This is an old revision of this page, as edited by 207.118.29.95 (talk) at 19:14, 13 October 2011 (Removed blatant advertisement.). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 19:14, 13 October 2011 by 207.118.29.95 (talk) (Removed blatant advertisement.)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)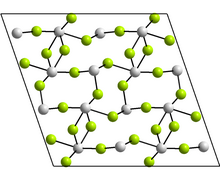 | |
| Names | |
|---|---|
| IUPAC name Tin(II) fluoride | |
| Other names Stannous fluoride | |
| Identifiers | |
| CAS Number | |
| ECHA InfoCard | 100.029.090 |
| RTECS number |
|
| UN number | 3288 |
| CompTox Dashboard (EPA) | |
| Properties | |
| Chemical formula | SnF2 |
| Molar mass | 156.69 g/mol |
| Appearance | colorless solid |
| Density | 4.57 g/cm |
| Melting point | 215 °C |
| Boiling point | 850 °C |
| Solubility in water | ca. 350 g/L (20 °C) |
| Structure | |
| Crystal structure | Monoclinic, mS48 |
| Space group | C2/c, No. 15 |
| Hazards | |
| NFPA 704 (fire diamond) |
 |
| Flash point | Non-flammable |
| Related compounds | |
| Other anions | Tin(II) chloride Tin(II) bromide Tin(II) iodide |
| Other cations | Germanium tetrafluoride Tin tetrafluoride Lead(II) fluoride |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Tin(II) fluoride, known by the common name stannous fluoride, is a chemical compound with the formula SnF2. It is a colorless solid used as an ingredient in toothpastes that are typically more expensive than those that use sodium fluoride. Stannous fluoride converts the calcium mineral apatite into fluorapatite, which makes tooth enamel more resistant to bacteria generated acid attacks. Sodium fluoride and sodium fluorophosphate, on the other hand, become biologically inactive when combined with calcium. Used in combination with calcium minerals, sodium fluoride is ineffective while stannous fluoride remains effective in strengthening tooth enamel. Stannous fluoride has also been shown to be more effective than sodium fluoride in controlling gingivitis.
Stannous fluoride was used, under the trade name "Fluoristan," in the original formulation of the toothpaste Crest, though it was later replaced with sodium monofluorophosphate, or "Fluoristat." It is the active ingredient in Crest Pro Health brand toothpaste. Crest Pro Health issues a warning on the tube that stannous fluoride may cause staining which can be avoided by proper brushing, and that its particular formulation is resistant to staining. Any stannous fluoride staining that occurs due to improper brushing is not permanent.
Stannous fluoride is also readily available in over-the-counter rinses.
SnF2 can be prepared by evaporating a solution of SnO in 40% HF.
Aqueous solutions
Readily soluble in water SnF2 is hydrolysed forming at low concentration species such as SnOH, Sn(OH)2 and Sn(OH)3 and at higher concentrations, predominantly polynuclear species, Sn2(OH)2 and Sn3(OH)4. Aqueous solutions readily oxidise to form insoluble precipitates of Sn which are ineffective as a dental prophylactic. Studies of the oxidation using Mössbauer spectroscopy on frozen samples suggests that O2 is the oxidizing species.
Lewis acidity
SnF2 is a Lewis acid forming, for example, a 1:1 complex (CH3)3NSnF2 and 2:1 complex 2SnF2 with trimethylamine, and a 1:1 complex with dimethylsulfoxide, (CH3)2SO.SnF2.
In solutions containing fluoride ion, F it forms fluoride complexes SnF3, Sn2F5, SnF2(OH2). Crystallization from an aqueous solution containing NaF produces compounds containing polynuclear anions, e.g. NaSn2F5 or Na4Sn3F10 depending on the reaction conditions, rather than NaSnF3. The compound NaSnF3 containing the pyramidal SnF3 anion can however be produced from a pyridine – water solution.
Other compounds containing the pyramidal SnF3 anion are known for example Ca(SnF3)2
Reducing properties
SnF2 is a reducing agent, with a standard reduction potential E (Sn/ Sn) = +0.15V. Solutions in HF are readily oxidised by a range of oxidizing agents, O2, SO2 or F2, to form the mixed valence compound, Sn3F8 (containing Sn and Sn and no Sn – Sn bonds).
Structure
The monoclinic form contains tetramers, Sn4F8, where there are two distinct coordination environments for the Sn atoms but in each case there are three nearest neighbours with Sn at the apex of a trigonal pyramid and the lone pair of electrons is sterically active. Other forms reported have the GeF2 and TeO2 structures.
Molecular SnF2
In the vapour phase SnF2 forms monomers as well as dimers and trimers. Monomeric SnF2 is a non-linear molecule with an Sn-F bond length of 206 pm.
Complexes of SnF2, sometimes called difluorostannylene, with an alkyne and aromatic compounds deposited in an argon matrix at 12 K have been reported
References
- Hattab, F. (April 1989). "The State of Fluorides in Toothpastes". Journal of Dentistry. 17 (2): 47–54. doi:10.1016/0300-5712(89)90129-2. PMID 2732364.
- Perlich, MA; Bacca, LA; Bollmer, BW; Lanzalaco, AC; McClanahan, SF; Sewak, LK; Beiswanger, BB; Eichold, WA; Hull, JR (1995). "The clinical effect of a stabilized stannous fluoride dentifrice on plaque formation, gingivitis and gingival bleeding: a six-month study". The Journal of Clinical Dentistry. 6 (Special Issue): 54–58. PMID 8593194.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- Séby F., Potin-Gautier M., Giffaut E., Donard O. F. X. (2001). "A critical review of thermodynamic data for inorganic tin species". Geochimica et Cosmochimica Acta. 65 (18): 3041–3053. Bibcode:2001GeCoA..65.3041S. doi:10.1016/S0016-7037(01)00645-7.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - David B. Troy, 2005, Remington: The Science and Practice of Pharmacy, Lippincott Williams & Wilkins, ISBN 0781746736, 9780781746731
- Denes G; Lazanas G. (1994). "Oxidation of SnF2 stannous fluoride in aqueous solutions". Hyperfine Interactions. 90 (1): 435–439. doi:10.1007/BF02069152.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Chung Chun Hsu and R. A. Geanangel (1977). "Synthesis and studies of trimethylamine adducts with tin(II) halides". Inorg. Chem. 16 (1): 2529–2534. doi:10.1021/ic50176a022.
- Chung Chun Hsu and R. A. Geanangel (1980). "Donor and acceptor behavior of divalent tin compounds". Inorg. Chem. 19 (1): 110–119. doi:10.1021/ic50203a024.
- ^ Egon Wiberg, Arnold Frederick Holleman (2001) Inorganic Chemistry, Elsevier ISBN 0123526515
- Salami T.O. , Zavalij P.Y. and Oliver S.R.J. (2004). "Synthesis and crystal structure of two tin fluoride materials: NaSnF3 (BING-12) and Sn3F3PO4". Journal of Solid State Chemistry. 177 (3): 800–805. doi:10.1016/j.jssc.2003.09.013.
- Kokunov Y.V., Detkov D. G., Gorbunova Yu. E.,Ershova M. M. , Mikhailov Yu. N. (2001). "Synthesis and Crystal Structure of Calcium Trifluorostannate(II)". Doklady Chemistry. 376 (4–6): 52–54. doi:10.1023/A:1018855109716.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Housecroft, C. E.; Sharpe, A. G. (2004). Inorganic Chemistry (2nd ed.). Prentice Hall. ISBN 978-0-13-039913-7.
- ^ Wells A.F. (1984) Structural Inorganic Chemistry 5th edition Oxford Science Publications ISBN 0-19-855370-6
- S. E. Boganov, V. I. Faustov, M. P. Egorov and O. M. Nefedov (1994). "Matrix IR spectra and quantum chemical studies of the reaction between difluorostannylene and hept-1-yne. The first direct observation of a carbene analog π-complex with alkyne". Russian Chemical Bulletin Volume. 43 (1): 47–49. doi:10.1007/BF00699133.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - S. E. Boganov, M. P. Egorov and O. M. Nefedov (1999). "Study of complexation between difluorostannylene and aromatics by matrix IR spectroscopy". Russian Chemical Bulletin. 48 (1): 98–103. doi:10.1007/BF02494408.
| Tin compounds | |
|---|---|
| Sn(II) | |
| Sn(IV) | |