This is an old revision of this page, as edited by CheMoBot (talk | contribs) at 06:13, 20 April 2011 (Updating {{chembox}} (no changed fields - added verified revid - updated 'UNII_Ref', 'ChemSpiderID_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref', 'ChEMBL_Ref', 'KEGG_Ref') per Chem/Drugbox validation (). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 06:13, 20 April 2011 by CheMoBot (talk | contribs) (Updating {{chembox}} (no changed fields - added verified revid - updated 'UNII_Ref', 'ChemSpiderID_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref', 'ChEMBL_Ref', 'KEGG_Ref') per Chem/Drugbox validation ()(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff) | |
| Names | |
|---|---|
| IUPAC name Oxepine | |
| Other names
Oxacycloheptatriene Benzene oxide | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
SMILES
| |
| Properties | |
| Chemical formula | C6H6O |
| Molar mass | 94.11 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
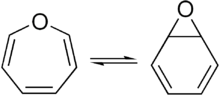
Oxepin is an oxygen-containing heterocycle consisting of a seven-membered ring with three double bonds. It exists as an equilibrium mixture with benzene oxide. The oxepin-benzene oxide system has fluctuating bonds in which the equilibrium can be shifted to one extreme or the other by suitable substituents. This compound is not aromatic.
References
- E. Vogel and H. Günther (1967). "Benzene Oxide-Oxepin Valence Tautomerism". Angewandte Chemie International Edition in English. 6 (5): 385–401. doi:10.1002/anie.196703851.