This is an old revision of this page, as edited by Kupirijo (talk | contribs) at 21:13, 20 April 2011 (|OtherNames= Orotidylic acid,<br>OMP). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 21:13, 20 April 2011 by Kupirijo (talk | contribs) (|OtherNames= Orotidylic acid,<br>OMP)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)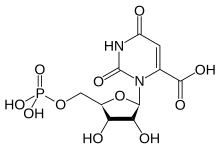 | |
| Names | |
|---|---|
| IUPAC name 3--2,6-dioxo-pyrimidine-4-carboxylic acid | |
| Other names
Orotidylic acid, OMP | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| MeSH | Orotidine+5'-monophosphate |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
SMILES
| |
| Properties | |
| Chemical formula | C10H13N2O11P |
| Molar mass | 368.191 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Orotidine 5'-monophosphate (OMP), also known as orotidylic acid, is a pyrimidine nucleotide which is the last intermediate in the biosynthesis of uridine monophosphate. OMP is formed from orotate and phosphoribosyl pyrophosphate by the enzyme Orotate phosphoribosyltransferase
In humans, the enzyme UMP synthase converts OMP into uridine 5'- monophosphate. If UMP synthase is defective, orotic aciduria can result.
| Nucleotide metabolic intermediates | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| purine metabolism |
| ||||||||||
| pyrimidine metabolism |
| ||||||||||
This biochemistry article is a stub. You can help Misplaced Pages by expanding it. |