 | |
 | |
 | |
| Identifiers | |
|---|---|
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.029.242 |
| EC Number |
|
| PubChem CID | |
| RTECS number |
|
| UNII | |
| UN number | 3077 |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | CoBr2, CoBr2.6H2O, CoBr2.2H2O |
| Molar mass | 218.7412 g/mol (anhydrous) 326.74 g/mol (hexahydrate) |
| Appearance | Bright green crystals (anhydrous) Red-purple crystals (hexahydrate) |
| Density | 4.909 g/cm (anhydrous) 2.46 g/cm (hexahydrate) |
| Melting point | 678 °C (1,252 °F; 951 K) (anhydrous) 47 °C (hexahydrate) |
| Solubility in water | anhydrous: 66.7 g/100 mL (59 °C) 68.1 g/100 mL (97 °C) hexahydrate: 113.2 g/100 mL (20 °C) |
| Solubility | 77.1 g/100 mL (ethanol, 20 °C) 58.6 g/100 mL (methanol, 30 °C) soluble in methyl acetate, ether, alcohol, acetone |
| Magnetic susceptibility (χ) | +13000·10 cm/mol |
| Structure | |
| Crystal structure | Rhombohedral, hP3, SpaceGroup = P-3m1, No. 164 |
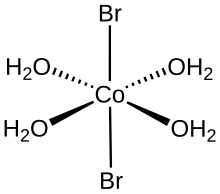
| Coordination geometry | octahedral |
| Hazards | |
| GHS labelling: | |
| Pictograms |   
|
| Signal word | Danger |
| Hazard statements | H302, H312, H315, H317, H319, H332, H334, H335, H350 |
| Precautionary statements | P201, P202, P261, P264, P270, P271, P272, P280, P281, P285, P301+P312, P302+P352, P304+P312, P304+P340, P304+P341, P305+P351+P338, P308+P313, P312, P321, P322, P330, P332+P313, P333+P313, P337+P313, P342+P311, P362, P363, P403+P233, P405, P501 |
| NFPA 704 (fire diamond) |
 |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) | 406 mg/kg (oral, rat) |
| Safety data sheet (SDS) | Fisher Scientific |
| Related compounds | |
| Other anions | cobalt(II) fluoride cobalt(II) chloride cobalt(II) iodide |
| Other cations | iron(II) bromide nickel(II) bromide |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Cobalt(II) bromide (CoBr2) is an inorganic compound. In its anhydrous form, it is a green solid that is soluble in water, used primarily as a catalyst in some processes.
Properties
When anhydrous, cobalt(II) bromide appears as green crystals. It is hygroscopic and eventually forms the hexahydrate in air, which appears as red-purple crystals. The hexahydrate loses four water of crystallization molecules at 100 °C forming the dihydrate:
- CoBr2·6H2O → CoBr2·2H2O + 4 H2O
Further heating to 130 °C produces the anhydrous form:
- CoBr2·2H2O → CoBr2 + 2 H2O
The anhydrous form melts at 678 °C. At higher temperatures, cobalt(II) bromide reacts with oxygen, forming cobalt(II,III) oxide and bromine vapor.
The tetrahydrate is molecular, with the formula trans-.
Preparation and reactions
Cobalt(II) bromide can be prepared as a hydrate by the reaction of cobalt hydroxide with hydrobromic acid:
- Co(OH)2 + 2HBr → CoBr2·6H2O
The classical coordination compound bromopentaamminecobalt(III) bromide is prepared by oxidation of an aqueous solution of cobalt(II) bromide and ammonia.
- 2 CoBr2 + 8 NH3 + 2 NH4Br + H2O2 → 2 Br2 + 2 H2O
Triphenylphosphine complexes of cobalt(II) bromide have been used as a catalysts in organic synthesis.
Safety
Exposure to large amounts of cobalt(II) can cause cobalt poisoning. Bromide is also mildly toxic.
References
- Perry, Dale L. (2011). Handbook of Inorganic Compounds (2nd ed.). Boca Raton: Taylor & Francis. p. 130. ISBN 978-1-4398-1461-1. OCLC 587104373.
- Cobalt Bromide Supplier & Tech Info American Elements
- WebElements Periodic Table of the Elements
- Waizumi, Kenji; Masuda, Hideki; Ohtaki, Hitoshi (1992). "X-ray Structural Studies of FeBr2·4H2O, CoBr2·4H2O, NiCl2·4H2O and CuBr2·4H2O. Cis/Trans Selectivity in Transition Metal(II) Dihalide Tetrahydrate". Inorganica Chimica Acta. 192 (2): 173–181. doi:10.1016/S0020-1693(00)80756-2.
- Diehl, Harvey; Clark, Helen; Willard, H. H.; Bailar, John C. (1939). "Bromopentamminocobalti Bromide". Inorganic Syntheses. Vol. 1. p. 186. doi:10.1002/9780470132326.ch66. ISBN 978-0-470-13232-6.
- "Cobalt Bromide (OUS)" (PDF). Archived from the original (PDF) on 2007-06-25. Retrieved 2008-04-10.
| Cobalt compounds | |
|---|---|
| Cobalt(I) | |
| Cobalt(II) | |
| Cobalt(0,III) | |
| Cobalt(II,III) | |
| Cobalt(III) | |
| Cobalt(III,IV) | |
| Cobalt(IV) | |
| Cobalt(V) | |
| Salts and covalent derivatives of the bromide ion | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||