| Revision as of 23:39, 28 December 2011 editRich Farmbrough (talk | contribs)Edit filter managers, Autopatrolled, Extended confirmed users, File movers, Pending changes reviewers, Rollbackers, Template editors1,726,081 editsm →References: Fix spelling Encyclopædia Britannica or similar; using AWB← Previous edit | Revision as of 09:19, 5 February 2012 edit undoNotWith (talk | contribs)61,905 edits Fungal infection in animalsNext edit → | ||
| Line 53: | Line 53: | ||
| Acriflavine was developed in 1912 by ], a German medical researcher and was used during the ] against ]. It is derived from ]. The ] form is more irritating than the neutral form. | Acriflavine was developed in 1912 by ], a German medical researcher and was used during the ] against ]. It is derived from ]. The ] form is more irritating than the neutral form. | ||
| Acriflavine is also used as treatment for external ]s of aquarium fish. In recent years Acriflavine has been shown to have anti-cancer activity. | Acriflavine is also used as treatment for external ]s of aquarium fish. In recent years Acriflavine has been shown to have anti-cancer activity. | ||
| == References == | == References == | ||
Revision as of 09:19, 5 February 2012
 | |
| Names | |
|---|---|
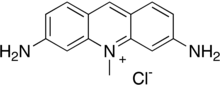
| IUPAC name 3,6-Diamino-10-methylacridin-10-ium chloride | |
| Identifiers | |
| CAS Number |
|
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.211.047 |
| EC Number |
|
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C14H14ClN3 |
| Molar mass | 259.74 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Acriflavine is a topical antiseptic. It has the form of an orange or brown powder. It may be harmful in the eyes or if inhaled. It is a dye and it stains the skin and may irritate. Commercial preparations are often mixtures with proflavine. It is known by a variety of commercial names.
Acriflavine was developed in 1912 by Paul Ehrlich, a German medical researcher and was used during the First World War against sleeping sickness. It is derived from acridine. The hydrochloride form is more irritating than the neutral form.
Acriflavine is also used as treatment for external fungal infections of aquarium fish. In recent years Acriflavine has been shown to have anti-cancer activity.
References
- Encyclopædia Britannica (accessed 2005-08-16)
- ChemExper Chemical Directory (accessed 2005-08-16)
- Houghton Mifflin definition (accessed 2005-08-16)