 | |
| Names | |
|---|---|
| IUPAC name (1R,2R,13S,15R,16R,23R)-7,9,21-triazahexacyclotetracosane | |
| Other names CHEBI:144243 | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| PubChem CID | |
InChI
| |
SMILES
| |
| Properties | |
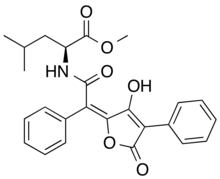
| Chemical formula | C25H25NO6 |
| Molar mass | 435.476 g·mol |
| Melting point | 162–163 °C (324–325 °F; 435–436 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Epanorin is a lichen secondary metabolite with the molecular formula C25H25NO6. Epanorin inhibits the proliferation of MCF-7 cancer cells.
References
- "Epanorin,18463-10-0 - LookChemical.com". www.lookchemical.com.
- ^ Palacios-Moreno, Juan; Rubio, Cecilia; Quilhot, Wanda; Cavieres, M. Fernanda; de la Peña, Eduardo; Quiñones, Natalia V.; Díaz, Hugo; Carrión, Flavio; Henríquez-Roldán, Carlos F.; Weinstein-Oppenheimer, Caroline R. (December 2019). "Epanorin, a lichen secondary metabolite, inhibits proliferation of MCF-7 breast cancer cells". Biological Research. 52 (1): 55. doi:10.1186/s40659-019-0261-4. PMC 6786276. PMID 31601259.
Further reading
- Issues in Life Sciences: Botany and Plant Biology Research: 2011 Edition. ScholarlyEditions. 9 January 2012. ISBN 978-1-4649-6343-8.
- Der Stoffwechsel Sekundärer Pflanzenstoffe / The Metabolism of Secondary Plant Products. Springer Science & Business Media. 11 November 2013. p. 572. ISBN 978-3-662-26784-4.
This article about an organic compound is a stub. You can help Misplaced Pages by expanding it. |