 | |
| Names | |
|---|---|
| Preferred IUPAC name 3-Sulfanylpropanoic acid | |
| Other names 3-MPA; 3-Mercaptopropanoic acid; β-Mercaptopropionic acid | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| Beilstein Reference | 773807 |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.003.216 |
| EC Number |
|
| Gmelin Reference | 101294 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C3H6O2S |
| Molar mass | 106.14 g·mol |
| Density | 1.218 |
| Melting point | 16.9 °C (62.4 °F; 290.0 K) |
| Boiling point | 111 °C (232 °F; 384 K) |
| Solubility in water | soluble |
| Solubility | ether benzene alcohol water |
| Acidity (pKa) | 4.34 |
| Refractive index (nD) | 1.4911 at 21 °C |
| Hazards | |
| GHS labelling: | |
| Pictograms |   
|
| Signal word | Danger |
| Hazard statements | H290, H301, H314, H332 |
| Precautionary statements | P234, P260, P261, P264, P270, P271, P280, P301+P310, P301+P330+P331, P303+P361+P353, P304+P312, P304+P340, P305+P351+P338, P310, P312, P321, P330, P363, P390, P404, P405, P501 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
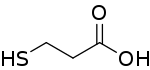
3-Mercaptopropionic acid (3-MPA) is an organosulfur compound with the formula HSCH2CH2CO2H. It is a bifunctional molecule, containing both carboxylic acid and thiol groups. It is a colorless oil. It is derived from the addition of hydrogen sulfide to acrylic acid.
Reactions and uses
It is competitive inhibitor of glutamate decarboxylase, and therefore acts as a convulsant. It has higher potency and faster onset of action compared to allylglycine.
It is used to prepare hydrophilic gold nanoparticles, exploiting the affinity of gold for sulfur ligands.
It is esterified with polyols to form thiol-based polymer cross-linking agents such as pentaerythritol-based pentaerythritol tetrakis(3-mercaptopropionate).
See also
- Allylglycine
- Thiolactic acid (2-mercaptopropionic acid)
References
- Horton, R. W; Meldrum, B. S (1973). "Seizures induced by allylglycine, 3-mercaptopropionic acid and 4-deoxypyridoxine in mice and photosensitive baboons, and different modes of inhibition of cerebral glutamic acid decarboxylase". British Journal of Pharmacology. 49 (1): 52–63. doi:10.1111/j.1476-5381.1973.tb08267.x. PMC 1776427. PMID 4207045.
- Subramanian, Vaidyanathan; Wolf, Eduardo E.; Kamat, Prashant V. (2004). "Catalysis with TiO2/Gold Nanocomposites. Effect of Metal Particle Size on the Fermi Level Equilibration". Journal of the American Chemical Society. 126 (15): 4943–4950. doi:10.1021/ja0315199. PMID 15080700.
- Hoyle, Charles E.; Bowman, Christopher N. (2010). "Thiol–Ene Click Chemistry". Angewandte Chemie International Edition. 49 (9): 1542–1543. doi:10.1002/anie.200903924. PMID 20166107.
| GABATooltip γ-Aminobutyric acid metabolism and transport modulators | |||||
|---|---|---|---|---|---|
| Transporter |
| ||||
| Enzyme |
| ||||
| Other |
| ||||
| Convulsants | |
|---|---|
| GABA receptor antagonists |
|
| GABA synthesis inhibitors | |
| Glycine receptor antagonists | |
| Glutamate receptor agonists | |
| Convulsant barbiturates | |
| Other | |
This article about an organic compound is a stub. You can help Misplaced Pages by expanding it. |