| ornithine cyclodeaminase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC no. | 4.3.1.12 | ||||||||
| CAS no. | 9054-76-6 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
The enzyme ornithine cyclodeaminase (EC 4.3.1.12) catalyzes the chemical reaction
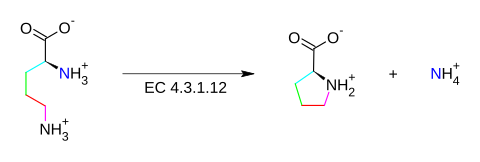
This enzyme belongs to the family of lyases, specifically ammonia lyases, which cleave carbon-nitrogen bonds. The systematic name of this enzyme class is Lornithine ammonia-lyase (cyclizing; L-proline-forming). Other names in common use include ornithine cyclase, ornithine cyclase (deaminating), and L-ornithine ammonia-lyase (cyclizing). This enzyme participates in arginine and proline biosynthesis. It employs one cofactor, NAD.
Structural studies
As of late 2007, two structures have been solved for this class of enzymes, with PDB accession codes 1U7H and 1X7D.
References
- Costilow RN, Laycock L (1971). "Ornithine cyclase (deaminating). Purification of a protein that converts ornithine to proline and definition of the optimal assay conditions". Journal of Biological Chemistry. 246 (21): 6655–60. doi:10.1016/S0021-9258(19)34165-1. PMID 4399881.
- Muth WL, Costilow RN (1974). "Ornithine cyclase (deaminating). II. Properties of the homogeneous enzyme". Journal of Biological Chemistry. 249 (23): 7457–62. doi:10.1016/S0021-9258(19)81260-7. PMID 4373469.
- Espineda CE, Linford AS, Devine D, Brusslan JA (1999). "The AtCAO gene, encoding chlorophyll a oxygenase, is required for chlorophyll b synthesis in Arabidopsis thaliana". Proceedings of the National Academy of Sciences of the United States of America. 96 (18): 10507–11. Bibcode:1999PNAS...9610507E. doi:10.1073/pnas.96.18.10507. PMC 17919. PMID 10468639.
- Goodman JL, Wang S, Alam S, Ruzicka FJ, Frey PA, Wedekind JE (2004). "Ornithine cyclodeaminase: structure, mechanism of action, and implications for the mu-crystallin family". Biochemistry. 43 (44): 13883–91. doi:10.1021/bi048207i. PMID 15518536.
- Alam S, Wang SC, Ruzicka FJ, Frey PA, Wedekind JE (2004). "Crystallization and X-ray diffraction analysis of ornithine cyclodeaminase from Pseudomonas putida". Acta Crystallographica Section D. 60 (Pt 5): 941–4. doi:10.1107/S0907444904005256. PMID 15103146.
| Carbon–nitrogen lyases (EC 4.3) | |
|---|---|
| 4.3.1: ammonia-lyases | |
| 4.3.2: amidine-lyases | |
| Enzymes | |
|---|---|
| Activity | |
| Regulation | |
| Classification | |
| Kinetics | |
| Types |
|
This enzyme-related article is a stub. You can help Misplaced Pages by expanding it. |
 L-proline + NH4
L-proline + NH4