 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Gidazepam IC |
| Routes of administration | Oral |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Hepatic |
| Elimination half-life | ~87 hours |
| Excretion | Renal |
| Identifiers | |
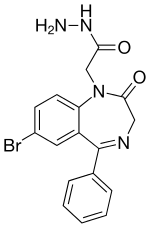
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C17H15BrN4O2 |
| Molar mass | 387.237 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (what is this?) (verify) | |
Gidazepam, also known as hydazepam or hidazepam, is a drug which is an atypical benzodiazepine derivative, developed in the Soviet Union. It is a selectively anxiolytic benzodiazepine. It also has therapeutic value in the management of certain cardiovascular disorders.
Pharmacology
Gidazepam and several of its analogs, in contrast to other benzodiazepines, are comparatively more selective agonists of TSPO (formerly the peripheral benzodiazepine receptor) than the benzodiazepine receptor.
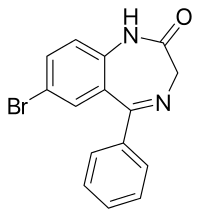
Gidazepam acts as a prodrug to its active metabolite 7-bromo-2,3-dihydro-5-phenyl-1H-1,4-benzodiazepin-2-one (desalkylgidazepam or bromo-nordazepam). Its anxiolytic effects can take several hours to manifest presumably due to its slow metabolism (half-life 87 hours). The onset and intensity of anxiolytic effects correlate with blood levels of desalkylgidazepam.
See also
- Phenazepam—another benzodiazepine widely used in Russia and other CIS countries
- Cinazepam
- Cloxazolam
- List of Russian drugs
References
- https://mozdocs.kiev.ua/likiview.php?id=33305 "Категорія відпуску. За рецептом."/"Leave Status: By Prescription"
- Library of Congress (2006). Library of Congress Subject Headings. Library of Congress. pp. 3300–.
- Spasennikov BA, Spasennikova MG (September 1991). "". Fel'dsher I Akusherka. 56 (9): 35–7. PMID 1684941.
- Neznamov GG, Koshelev VV, Voronina TA, Trofimov SS (Mar 2002). "". Eksperimental'naia i Klinicheskaia Farmakologiia. 65 (2): 12–6. PMID 12109283.
- ^ Korkhov VM, Tkachuk NA, Makan SY, Pavlovsky VI, Andronati SA (Feb 2002). "Affinities of gidazepam and its analogs for mitochondrial benzodiazepine receptors". Journal of Receptor and Signal Transduction Research. 22 (1–4): 411–20. doi:10.1081/RRS-120014610. PMID 12503630. S2CID 25403613.
- Morozov IS, Barchukov VG, Neznamov GG (Jan 1998). "". Eksperimental'naia i Klinicheskaia Farmakologiia. 61 (1): 30–2. PMID 9575408.
- Petrova TR, Tatarkin AN (1994). "". Terapevticheskii Arkhiv. 66 (9): 65–8. PMID 7992218.
- Skibitskiĭ VV, Kanorskiĭ SG (Sep 1993). "". Eksperimental'naia i Klinicheskaia Farmakologiia. 56 (5): 23–7. PMID 7906171.
- Skibitskiĭ VV, Petrova TR, Kanorskiĭ SG (June 1992). "". Kardiologiia. 32 (6): 35–7. PMID 1405290.
- Meerson FZ, Skibitskiĭ VV (April 1992). "". Kardiologiia. 32 (4): 25–30. PMID 1328745.
- Andronati SA, Zin'kovskiĭ VG, Totrova MI, Golovenko NI, Stankevich EA, Zhuk OV (January 1992). "". Biulleten' Eksperimental'noi Biologii I Meditsiny. 113 (1): 45–7. PMID 1356504.
- Kolyvanov GB, Zherdev VP, Chirkov AM, Otabekova SG, Litvin AA (May 1993). "". Eksperimental'naia i Klinicheskaia Farmakologiia. 56 (3): 48–50. PMID 8106054.
- Zherdev VP, Neznamov GG, Kolyvanov GB, Litvin AA, Otabekova SG (May 1993). "". Eksperimental'naia i Klinicheskaia Farmakologiia. 56 (3): 50–2. PMID 8106055.
This sedative-related article is a stub. You can help Misplaced Pages by expanding it. |