| |||
| Names | |||
|---|---|---|---|
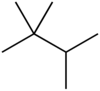
| Preferred IUPAC name 2,2,3-Trimethylbutane | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| Beilstein Reference | 1730756 | ||
| ChemSpider | |||
| ECHA InfoCard | 100.006.680 | ||
| EC Number |
| ||
| PubChem CID | |||
| UNII | |||
| UN number | 1206 | ||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | C7H16 | ||
| Molar mass | 100.205 g·mol | ||
| Appearance | Colorless liquid | ||
| Odor | Odorless | ||
| Density | 0.693 g mL | ||
| Melting point | −26 to −24 °C; −15 to −11 °F; 247 to 249 K | ||
| Boiling point | 80.8 to 81.2 °C; 177.3 to 178.1 °F; 353.9 to 354.3 K | ||
| Vapor pressure | 23.2286 kPa (at 37.7 °C) | ||
| Henry's law constant (kH) |
4.1 nmol Pa kg | ||
| Magnetic susceptibility (χ) | -88.36·10 cm/mol | ||
| Refractive index (nD) | 1.389 | ||
| Thermochemistry | |||
| Heat capacity (C) | 213.51 J K mol | ||
| Std molar entropy (S298) |
292.25 J K mol | ||
| Std enthalpy of formation (ΔfH298) |
−238.0 – −235.8 kJ mol | ||
| Std enthalpy of combustion (ΔcH298) |
−4.80449 – −4.80349 MJ mol | ||
| Hazards | |||
| GHS labelling: | |||
| Pictograms |    
| ||
| Signal word | Danger | ||
| Hazard statements | H225, H302, H305, H315, H336, H400 | ||
| Precautionary statements | P210, P261, P273, P301+P310, P331 | ||
| NFPA 704 (fire diamond) |
 | ||
| Flash point | −7 °C (19 °F; 266 K) | ||
| Autoignition temperature |
450 °C (842 °F; 723 K) | ||
| Explosive limits | 1–7% | ||
| Related compounds | |||
| Related alkanes | |||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |||
Triptane, or 2,2,3-trimethylbutane, is an organic chemical compound with the molecular formula C7H16 or (H3C-)3C-C(-CH3)2H. It is therefore an alkane, specifically the most compact and heavily branched of the heptane isomers, the only one with a butane (C4) backbone.
It was first synthesized in 1922 by Belgian chemists Georges Chavanne (1875–1941) and B. Lejeune, who called it trimethylisopropylmethane.
Due to its high octane rating (112–113 RON, 101 MON) triptane was produced on alkylation units starting from 1943 for use as an anti-knock additive in gasoline. It was extensively researched for this role and received the modern name in the late 1930s at a joint laboratory of NACA, National Bureau of Standards, US Army Air Corps and the Bureau of Aeronautics.
As of 2011, it was not a significant component of US automobile gasoline, present only in trace amounts (0.05–0.1%).
See also
References
- "Triptan - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 26 March 2005. Identification and Related Records. Retrieved 11 March 2012.
- Chavanne, G.; Lejeune, B. (March 1922). "Un nouvel heptane : le triméthylisopropylméthane". Bulletin de la Société Chimique de Belgique. 31 (3): 99–102 – via Internet Archive.
- https://webbook.nist.gov/cgi/cbook.cgi?Source=1922CHA%2FLEJ98
- Nash, Connor P.; Dupuis, Daniel P.; Kumar, Anurag; Farberow, Carrie A.; To, Anh T.; Yang, Ce; Wegener, Evan C.; Miller, Jeffrey T.; Unocic, Kinga A.; Christensen, Earl; Hensley, Jesse E.; Schaidle, Joshua A.; Habas, Susan E.; Ruddy, Daniel A. (2022-02-01). "Catalyst design to direct high-octane gasoline fuel properties for improved engine efficiency". Applied Catalysis B: Environmental. 301: 120801. Bibcode:2022AppCB.30120801N. doi:10.1016/j.apcatb.2021.120801. ISSN 0926-3373. OSTI 1827631.
- Perdih, A.; Perdih, F. (2006). "Chemical Interpretation of Octane Number". Acta Chimica Slovenica. S2CID 55494502.
- stason.org, Stas Bekman: stas (at). "10.1 The myth of Triptane". stason.org. Retrieved 2024-11-16.
- Annual Report of the National Advisory Committee for Aeronautics. U.S. Government Printing Office. 1938. p. 28.
- "Hydrocarbon Composition of Gasoline Vapor Emissions from Enclosed Fuel Tanks". nepis.epa.gov. United States Environmental Protection Agency. 2011.
This article about a hydrocarbon is a stub. You can help Misplaced Pages by expanding it. |