 | |
 | |
| Names | |
|---|---|
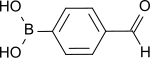
| Preferred IUPAC name 4-(Dihydroxyboranyl)benzaldehyde | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.103.550 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C7H7BO3 |
| Molar mass | 149.94 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
4-Formylphenylboronic acid (4-FPBA) is a versatile synthetic building block and an important intermediate in the preparation of agrochemical and pharmaceutical active ingredients. The substance finds industrial application as a stabilizer and inhibitor for enzymes and as a bactericide.
Synthesis
The synthesis of 4-formylyphenylboronic acid was reported by the group of Heinrich Nöth in 1990. 4-Bromobenzaldehyde was used as starting material. The acetalization of the aldehyde group was carried out by standard methods using diethoxymethoxyethane and ethanol to give 1-bromo-4-(diethoxymethyl)benzene. The formation of the Grignard compound with magnesium requires 1,2-dibromoethane and activation with ultrasound. Reaction with tri-n-butyl borate leads to the protected aryl boronic ester which gives after acidic work-up the target product in 78% yield.

The same reactants are forming with the aryl boronic ester at -60 °C 4-formylyphenylboronic acid with a 99% yield when activated with sodium bis(2-methoxyethoxy)aluminiumhydride, also on the kilogram scale.
When the aryllithium compound of 1-bromo-4-(diethoxymethyl)benzene is used with triisopropylborate at -78 °C instead of the Grignard compound (via n-butyllithium) 4-formylphenylboronic acid is obtained in 99% crude yield.

Disadvantages of both routes are the high price of the educts used (such as 4-bromobenzaldehyde, boronic esters of higher alcohols and butyllithium) as well as in the Nöth route the difficult workup after the hydrolysis by n-butanol. More recently, an improved process has been patented using less expensive starting materials such as 4-chlorobenzaldehyde, metallic lithium and trimethyl borate.

4-Formylphenylboronic acid can also be prepared by hydrolysis of potassium 4-formylphenyl-trifluoroborate by means of acidic alumina or silicon dioxide. As a rule, phenylboronic acids serve as starting compounds for the corresponding potassium aryl trifluoroborates.
Properties
4-Formylphenyl boronic acid crystallizes in colorless needles or is obtained as an odorless, whitish powder, which dissolves little in cold but better in hot water. The compound is quite stable and readily forms dimers and cyclic trimeric anhydrides, which complicate purification and tend to protodeboronize, a secondary reaction that occurs frequently in the Suzuki coupling, with elimination of the boronic acid function.
Applications
4-Formylphenylboronic acid is used in Suzuki couplings, for example in the build up of pharmacologically active biphenyl compounds such as a precursor of the antihypertensive AT1 antagonist telmisartan in an improved synthesis:
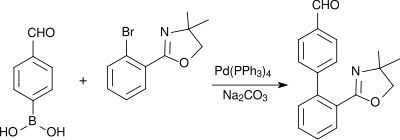
Also palladium-catalyzed aryl heteroaryl linkages after Suzuki use 4-formylphenylboronic acid as a molecular building block, as for instance in the synthesis of aryl-benzimidazole derivatives (which bind to peroxisome-proliferator-activated receptors (PPARγ) and activate the expression of a variety of genes):

In a copper-mediated fluoroalkylation reaction, the boronic acid group of the 4-FPBA can be replaced with perfluorinated alkyl iodides (Rf-I) by a perfluoroalkyl chain under mild conditions.

4-Formyphenylboronic acid is used industrially as an enzyme stabilizer for proteases and in particular for lipases in liquid detergent preparations. The addition of 4-FPBA in amounts < 0.08 wt% in the formulation reduces the loss of hydrolytic activity of the enzymes used and increases the storage stability of enzyme-containing liquid detergents.
References
- ^ US 5972873, L.K. Nielsen, A. Deane-Wray, "4-Substituted-phenyl-boronic acids as enzyme stabilizers", published 1999-10-26, assigned to Novo Nordisk A/S
- ^ H. Feulner; G. Linti; H. Nöth (1990), "Beiträge zur Chemie des Bors, 206. Darstellung und strukturelle Charakterisierung der p-Formylbenzolboronsäure", Chem. Ber. (in German), 123 (9): 1841–1843, doi:10.1002/cber.19901230915
- Autorenkollektiv, Organikum, 24. Auflage, S. 481, Wiley-VCH, Weinheim, 2001, ISBN 978-3-527-33968-6
- ^ H. Jendralla; A. Wagner; M. Mollath; J. Wunner (1995), "Efficient, simple procedures for the large-scale preparation of buildings blocks for angiotensin (II) receptor antagonists", Liebigs Ann. Chem., 1995 (7): 1253–1257, doi:10.1002/jlac.1995199507166
- Y. Kobayashi; Y. Tokoro; K. Watatani (1998), "Preparation of functionalized zinc borates and their coupling reactions with allylic acetates", Tetrahedron Lett., 39 (41): 7537–7540, doi:10.1016/S0040-4039(98)01639-6
- US 6833470, A. Meudt, S. Scherer, F. Vollmüller, M. Erbes, "Method for producing formylphenylboronic acids", published 2004-12-21, assigned to Clariant GmbH
- G.W. Kabalka; V. Coltuclu (2009), "Thermal and microwave hydrolysis of organotrifluoroborates mediated by alumina", Tetrahedron Lett., 50 (46): 6271–6272, doi:10.1016/j.tetlet.2009.09.008
- G.A. Molander; L.N. Cavalcanti; B. Canturk; P.-S. Pan; L.E. Kennedy (2009), "Efficient hydrolysis of organotrifluoroborates via silicagel and water", J. Org. Chem., 74 (19): 7364–7369, doi:10.1021/jo901441u, PMC 2763364, PMID 19743828
- E. Vedejs; R.W. Chapman; S.C. Fields; S. Lin; M.R. Schimpf (1995), "Conversion of arylboronic acids into potassium aryltrifluoroborates: convenient precursors of arylboron difluoride Lewis acids", J. Org. Chem., 60 (10): 3020–3027, doi:10.1021/jo00115a016
- G.K. Surya Prakash; F. Pertusati; G.A. Olah (2011), "HF-free, direct synthesis of tetrabutylammonium trifluoroborates", Synthesis, 2011 (2): 292–302, doi:10.1055/s-0030-1258370
- A. S. Kumar; S. Ghosh; G.N. Mehta (2010), "Efficient and improved synthesis of Telmisartan", Beilstein J. Org. Chem., 25: 6, doi:10.3762/bjoc.6.25, PMC 2874342, PMID 20502601
- Q. Qi; Q. Shen; L. Lu (2012), "Copper-mediated aerobic fluoroalkylation of arylboronic acids with fluoroalkyl iodides at room temperature", J. Am. Chem. Soc., 134 (15): 6548–6551, Bibcode:2012JAChS.134.6548Q, doi:10.1021/ja301705z, PMID 22458339
- US 20130252315, T. O’Connell, S. Tondera, T. Weber, "Stabilized, liquid, enzyme-containing surfactant preparation", published 2013-9-26, assigned to Henkel AG & Co. KGaA