This is an old revision of this page, as edited by Citation bot 1 (talk | contribs) at 13:44, 18 March 2011 (Misc citation tidying.). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 13:44, 18 March 2011 by Citation bot 1 (talk | contribs) (Misc citation tidying.)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff) | |
 | |
| Names | |
|---|---|
| Other names
Ammonium perrhenate, Ammonium perrhenate(VII) | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.033.690 |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | NH4ReO4 |
| Molar mass | 268.2359 g/mol |
| Density | 3.97 g/cm³, solid |
| Melting point | °C |
| Solubility in water | soluble. |
| Structure | |
| Crystal structure | scheelite |
| Coordination geometry | N/A |
| Hazards | |
| Flash point | Non-flammable. |
| Related compounds | |
| Other anions | Ammonium manganate; ammonium pertechnetate |
| Other cations | Sodium perrhenate; perrhenic acid |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Ammonium perrhenate (APR) is the ammonium salt of perrhenic acid, NH4ReO4. This is the most common form in which rhenium is traded. Prices are quoted in US Dollars per kg Rhenium content. Rhenium content of APR is typically 69.0 - 69.4%.
Structure
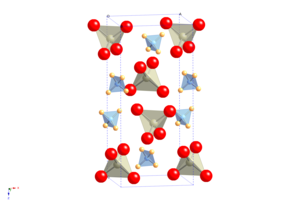
The crystal structure of APR is that of scheelite, in which the atomic cation is replaced by the ammonium molecular cation. It undergoes a molecular orientational ordering transition on cooling without change of space group, but with a highly anisotropic change in the shape of the unit cell, resulting in the unusual property of having a positive temperature and pressure Re NQR coefficient.
NH4ReO4 can be regarded as the prototype structure of a family of ammonium scheelites, which include the pertechnetate (NH4TcO4), periodate (NH4IO4), tetrachlorothallate (NH4TlCl4) and tetrachloroindate (NH4InCl4).
Preparation
Ammonium perrhenate may be prepared from elemental rhenium by dissolving in ammoniacal hydrogen peroxide (also known as base piranha).
Reactions
Pure rhenium powder can be produced from APR by reducing it with hydrogen:
- 2 NH4ReO4 + 7 H2 → 2 Re + 8 H2O + 2 NH3
References
- ^ I.P. Swainson and R.J.C. Brown (1997). "Refinement of ammonium perrhenate structure using a pseudo-spin model for the ammonium ion orientation". Acta Crystallographica. B53: 76–81. doi:10.1107/S0108768196011160.
- R.J.C. Brown and S.L. Segel (1977). "Re, N, and H nuclear quadrupole couplings in NH4ReO4: Evidence for a possible phase transition". Journal of Chemical Physics. 67 (7): 3163–7. doi:10.1063/1.435229.
- ^ Pradyot Patnaik (2003). Handbook of Inorganic Chemicals. New York: McGraw-Hill. pp. 789–790. ISBN 0-07-049439-8.
- Wm. T. Smith, S. Harmon Long (1948). "The Salts of Perrhenic Acid. I. The Alkali Metals and Ammonium". Journal of the American Chemical Society. 70 (1): 354–356. doi:10.1021/ja01181a110.
Further reading
- Richard J. Thompson, Tung-Ch'Ing Wang, Jacob Kleinberg, George M. Adams, Burl E. Bryant (1966). "Ammonium Perrhenate". Inorganic Syntheses. 8: 171–173. doi:10.1002/9780470132395.ch44.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
This inorganic compound–related article is a stub. You can help Misplaced Pages by expanding it. |