| |||
| Names | |||
|---|---|---|---|
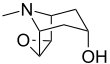
| IUPAC name 6β,7β-Epoxytropan-3α-ol | |||
| Systematic IUPAC name (1R,2R,4S,5S,7s)-9-Methyl-3-oxa-9-azatricyclononan-7-ol | |||
| Other names 6,7-Epoxytropine; Scopanol; Scopin; 6β,7β-Epoxy-1αH,5αH-tropan-3α-ol | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChEMBL | |||
| ChemSpider | |||
| PubChem CID | |||
| UNII | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | C8H13NO2 | ||
| Molar mass | 155.197 g·mol | ||
| Melting point | 75 to 76 °C (167 to 169 °F; 348 to 349 K) | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |||
Scopine is a tropane alkaloid found in a variety of plants including Mandragora root, Senecio mikanioides (Delairea odorata), Scopolia carniolica, and Scopolia lurida.
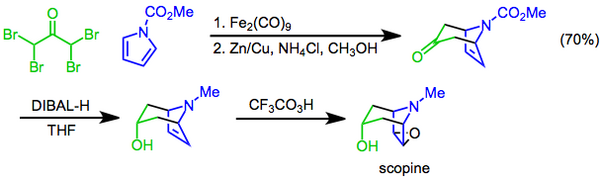
Scopine can be prepared by the hydrolysis of scopolamine. It can also be prepared in three steps from N-methoxycarbonylpyrrole and 1,1,3,3-tetrabromoacetone; the reagents are combined in a cycloaddition, followed by a diastereoselective reduction with diisobutylaluminum hydride, and finally a Prilezhaev epoxidation with trifluoroperacetic acid affords scopine.
See also
References
- ^ Werner, Gottfried; Schmidt, K.-H. (1967). "Die darstellung von scopin aus scopolamin". Tetrahedron Letters. 8 (14): 1283–1284. doi:10.1016/S0040-4039(00)90685-3. PMID 6044210.
- Staub, H. (1962). "The chemical constituents of the Mandragora root. II. The alkaloids". Helvetica Chimica Acta. 45 (7): 2297–2305. doi:10.1002/hlca.19620450703.
- Adams, Roger; Gianturco, Maurizio (1957). "Senecio alkaloids: mikanoidine, the alkaloid from Senecio mikanoides". Journal of the American Chemical Society. 79: 166–169. doi:10.1021/ja01558a045.
- Bendik, I.; Bauerova, O.; Bauer, S.; Mokry, J.; Tomko, J. (1958). "Alkaloids from Scopolia carniolica". Chemicke Zvesti. 12: 181–184.
- Szymanska, Miroslawa (1967). "Alkaloids in Scopolia lurida. Chromatographic analysis. Isolation of cuscohygrine". Acta Poloniae Pharmaceutica. 24 (1): 59–64.
- Meinwald, J.; Chapman, O. L. (1957). "Alkaline hydrolysis of scopolamine methoxymethochloride: a new route to scopine". Journal of the American Chemical Society. 79 (3): 665–666. doi:10.1021/ja01560a042.
- Willstatter, Richard; Berner, Endre (1923). "Hydrolysis of scopolamine". Berichte der Deutschen Chemischen Gesellschaft B. 56 (5): 1079–1082. doi:10.1002/cber.19230560515.
- Hayakawa, Y.; Baba, Y.; Makino, S.; Noyori, R. (1978). "Carbon-carbon bond formation promoted by transition metal carbonyls. 19. General synthesis of tropane alkaloids via the polybromo ketone-iron carbonyl reaction". J. Am. Chem. Soc. 100 (6): 1786–1791. doi:10.1021/ja00474a021.