This is an old revision of this page, as edited by Beetstra (talk | contribs) at 17:30, 15 February 2011 (Script assisted update of identifiers from ChemSpider, CommonChemistry and FDA for the Chem/Drugbox validation project - Updated: ChEMBL KEGG.). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 17:30, 15 February 2011 by Beetstra (talk | contribs) (Script assisted update of identifiers from ChemSpider, CommonChemistry and FDA for the Chem/Drugbox validation project - Updated: ChEMBL KEGG.)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)
| |||
| Names | |||
|---|---|---|---|
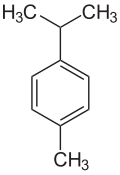
| IUPAC name 1-Methyl-4-(1-methylethyl)benzene | |||
| Other names 4-Isopropyltoluene; Paracymene | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.002.542 | ||
| EC Number |
| ||
| KEGG | |||
| PubChem CID | |||
| UNII | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | C10H14 | ||
| Molar mass | 134.21 g/mol | ||
| Appearance | Colourless liquid | ||
| Density | 0.857 g/cm | ||
| Melting point | -68°C | ||
| Boiling point | 177°C | ||
| Solubility in water | Negligible | ||
| Hazards | |||
| Flash point | 47°C | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Cymene, or p-cymene, is a naturally occurring aromatic organic compound. It is classified as a hydrocarbon related to a monoterpene. Its structure consists of a benzene ring para-substituted with a methyl group and an isopropyl group. It is insoluble in water, but miscible with ethanol and ether.
Cymene is a constituent of a number of essential oils, most commonly the oil of cumin and thyme.
There are two less common geometric isomers. o-Cymene, in which the alkyl groups are ortho-substituted, and m-cymene, in which they are meta-substituted. p-Cymene is the only natural isomer.
Cymene is a common ligand for ruthenium. The parent compound is 2. This half-sandwich compound is prepared by the reaction of ruthenium trichloride with the terpene α-phellandrene. The osmium complex is also known.
Significant amounts of cymene are formed in sulfite pulping process from the wood terpenes.
References
- Bennett, M. A.; Huang, T. N.; Matheson, T. W. and Smith, A. K. (1982). "(h6-Hexamethylbenzene)ruthenium complexes". Inorganic Syntheses. 21: 74–8. doi:10.1002/9780470132524.ch16.
{{cite journal}}: CS1 maint: multiple names: authors list (link)