This is an old revision of this page, as edited by CheMoBot (talk | contribs) at 03:47, 19 December 2010 (Updating {{chembox}} (no changed fields - added verified revid - updated 'UNII_Ref', 'ChemSpiderID_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref') per Chem/Drugbox validation (report [[Wikipedia_talk:Wi). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 03:47, 19 December 2010 by CheMoBot (talk | contribs) (Updating {{chembox}} (no changed fields - added verified revid - updated 'UNII_Ref', 'ChemSpiderID_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref') per Chem/Drugbox validation (report [[Wikipedia_talk:Wi)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)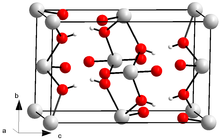 | |
| Identifiers | |
|---|---|
| CompTox Dashboard (EPA) | |
| Properties | |
| Chemical formula | H2O4U |
| Molar mass | 304.041 g·mol |
| Density | 5.73 - 6.73 g/cc |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Uranyl hydroxide is a hydroxide of uranium with the chemical formula UO2(OH)2 in the monomeric form and (UO2)2(OH)4 in the dimeric; both forms may exist in normal aqueous media. Uranyl hydroxide hydrate is precipitated as a colloidal yellowcake from oxidized uranium liquors near neutral pH.
Uranyl hydroxide was once used in glassmaking and ceramics in the colouring of the vitreous phases and the preparation of pigments for high temperature firing. The introduction of alkaline diuranates into glasses leads to yellow by transmission, green by reflection; moreover these glasses become dichroic and fluorescent under ultraviolet rays.
Uranyl hydroxide is teratogenic and radioactive, and should be handled with the appropriate care.
References
- The Structure of the a Form of Uranyl Hydroxide
- Alexander, C.A. (2005) "Volatilization of urania under strongly oxidizing conditions," Journal of Nuclear Materials, 346, 312–318.
External links
| Uranium compounds | |||
|---|---|---|---|
| U(II) | |||
| U(III) |
| ||
| U(IV) |
| ||
| U(IV,V) | |||
| U(IV,VI) | |||
| U(V) | |||
| U(VI) | |||
| U(XII) |
| ||
This inorganic compound–related article is a stub. You can help Misplaced Pages by expanding it. |