(Redirected from Uroporphyrinogen decarboxylase )
Mammalian protein found in Homo sapiens
UROD Available structures PDB Ortholog search: PDBe RCSB List of PDB id codes 1JPH , 1JPI , 1JPK , 1R3Q , 1R3R , 1R3S , 1R3T , 1R3V , 1R3W , 1R3Y , 1URO , 2Q6Z , 2Q71 , 3GVQ , 3GVR , 3GVV , 3GVW , 3GW0 , 3GW3
Identifiers Aliases UROD , PCT, UPD, uroporphyrinogen decarboxylaseExternal IDs OMIM : 613521 ; MGI : 98916 ; HomoloGene : 320 ; GeneCards : UROD ; OMA :UROD - orthologs Gene location (Mouse ) Chr. Chromosome 4 (mouse) Band 4 D1|4 53.41 cM Start 116,847,162 bp End 116,851,610 bp
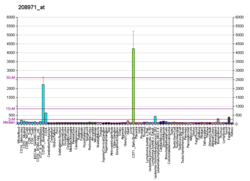
RNA expression patternBgee Human Mouse (ortholog)Top expressed in trabecular bone parotid gland right adrenal gland right adrenal cortex parietal pleura left adrenal cortex endothelial cell middle temporal gyrus Brodmann area 23 bone marrow
Top expressed in fetal liver hematopoietic progenitor cell internal carotid artery endocardial cushion human fetus external carotid artery facial motor nucleus atrioventricular valve Epithelium of choroid plexus medullary collecting duct renal corpuscle
More reference expression data
BioGPS
Wikidata
Uroporphyrinogen III decarboxylase (uroporphyrinogen decarboxylase , or UROD ) is an enzyme (EC 4.1.1.37 ) that in humans is encoded by the UROD gene .
Function
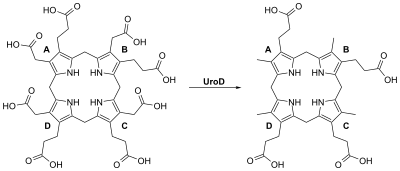
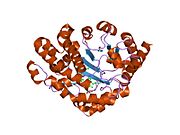
Uroporphyrinogen III decarboxylase is a homodimeric enzyme (PDB : 1URO ) that catalyzes the fifth step in heme biosynthesis, which corresponds to the elimination of carboxyl groups from the four acetate side chains of uroporphyrinogen III to yield coproporphyrinogen III :
uroporphyrinogen III
⇌
{\displaystyle \rightleftharpoons }
coproporphyrinogen III + 4 CO2 Clinical significance
Mutations and deficiency in this enzyme are known to cause familial porphyria cutanea tarda and hepatoerythropoietic porphyria . At least 65 disease-causing mutations in this gene have been discovered.
Mechanism
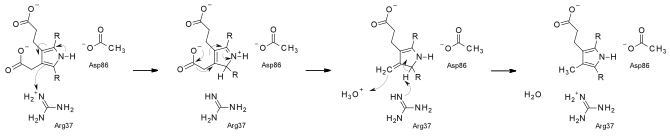
At low substrate concentrations, the reaction is believed to follow an ordered route, with the sequential removal of CO2 from the D, A, B, and C rings, whereas at higher substrate/enzyme levels a random route seems to be operative. The enzyme functions as a dimer in solution, and both the enzymes from human and tobacco have been crystallized and solved at good resolutions.
The reaction catalyzed by UroD UroD is regarded as an unusual decarboxylase, since it performs decarboxylations without the intervention of any cofactors, unlike the vast majority of decarboxylases. Its mechanism has recently been proposed to proceed through substrate protonation by an arginine residue. A 2008 report demonstrated that the uncatalyzed rate for UroD's reaction is 10 s, so at pH 10 the rate acceleration of UroD relative to the uncatalyzed rate, i.e. catalytic proficiency, is the largest for any enzyme known, 6 x 10 M.
Proposed reaction mechanism of uroporphyrinogen III decarboxylase References
^ GRCh38: Ensembl release 89: ENSG00000126088 – Ensembl , May 2017
^ GRCm38: Ensembl release 89: ENSMUSG00000028684 – Ensembl , May 2017
"Human PubMed Reference:" . National Center for Biotechnology Information, U.S. National Library of Medicine ."Mouse PubMed Reference:" . National Center for Biotechnology Information, U.S. National Library of Medicine .^ "Entrez Gene: UROD uroporphyrinogen decarboxylase" .
Šimčíková D, Heneberg P (December 2019). "Refinement of evolutionary medicine predictions based on clinical evidence for the manifestations of Mendelian diseases" . Scientific Reports . 9 (1): 18577. Bibcode :2019NatSR...918577S . doi :10.1038/s41598-019-54976-4 . PMC 6901466 . PMID 31819097 .
Silva PJ, Ramos MJ (2005). "Density-functional study of mechanisms for the cofactor-free decarboxylation performed by uroporphyrinogen III decarboxylase". J Phys Chem B . 109 (38): 18195–200. doi :10.1021/jp051792s . PMID 16853337 .
Lewis CA, Wolfenden R (November 2008). "Uroporphyrinogen decarboxylation as a benchmark for the catalytic proficiency of enzymes" . Proc. Natl. Acad. Sci. U.S.A . 105 (45): 17328–33. Bibcode :2008PNAS..10517328L . doi :10.1073/pnas.0809838105 . PMC 2582308 . PMID 18988736 .
Further reading
Elder GH, Lee GB, Tovey JA (1978). "Decreased activity of hepatic uroporphyrinogen decarboxylase in sporadic porphyria cutanea tarda". N. Engl. J. Med . 299 (6): 274–8. doi :10.1056/NEJM197808102990603 . PMID 661926 . de Verneuil H, Bourgeois F, de Rooij F, et al. (1992). "Characterization of a new mutation (R292G) and a deletion at the human uroporphyrinogen decarboxylase locus in two patients with hepatoerythropoietic porphyria" . Hum. Genet . 89 (5): 548–52. doi :10.1007/bf00219182 . hdl :1765/58484 . PMID 1634232 . S2CID 31811381 . Romana M, Grandchamp B, Dubart A, et al. (1991). "Identification of a new mutation responsible for hepatoerythropoietic porphyria". Eur. J. Clin. Invest . 21 (2): 225–9. doi :10.1111/j.1365-2362.1991.tb01814.x . PMID 1905636 . S2CID 22358220 . Garey JR, Harrison LM, Franklin KF, et al. (1990). "Uroporphyrinogen decarboxylase: a splice site mutation causes the deletion of exon 6 in multiple families with porphyria cutanea tarda" . J. Clin. Invest . 86 (5): 1416–22. doi :10.1172/JCI114856 . PMC 296884 . PMID 2243121 . Garey JR, Hansen JL, Harrison LM, et al. (1989). "A point mutation in the coding region of uroporphyrinogen decarboxylase associated with familial porphyria cutanea tarda" . Blood . 73 (4): 892–5. doi :10.1182/blood.V73.4.892.892 . PMID 2920211 . Roméo PH, Raich N, Dubart A, et al. (1986). "Molecular cloning and nucleotide sequence of a complete human uroporphyrinogen decarboxylase cDNA" . J. Biol. Chem . 261 (21): 9825–31. doi :10.1016/S0021-9258(18)67589-1 . PMID 3015909 . Dubart A, Mattei MG, Raich N, et al. (1986). "Assignment of human uroporphyrinogen decarboxylase (URO-D) to the p34 band of chromosome 1". Hum. Genet . 73 (3): 277–9. doi :10.1007/BF00401245 . PMID 3460962 . S2CID 34478515 . Romana M, Dubart A, Beaupain D, et al. (1987). "Structure of the gene for human uroporphyrinogen decarboxylase" . Nucleic Acids Res . 15 (18): 7343–56. doi :10.1093/nar/15.18.7343 . PMC 306252 . PMID 3658695 . de Verneuil H, Grandchamp B, Beaumont C, et al. (1986). "Uroporphyrinogen decarboxylase structural mutant (Gly281----Glu) in a case of porphyria". Science . 234 (4777): 732–4. Bibcode :1986Sci...234..732D . doi :10.1126/science.3775362 . PMID 3775362 . Roberts AG, Elder GH, De Salamanca RE, et al. (1995). "A mutation (G281E) of the human uroporphyrinogen decarboxylase gene causes both hepatoerythropoietic porphyria and overt familial porphyria cutanea tarda: biochemical and genetic studies on Spanish patients" . J. Invest. Dermatol . 104 (4): 500–2. doi :10.1111/1523-1747.ep12605953 . PMID 7706766 . Maruyama K, Sugano S (1994). "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides". Gene . 138 (1–2): 171–4. doi :10.1016/0378-1119(94)90802-8 . PMID 8125298 . Meguro K, Fujita H, Ishida N, et al. (1994). "Molecular defects of uroporphyrinogen decarboxylase in a patient with mild hepatoerythropoietic porphyria". J. Invest. Dermatol . 102 (5): 681–5. doi :10.1111/1523-1747.ep12374134 . PMID 8176248 . Moran-Jimenez MJ, Ged C, Romana M, et al. (1996). "Uroporphyrinogen decarboxylase: complete human gene sequence and molecular study of three families with hepatoerythropoietic porphyria" . Am. J. Hum. Genet . 58 (4): 712–21. PMC 1914669 . PMID 8644733 . McManus JF, Begley CG, Sassa S, Ratnaike S (1996). "Five new mutations in the uroporphyrinogen decarboxylase gene identified in families with cutaneous porphyria" . Blood . 88 (9): 3589–600. doi :10.1182/blood.V88.9.3589.bloodjournal8893589 . PMID 8896428 . Suzuki Y, Yoshitomo-Nakagawa K, Maruyama K, et al. (1997). "Construction and characterization of a full length-enriched and a 5'-end-enriched cDNA library". Gene . 200 (1–2): 149–56. doi :10.1016/S0378-1119(97)00411-3 . PMID 9373149 . Whitby FG, Phillips JD, Kushner JP, Hill CP (1998). "Crystal structure of human uroporphyrinogen decarboxylase" . EMBO J . 17 (9): 2463–71. doi :10.1093/emboj/17.9.2463 . PMC 1170588 . PMID 9564029 . Mendez M, Sorkin L, Rossetti MV, et al. (1998). "Familial porphyria cutanea tarda: characterization of seven novel uroporphyrinogen decarboxylase mutations and frequency of common hemochromatosis alleles" . Am. J. Hum. Genet . 63 (5): 1363–75. doi :10.1086/302119 . PMC 1377546 . PMID 9792863 . Wang H, Long Q, Marty SD, et al. (1998). "A zebrafish model for hepatoerythropoietic porphyria". Nat. Genet . 20 (3): 239–43. doi :10.1038/3041 . PMID 9806541 . S2CID 28379777 . McManus JF, Begley CG, Sassa S, Ratnaike S (1999). "Three new mutations in the uroporphyrinogen decarboxylase gene in familial porphyria cutanea tarda. Mutation in brief no. 237. Online" . Hum. Mutat . 13 (5): 412–413. doi :10.1002/(SICI)1098-1004(1999)13:5<412::AID-HUMU13>3.0.CO;2-N . PMID 10338097 . Christiansen L, Ged C, Hombrados I, et al. (1999). "Screening for mutations in the uroporphyrinogen decarboxylase gene using denaturing gradient gel electrophoresis. Identification and characterization of six novel mutations associated with familial PCT" . Hum. Mutat . 14 (3): 222–32. doi :10.1002/(SICI)1098-1004(1999)14:3<222::AID-HUMU5>3.0.CO;2-V . PMID 10477430 . S2CID 245442 . External links
Overview of all the structural information available in the PDB for UniProt : P06132 PDBe-KB . PDB gallery
1jph : Ile260Thr mutant of Human UroD, human uroporphyrinogen III decarboxylase
1jpi : Phe232Leu mutant of human UROD, human uroporphyrinogen III decarboxylase
1jpk : Gly156Asp mutant of Human UroD, human uroporphyrinogen III decarboxylase
1r3q : Uroporphyrinogen Decarboxylase in complex with coproporphyrinogen-I
1r3r : Uroporphyrinogen Decarboxylase with mutation D86N
1r3s : Uroporphyrinogen Decarboxylase single mutant D86G in complex with coproporphyrinogen-I
1r3t : Uroporphyrinogen Decarboxylase single mutant D86G in complex with coproporphyrinogen-III
1r3v : Uroporphyrinogen Decarboxylase single mutant D86E in complex with coproporphyrinogen-I
1r3w : Uroporphyrinogen Decarboxylase Y164F mutant in complex with coproporphyrinogen-III
1r3y : Uroporphyrinogen Decarboxylase in complex with coproporphyrinogen-III
1uro : UROPORPHYRINOGEN DECARBOXYLASE
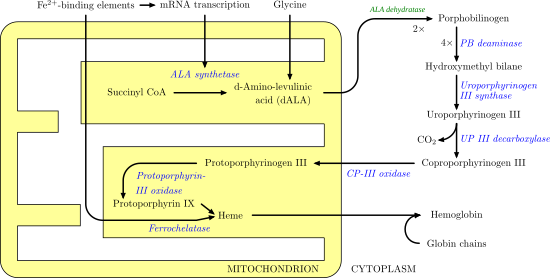
Heme synthesis—note that some reactions occur in the cytoplasm and some in the mitochondrion (yellow) Enzymes Activity
Regulation
Classification
Kinetics
Types
Portal :
Categories :
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.
**DISCLAIMER** We are not affiliated with Wikipedia, and Cloudflare.
The information presented on this site is for general informational purposes only and does not constitute medical advice.
You should always have a personal consultation with a healthcare professional before making changes to your diet, medication, or exercise routine.
AI helps with the correspondence in our chat.
We participate in an affiliate program. If you buy something through a link, we may earn a commission 💕
↑