This is an old revision of this page, as edited by Citation bot 1 (talk | contribs) at 07:31, 21 October 2011 (Add: issue. You can use this bot yourself. Report bugs here.). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 07:31, 21 October 2011 by Citation bot 1 (talk | contribs) (Add: issue. You can use this bot yourself. Report bugs here.)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff) | |
| Names | |
|---|---|
| IUPAC name Boron carbide | |
| Other names Tetrabor | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.031.907 |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | B4C |
| Molar mass | 55.255 g/mol |
| Appearance | dark gray or black powder, odorless |
| Density | 2.52 g/cm, solid. |
| Melting point | 2,763 °C (5,005 °F; 3,036 K) |
| Boiling point | 3,500 °C (6,330 °F; 3,770 K) |
| Solubility in water | insoluble |
| Acidity (pKa) | 6–7 (20 °C) |
| Structure | |
| Crystal structure | Rhombohedral |
| Related compounds | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Boron carbide (chemical formula approximately B4C) is an extremely hard boron-carbon ceramic material used in tank armor, bulletproof vests, and numerous industrial applications. With a Mohs hardness of above 9, it is one of the hardest materials known, behind cubic boron nitride and diamond.
Boron carbide was discovered in the 19th century as a by-product of reactions involving metal borides, however, its chemical formula was unknown. It was not until the 1930s that the chemical composition was estimated as B4C. There remained, however, controversy as to whether or not the material had this exact 4:1 stoichiometry, as in practice the material is always slightly carbon-deficient with regard to this formula, and X-ray crystallography shows that its structure is highly complex, with a mixture of C-B-C chains and B12 icosahedra. These features argued against a very simple exact B4C empirical formula. Because of the B12 structural unit, the chemical formula of "ideal" boron carbide is often written not as B4C, but as B12C3, and the carbon deficiency of boron carbide described in terms of a combination of the B12C3 and B12C2 units.
The ability of boron carbide to absorb neutrons without forming long lived radionuclides makes it attractive as an absorbent for neutron radiation arising in nuclear power plants. Nuclear applications of boron carbide include shielding, control rod and shut down pellets. Within control rods, boron carbide is often powdered, to increase its surface area.
Crystal structure
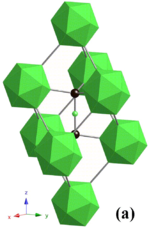

Boron carbide has a complex crystal structure typical of icosahedron-based borides. There, B12 icosahedra form a rhombohedral lattice unit (space group: R3m (No. 166), lattice constants: a = 0.56 nm and c = 1.212 nm) surrounding a C-B-C chain that resides at the center of the unit cell, and both carbon atoms bridge the neighboring three icosahedra. This structure is layered: the B12 icosahedra and bridging carbons form a network plane that spreads parallel to the c-plane and stacks along the c-axis. The lattice has two basic structure units – the B12 icosahedron and the B6 octahedron. Because of the small size of the B6 octahedra, they cannot interconnect. Instead, they bond to the B12 icosahedra in the neighboring layer, and this decreases bonding strength in the c-plane.
Because of the B12 structural unit, the chemical formula of "ideal" boron carbide is often written not as B4C, but as B12C3, and the carbon deficiency of boron carbide described in terms of a combination of the B12C3 and B12C2 units.
Properties
Boron carbide is known as a robust material having high hardness, high cross section for absorption of neutrons (i.e. good shielding properties against neutrons), stability to ionizing radiation and most chemicals. Its Vickers hardness (38 GPa) and fracture toughness (3.5 MPa·m) approach the corresponding values for diamond (115 GPa and 5.3 MPa·m).
Preparation
Boron carbide was first synthesized by Henri Moissan in 1899, by reduction of boron trioxide either with carbon or magnesium in presence of carbon in an electric arc furnace. In the case of carbon, the reaction occurs at temperatures above the melting point of B4C and is accompanied by liberation of large amount of carbon monoxide:
- 2 B2O3 + 7 C → B4C + 6 CO
If magnesium is used, the reaction can be carried out in a graphite furnace, and the magnesium byproducts are removed by treatment with acid.
Uses
- Padlocks
- Personal and vehicle anti-ballistic armor plating.
- Grit blasting nozzles.
- High-pressure water jet cutter nozzles.
- Scratch and wear resistant coatings.
- Cutting tools and dies.
- Abrasives.
- Neutron absorber in nuclear reactors.
- Metal matrix composites.
- High energy fuel for solid fuel Ramjets.
References
- Ridgway, Ramond R "Boron Carbide", European Patent CA339873 (A), publication date: 1934-03-06
- ^ Musiri M. Balakrishnarajan, Pattath D. Pancharatna and Roald Hoffmann (2007). "Structure and bonding in boron carbide: The invincibility of imperfections". New J. Chem. 31 (4): 473. doi:10.1039/b618493f.
- ^ Weimer, p. 330
- ^ Zhang F X, Xu F F, Mori T, Liu Q L, Sato A and Tanaka T (2001). "Crystal structure of new rare-earth boron-rich solids: REB28.5C4". J. Alloys Compd. 329: 168. doi:10.1016/S0925-8388(01)01581-X.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 149. ISBN 978-0-08-037941-8.
- Solozhenko, V. L.; Kurakevych, Oleksandr O.; Le Godec, Yann; Mezouar, Mohamed; Mezouar, Mohamed (2009). "Ultimate Metastable Solubility of Boron in Diamond: Synthesis of Superhard Diamondlike BC5". Phys. Rev. Lett. 102 (1): 015506. Bibcode:2009PhRvL.102a5506S. doi:10.1103/PhysRevLett.102.015506. PMID 19257210.
- Weimer, p. 131
- Pradyot Patnaik. Handbook of Inorganic Chemicals. McGraw-Hill, 2002, ISBN 0070494398
Bibliography
- Alan W. Weimer (1997). Carbide, Nitride and Boride Materials Synthesis and Processing. Chapman & Hall (London, New York). ISBN 0-412-54060-6.
External links
| Boron compounds | |
|---|---|
| Boron pnictogenides | |
| Boron halides | |
| Acids | |
| Boranes | |
| Boron oxides and sulfides | |
| Carbides | |
| Organoboron compounds | |