This is an old revision of this page, as edited by Beetstra (talk | contribs) at 07:52, 9 August 2011 (Script assisted update of identifiers for the Chem/Drugbox validation project (updated: 'UNII', 'ChEBI').). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 07:52, 9 August 2011 by Beetstra (talk | contribs) (Script assisted update of identifiers for the Chem/Drugbox validation project (updated: 'UNII', 'ChEBI').)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)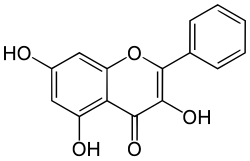 | |
| Names | |
|---|---|
| IUPAC name 3,5,7-trihydroxy-2-phenylchromen-4-one | |
| Other names
Norizalpinin 3,5,7-Trihydroxyflavone 3,5,7-triOH-Flavone | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.008.147 |
| IUPHAR/BPS | |
| KEGG | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C15H10O5 |
| Molar mass | 270.24 g/mol |
| Melting point | 214-215 °C |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Galangin is a flavonol found in high concentrations in Alpinia officinarum (lesser galangal). It is also found in the galangal rhizome (Alpinia galanga) and in propolis. Galangin has been shown to slow the increase and growth of breast tumor cells.
References
- Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1038/sj.bjc.6690216, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1038/sj.bjc.6690216instead. - Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 21046987, please use {{cite journal}} with
|pmid=21046987instead. - Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1016/j.foodchem.2007.01.011, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1016/j.foodchem.2007.01.011instead. - Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 8875554, please use {{cite journal}} with
|pmid=8875554instead. - Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1016/S0304-3835(96)04557-0, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1016/S0304-3835(96)04557-0instead.