This is an old revision of this page, as edited by CheMoBot (talk | contribs) at 06:12, 11 December 2011 (Updating {{chembox}} (no changed fields - added verified revid - updated 'DrugBank_Ref', 'UNII_Ref', 'ChEMBL_Ref', 'ChEBI_Ref', 'KEGG_Ref', 'CASNo_Ref') per Chem/Drugbox validation (report [[Wikipedia_talk:WikiProject_Chemicals|er...). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 06:12, 11 December 2011 by CheMoBot (talk | contribs) (Updating {{chembox}} (no changed fields - added verified revid - updated 'DrugBank_Ref', 'UNII_Ref', 'ChEMBL_Ref', 'ChEBI_Ref', 'KEGG_Ref', 'CASNo_Ref') per Chem/Drugbox validation (report [[Wikipedia_talk:WikiProject_Chemicals|er...)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff) | |
| Identifiers | |
|---|---|
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C12H12 |
| Molar mass | 156.22 g mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Tricyclobutabenzene is an aromatic hydrocarbon consisting of a benzene core with three cyclobutane rings fused onto it. This compound and related compounds are studied in the laboratory because they are often display unusual conformations and because of their unusual reactivity. Tricyclobutabenzenes are isomers of radialenes with which they are able to interconvert.
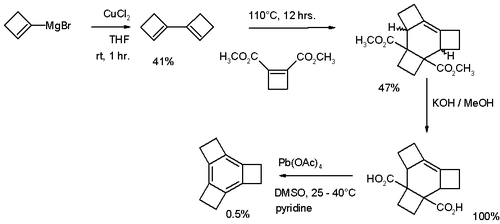
The parent tricyclobutabenzene (C12H12) was first synthesized in 1979 via a procedure depicted in Scheme 1. This compound is stable up to 250 °C.
A polyoxygenated tricyclobutabenzene with an extraordinary bond length of 160 pm for the bond connecting two carbonyl groups was synthesized according to scheme 2.
An ordinary bond of this type is only 148 pm and for comparison the C-C bond in isatin is 154 pm long. On the other hand, no change is recorded in the aromatic bond length alternation.
Similar chemistry yielded the six-fold ketone hexaoxotricyclobutabenzene C12O6, which happens to be a novel oxide of carbon. A key starting material is the iodo triflate depicted below which is a benzotriyne synthon.
See also
- Biphenyl
- Triphenyl
- Terpyridine
- Terthiophene
- Naphthalene where the rings are fused
References
- Tricyclobutabenzene Wutichai Nutakul, Randolph P. Thummel, Austin D. Taggart J. Am. Chem. Soc.; 1979; 101(3); 770-771. Abstract
- Reaction sequence: coupling reaction of cyclobutene Grignard reagents followed by Diels-Alder reaction with dimethylcyclobutene-1,2-dicarboxylate, followed by ester hydrolysis to dicarboxylic acid with potassium hydroxide in methanol followed by decarboxylation and aromatization with lead tetraacetate
- Poly-Oxygenated Tricyclobutabenzenes via Repeated Cycloaddition of Benzyne and Ketene Silyl Acetal Toshiyuki Hamura, Yousuke Ibusuki, Hidehiro Uekusa, Takashi Matsumoto, and Keisuke SuzukiJ. Am. Chem. Soc.; 2006; 128(11) pp 3534 - 3535; Abstract
- Reaction sequence: the key step is a cycloaddition between an aryne formed from the iodotriflate by action of n-butyllithium and a ketene silyl acetal. The silyl acetal is then converted to a ketone group by hydrofluoric acid and the remaining acetal groups by reaction with boron trifluoride
- Dodecamethoxy- and Hexaoxotricyclobutabenzene: Synthesis and Characterization Toshiyuki Hamura, Yousuke Ibusuki, Hidehiro Uekusa, Takashi Matsumoto, Jay S. Siegel, Kim K. Baldridge, and Keisuke Suzuki J. Am. Chem. Soc.; 2006; 128(31) pp 10032 - 10033; (Communication) doi:10.1021/ja064063e