynamic Behavior of Structural and Electronic Features. Angewandte Chemie International Edition, 49: 6333–6337. doi:10.1002/anie.201002626
- Zabula, A. V., Spisak, S. N., Filatov, A. S., Rogachev, A. Y. and Petrukhina, M. A. (2011), A Strain-Releasing Trap for Highly Reactive Electrophiles: Structural Characterization of Bowl-Shaped Arenium Carbocations. Angewandte Chemie International Edition, 50: 2971–2974. doi:10.1002/anie.201007762
- Template:Cite DOI
- Eisenberg, D., Quimby, J. M., Jackson, E. A., Scott, L. T. and Shenhar, R. (2010), The Bicorannulenyl Dianion: A Charged Overcrowded Ethylene. Angewandte Chemie International Edition, 49: 7538–7542. doi:10.1002/anie.201002515
- Eisenberg, D., Quimby, J. M., Jackson, E. A., Scott, L. T. and Shenhar, R. (2010), Highly Charged Supramolecular Oligomers Based on the Dimerization of Corannulene Tetraanion. Chemical Communications, 46: 9010–9012. doi:10.1039/c0cc03965a
- Template:Cite DOI
- Template:Cite DOI
- Template:Cite DOI
- Template:Cite DOI
- Hexahapto Metal Coordination to Curved Polyaromatic Hydrocarbon Surfaces: The First Transition Metal Corannulene Complex T. Jon Seiders, Kim K. Baldridge, Joseph M. O'Connor, and Jay S. Siegel J. Am. Chem. Soc., 1997, 119 (20), pp 4781–4782 doi:10.1021/ja964380t
- d8 Rhodium and Iridium Complexes of Corannulene Jay S. Siegel, Kim K. Baldridge, Anthony Linden, and Reto Dorta J. Am. Chem. Soc., 2006, 128 (33), pp 10644–10645 doi:10.1021/ja062110x
- Template:Cite DOI
- Template:Cite DOI
- Template:Cite DOI
- Template:Cite DOI
- Bandera, D., Baldridge, K. K., Linden, A., Dorta, R. and Siegel, J. S. (2011), Stereoselective Coordination of C5-Symmetric Corannulene Derivatives with an Enantiomerically Pure Metal Complex. Angewandte Chemie International Edition, 50: 865–867. doi: 10.1002/anie.201006877
- Template:Cite DOI
- Template:Cite DOI
| Polycyclic aromatic hydrocarbons | |
|---|---|
| 2 rings | |
| 3 rings | |
| 4 rings | |
| 5 rings | |
| 6 rings | |
| 7+ rings | |
| General classes | |