 __ Li __ Po | |
| Names | |
|---|---|
| Preferred IUPAC name Lithium polonide | |
| Identifiers | |
| 3D model (JSmol) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | Li2Po |
| Molar mass | 222.86 g/mol |
| Appearance | greyish |
| Related compounds | |
| Other anions | Lithium oxide Lithium sulfide Lithium selenide Lithium telluride |
| Other cations | Polonium hydride Sodium polonide Potassium polonide |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Lithium polonide is a chemical compound with the formula Li2Po. It is a polonide, a set of very chemically stable compounds of polonium.
Production
Lithium polonide may be produced from a redox reaction between aqueous polonium hydride and lithium metal or from an acid-base reaction of H2Po with strong lithium-containing bases:
- H2Po + 2 Li → Li2Po + H2
It may also be produced by heating lithium and polonium together at 300–400 °C.
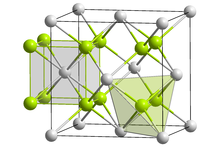
Crystal structure
Like sodium polonide, lithium polonide has the antifluorite structure.
References
- ^ Bagnall, K. W. (1962). "The Chemistry of Polonium". Advances in Inorganic Chemistry and Radiochemistry. New York: Academic Press. pp. 197–230. ISBN 9780120236046. Retrieved June 17, 2012.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1984). Chemistry of the Elements. Oxford: Pergamon Press. p. 899. ISBN 978-0-08-022057-4.
- ^ Moyer, Harvey V. (1956), "Chemical Properties of Polonium", in Moyer, Harvey V. (ed.), Polonium, Oak Ridge, Tenn.: United States Atomic Energy Commission, pp. 33–96, doi:10.2172/4367751, TID-5221.
| Polonium compounds | |
|---|---|
| Polonium(−II) | |
| Polonium(II) | |
| Polonium(IV) | |
| Polonium(VI) | |
This inorganic compound–related article is a stub. You can help Misplaced Pages by expanding it. |