 | |
| Names | |
|---|---|
| Preferred IUPAC name 1-(Pyridin-2-yl)-N-methanamine | |
| Other names Di-(2-picolyl)amine, DPA | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.014.788 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C12H13N3 |
| Molar mass | 199.25 |
| Appearance | yellow liquid |
| Density | 1.107 g/cm |
| Boiling point | 139 to 141 °C at 1 mmHg |
| Solubility in water | low |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
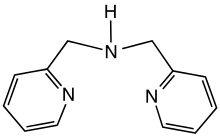
Dipicolylamine is an organic compound with the formula HN(CH2C5H4N)2. It is a yellow liquid that is soluble in polar organic solvents. The molecule is a secondary amine with two picolyl substituents. The compound is a common tridentate ligand in coordination chemistry.
The compound can be prepared by many methods, alkylation of picolinylamine with picolinyl chloride, deamination of picolinylamine, and reductive amination of picolinyl amine and pyridine-2-carboxaldehyde. It is commonly used to bind to bacteria in purifying mixtures that require separation.
Related compounds
References
- Sakamoto, Takashi; Ojida, Akio; Hamachi, Itaru"Molecular recognition, fluorescence sensing, and biological assay of phosphate anion derivatives using artificial Zn(II)-Dpa complexes" Chemical Communications 2009, pp.141-152. doi:10.1039/B812374H
- Huy Tien Ngo, Xuejian Liu, Katrina A. Jolliffe "Anion recognition and sensing with Zn(II)–dipicolylamine complexes" Chem. Soc. Rev., 2012,41, 4928-4965. doi:10.1039/C2CS35087D