 | |
 | |
| Names | |
|---|---|
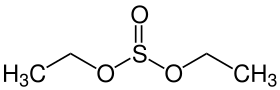
| Preferred IUPAC name Diethyl sulfite | |
| Other names
Diethyl sulphite Sulfurous acid, diethyl ester | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.009.832 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C4H10O3S |
| Molar mass | 138.18 g·mol |
| Appearance | Clear liquid |
| Density | 1.88 g/cm |
| Boiling point | 158 to 160 °C (316 to 320 °F; 431 to 433 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Diethyl sulfite (C4H10O3S) is an ester of sulfurous acid. Among other properties, diethyl sulfite inhibits the growth of mold spores during grain storage.
Diethyl sulfite is used as an additive in some polymers to prevent oxidation.
See also
References
- Pasiut, Lad A.; DeMarinis, F. (1966). "Inhibition of growth of spores of Penicillium and Aspergillus isolated from the white molds of silages". Ohio Journal of Science. 66 (1): 64–68.
- Guenther, A.; Koenig, T.; Habicher, W. D.; Schwetlick, K. (1997). "Antioxidant action of organic sulfites. I. Esters of sulfurous acid as secondary antioxidants". Polymer Degradation and Stability. 55 (2): 209–216. doi:10.1016/S0141-3910(96)00150-4.
External links
This article about an ester is a stub. You can help Misplaced Pages by expanding it. |