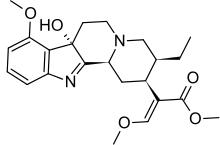
 | |
 | |
| Clinical data | |
|---|---|
| Other names | 7α-Hydroxy-7H-mitragynine; 9-Methoxycorynantheidine hydroxyindolenine |
| Routes of administration | By mouth |
| Drug class | Opioid |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolites | Mitragynine pseudoindoxyl |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C23H30N2O5 |
| Molar mass | 414.502 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
7-Hydroxymitragynine (7-OH) is a terpenoid indole alkaloid from the plant Mitragyna speciosa, commonly known as kratom. It was first described in 1994 and is a human metabolite metabolized from mitragynine present in the Mitragyna speciosa, commonly known as kratom. 7-OH binds to opioid receptors like mitragynine, but research suggests that 7-OH binds with greater efficacy.
7-Hydroxymitragynine (7-OH), a metabolite of the psychoactive botanical kratom, exhibits significantly higher binding affinity to mu-opioid receptors (MOR) than morphine although this is somewhat meaningless as it's receptor activation is only partial.
The national epidemic of opioid abuse has claimed more than 300,000 lives in the U.S. over the last 16 years -- and some researchers claim that kratom, an herbal psychoactive drug that is currently unregulated, could help people struggling with addiction. But federal drug policy-makers may classify kratom as an illegal drug, which would slow down its sale, research and development.4
Pharmacology
7-Hydroxymitragynine, like mitragynine, appears to be a mixed opioid receptor agonist/antagonist, with recent research indicating that it acts as a partial agonist at μ-opioid receptors and as a competitive antagonist at δ- and κ-opioid receptors. 7-OH does not appear to activate the β-arrestin pathway, distinguising it from traditional opiate & opioid chemicals. It shares this trait with mitragynine.
References
- ^ Chemical Abstracts Service: Columbus, OH, 2004; RN 174418-82-7 (accessed via SciFinder Scholar, version 2007.3; November 30, 2011)
- Matsumoto K, Horie S, Ishikawa H, Takayama H, Aimi N, Ponglux D, Watanabe K (March 2004). "Antinociceptive effect of 7-hydroxymitragynine in mice: Discovery of an orally active opioid analgesic from the Thai medicinal herb Mitragyna speciosa". Life Sciences. 74 (17): 2143–2155. doi:10.1016/j.lfs.2003.09.054. PMID 14969718.
- Ponglux D, Wongseripipatana S, Takayama H, Kikuchi M, Kurihara M, Kitajima M, et al. (December 1994). "A New Indole Alkaloid, 7 alpha-Hydroxy-7H-mitragynine, from Mitragyna speciosa in Thailand". Planta Medica. 60 (6): 580–581. doi:10.1055/s-2006-959578. PMID 17236085. S2CID 260252538.
- Kruegel AC, Grundmann O (May 2018). "The medicinal chemistry and neuropharmacology of kratom: A preliminary discussion of a promising medicinal plant and analysis of its potential for abuse". Neuropharmacology. 134 (Pt A): 108–120. doi:10.1016/j.neuropharm.2017.08.026. PMID 28830758. S2CID 24009429.
- ^ Eastlack SC, Cornett EM, Kaye AD (June 2020). "Kratom-Pharmacology, Clinical Implications, and Outlook: A Comprehensive Review". Pain and Therapy. 9 (1): 55–69. doi:10.1007/s40122-020-00151-x. PMC 7203303. PMID 31994019.
- Chang-Chien GC, Odonkor CA, Amorapanth P (2017). "Is Kratom the New 'Legal High' on the Block?: The Case of an Emerging Opioid Receptor Agonist with Substance Abuse Potential". Pain Physician. 20 (1): E195 – E198. doi:10.36076/ppj.2017.1.E195. PMID 28072812.