This is an old revision of this page, as edited by Free Bear (talk | contribs) at 18:28, 17 May 2011 (Reverted edits by 119.152.27.175 (talk) to last revision by MystBot (HG)). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 18:28, 17 May 2011 by Free Bear (talk | contribs) (Reverted edits by 119.152.27.175 (talk) to last revision by MystBot (HG))(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)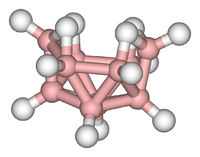 | |
| Names | |
|---|---|
| Other names
decaborane decaboron tetradecahydride | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.037.904 |
| EC Number |
|
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Molar mass | 122.22 g/mol |
| Appearance | White crystals |
| Melting point | 99.6 °C |
| Boiling point | 213 °C |
| Solubility in other solvents | Slightly, in cold water. |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Decaborane, also called decaborane(14), is the borane with the chemical formula B10H14. This white crystalline compound is one of the principal boron hydride clusters, both as a reference structure and as a precursor to other boron hydrides.
Handling properties and structure
B10H14 possesses a strong characteristic odor, sometimes described as musty or intensely bitter, chocolate-like that is unique to boranes. The physical characteristics of decaborane(14) resemble those of the organic compounds, such as naphthalene and anthracene, in that it can be sublimed under vacuum at moderate temperatures. Sublimation is the common method of purification. Like organic compounds, decaborane is highly flammable, but, like other boron hydrides, it burns with a bright green flame. It is not sensitive to moist air, although it hydrolyzes in boiling water, releasing hydrogen and giving a solution of boric acid. It is soluble in cold water as well as a variety of non-polar and moderately polar solvents.
In decaborane, the B10 framework resembles an incomplete octadecahedron. Each boron has one "radial" hydride, and four boron atoms near the open part of the cluster feature extra hydrides. In the language of cluster chemistry, the structure is classified as "nido".
Synthesis and reactions
It is commonly synthesized via the pyrolysis of smaller boron hydride clusters. For example, heating B2H6 or B5H9 gives decaborane, with loss of H2.
It reacts with Lewis bases (L) such as CH3CN and Me2S, to form derivatives with the formula B10H12·2L.
- B10H14 + 2 L → B10H12L2 + H2
These species, which are classified as "arachno" clusters, in turn react with acetylene to give the "closo" ortho-carborane:
- B10H12·2L + C2H2 → C2B10H12 + 2 L + H2
It is a weak Brønsted acid. Monodeprotonation generates the anion , with again a nido structure.
Applications
Decaborane has no significant applications, although it has often been investigated. Since the molecule decomposes in a plasma, yielding monoatomic boron ions, decaborane is potentially useful as a fuel for aneutronic fusion, and for low energy ion implantation of boron in the manufacture of semiconductors. It has also been considered for plasma-assisted chemical vapor deposition for the manufacture boron-containing thin films. In fusion research, the neutron-absorbing nature of boron has led to the use of these thin boron-rich films to "boronize" the walls of the tokamak vacuum vessel to reduce recycling of particles and impurities into the plasma and improve overall performance.
Decaborane was also developed as an additive to special high-performance rocket fuels. Its derivates were investigated as well, e.g. ethyl decaborane. One patented fuel composition included vinyl decaborane-polyester copolymer. Vinyl decaborane ("dekene") is prepared by reacting decaborane with acetylene.
Safety
Decaborane, like pentaborane, is a powerful toxin affecting central nervous system, although decaborane is less toxic than pentaborane. It can be absorbed through skin. It forms an explosive mixture with carbon tetrachloride, which caused an often quoted explosion in a Malta, NY manufacturing facility in 1948 when CCl4 was used to clean the equipment.
References
- Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry of the Elements (2nd Edn.), Oxford:Butterworth-Heinemann. ISBN 0-7506-3365-4.
- Advances towards pB11 fusion with the dense plasma focus
- Boronization effects using deuterated-decaborane (B10D14) in JT-60U 15th PSI Gifu
- Hawthorne, Marion F. (1976). "High energy propellant compositions including vinyl decaborane-polyester copolymer binder (United States Patent 3967989)". US Patent Office. Retrieved 2009-09-25.
- UCLA, Condensed version of the 79th Faculty Research Lecture Presented by Professor M. Frederick Hawthorne, accessed 23 Oct 2006.
Further reading
- NIST Chemistry Webbook, decaborane, accessed 23 October 2006.
- National Pollutant Inventory, Boron and compounds, accessed 23 October 2006.
- Decaborane in Organic Synthesis
- Webelements, Compounds of boron, accessed 23 October 2006.