| Revision as of 08:36, 4 November 2011 editBeetstra (talk | contribs)Edit filter managers, Administrators172,084 edits Script assisted update of identifiers for the Chem/Drugbox validation project (updated: 'DrugBank').← Previous edit |
Latest revision as of 00:22, 10 December 2024 edit undoCitation bot (talk | contribs)Bots5,459,787 edits Altered issue. Formatted dashes. | Use this bot. Report bugs. | Suggested by Dominic3203 | Linked from User:Marbletan/sandbox | #UCB_webform_linked 783/2664 |
| (79 intermediate revisions by 60 users not shown) |
| Line 1: |
Line 1: |
|
|
{{Short description|Muscle relaxant}} |
|
|
{{cs1 config|name-list-style=vanc|display-authors=6}} |
|
{{Drugbox |
|
{{Drugbox |
|
| verifiedrevid = 443520405 |
|
| verifiedrevid = 458941862 |
| ⚫ |
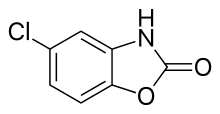
| IUPAC_name = 5-chloro-3''H''-benzooxazol-2-one |
|
|
| image = Chlorzoxazone.svg |
|
| image = Chlorzoxazone.svg |
|
|
|
|
|
<!--Clinical data--> |
|
<!--Clinical data--> |
|
| tradename = Parafonforte |
|
| tradename = Lorzone, Paraflex, Muscol |
|
| Drugs.com = {{drugs.com|monograph|chlorzoxazone}} |
|
| Drugs.com = {{drugs.com|monograph|chlorzoxazone}} |
|
| MedlinePlus = a682577 |
|
| MedlinePlus = a682577 |
|
|
| DailyMedID = Chlorzoxazone |
|
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> |
|
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> |
|
| pregnancy_US = <!-- A / B / C / D / X --> |
|
| pregnancy_US = N |
|
| pregnancy_category = |
|
| pregnancy_category = |
|
⚫ |
| routes_of_administration = ] |
|
| legal_AU = <!-- Unscheduled / S2 / S4 / S8 --> |
|
|
⚫ |
| ATC_prefix = M03 |
|
| legal_UK = <!-- GSL / P / POM / CD --> |
|
|
⚫ |
| ATC_suffix = BB03 |
| ⚫ |
| legal_US = <!-- OTC / Rx-only --> |
|
|
| legal_status = |
|
| ATC_supplemental = |
|
|
| class = ]s |
| ⚫ |
| routes_of_administration = oral |
|
|
|
|
|
|
<!-- Legal status --> |
|
|
| legal_AU = <!-- S2, S3, S4, S5, S6, S7, S8, S9 or Unscheduled --> |
|
|
| legal_AU_comment = |
|
|
| legal_BR = <!-- OTC, A1, A2, A3, B1, B2, C1, C2, C3, C4, C5, D1, D2, E, F --> |
|
|
| legal_BR_comment = |
|
|
| legal_CA = <!-- OTC, Rx-only, Schedule I, II, III, IV, V, VI, VII, VIII --> |
|
|
| legal_CA_comment = |
|
|
| legal_DE = <!-- Anlage I, II, III or Unscheduled --> |
|
|
| legal_DE_comment = |
|
|
| legal_NZ = <!-- Class A, B, C --> |
|
|
| legal_NZ_comment = |
|
|
| legal_UK = <!-- GSL, P, POM, CD, CD Lic, CD POM, CD No Reg POM, CD (Benz) POM, CD (Anab) POM or CD Inv POM / Class A, B, C --> |
|
|
| legal_UK_comment = |
|
⚫ |
| legal_US = Rx-only |
|
|
| legal_US_comment = <ref>{{cite web | title=Parafon DSC- chlorzoxazone tablet | website=DailyMed | date=9 February 2010 | url=https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5af155d7-3780-435b-9a87-0ce01f1ed6ec | access-date=5 November 2020}}</ref><ref>{{cite web | title=Lorzone- chlorzoxazone tablet | website=DailyMed | date=21 June 2019 | url=https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=bdd61b83-5bcd-4d23-8ef5-aeb9ca2f7c14 | access-date=5 November 2020}}</ref> |
|
|
| legal_EU = |
|
|
| legal_EU_comment = |
|
|
| legal_UN = <!-- N I, II, III, IV / P I, II, III, IV --> |
|
|
| legal_UN_comment = |
|
|
| legal_status = <!-- For countries not listed above --> |
|
|
|
|
|
<!--Pharmacokinetic data--> |
|
<!-- Pharmacokinetic data --> |
|
| bioavailability = well absorbed |
|
| bioavailability = Well absorbed |
|
| protein_bound = 13–18% |
|
| protein_bound = 13–18% |
|
| metabolism = hepatic |
|
| metabolism = Hepatic |
|
| elimination_half-life = 1.1 hr |
|
| elimination_half-life = 1.1 hours |
|
|
| duration_of_action = 3–4 hours |
|
| excretion = urine (<1%) |
|
| excretion = urine (<1%) |
|
|
|
|
|
<!--Identifiers--> |
|
<!--Identifiers--> |
|
| CASNo_Ref = {{cascite|correct|CAS}} |
|
|
| CAS_number_Ref = {{cascite|correct|??}} |
|
| CAS_number_Ref = {{cascite|correct|??}} |
|
| CAS_number = 95-25-0 |
|
| CAS_number = 95-25-0 |
| ⚫ |
| ATC_prefix = M03 |
|
| ⚫ |
| ATC_suffix = BB03 |
|
|
| ATC_supplemental = |
|
|
| PubChem = 2733 |
|
| PubChem = 2733 |
|
|
| IUPHAR_ligand = 2322 |
|
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} |
|
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} |
|
| DrugBank = DB00356 |
|
| DrugBank = DB00356 |
| Line 46: |
Line 67: |
|
|
|
|
|
<!--Chemical data--> |
|
<!--Chemical data--> |
|
⚫ |
| IUPAC_name = 5-chloro-3''H''-benzooxazol-2-one |
|
| C=7 | H=4 | Cl=1 | N=1 | O=2 |
|
| C=7 | H=4 | Cl=1 | N=1 | O=2 |
|
| molecular_weight = 169.565 g/mol |
|
|
| smiles = Clc2cc1c(OC(=O)N1)cc2 |
|
| smiles = Clc2cc1c(OC(=O)N1)cc2 |
|
| InChI = 1/C7H4ClNO2/c8-4-1-2-6-5(3-4)9-7(10)11-6/h1-3H,(H,9,10) |
|
|
| InChIKey = TZFWDZFKRBELIQ-UHFFFAOYAQ |
|
|
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
|
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
|
| StdInChI = 1S/C7H4ClNO2/c8-4-1-2-6-5(3-4)9-7(10)11-6/h1-3H,(H,9,10) |
|
| StdInChI = 1S/C7H4ClNO2/c8-4-1-2-6-5(3-4)9-7(10)11-6/h1-3H,(H,9,10) |
| Line 56: |
Line 75: |
|
| StdInChIKey = TZFWDZFKRBELIQ-UHFFFAOYSA-N |
|
| StdInChIKey = TZFWDZFKRBELIQ-UHFFFAOYSA-N |
|
}} |
|
}} |
|
|
|
|
'''Chlorzoxazone''' is a centrally acting ] used to treat muscle ] and the resulting pain or discomfort. It acts on the spinal cord by depressing reflexes. It is sold as '''Muscol''' or '''Parafon Forte''', a combination of chlorzoxazone and ] (Paracetamol). Possible side effects include ], ], ], ], ], and liver dysfunction. Used with acetaminophen it has added risk of ], which is why the combination is not recommended. |
|
|
|
'''Chlorzoxazone''' (]) is a centrally acting ] used to treat muscle ] and the resulting pain or discomfort. It can also be administered for acute pain in general and for tension headache (muscle contraction headache). It acts on the spinal cord by depressing reflexes. It is sold under the brand names '''Lorzone''', '''Paraflex''' and '''Muscol''' and in combination form as '''Parafon Forte''', a combination of chlorzoxazone and ] (paracetamol). Possible side effects include ], ], ], ], ].{{medcn|date=December 2013}} In rare cases, chlorzoxazone may cause severe liver dysfunction.<ref name="f298">{{cite book | title=LiverTox | chapter = Chlorzoxazone | publisher=National Institute of Diabetes and Digestive and Kidney Diseases | date=2017-01-30 | pmid=31643467 | chapter-url=https://www.ncbi.nlm.nih.gov/books/NBK548137/ | access-date=2024-05-10}}</ref> On the other hand, chlorzoxazone may reduce the liver toxicity of ] by competitive inhibition.<ref name="o489">{{cite journal | vauthors = Pingili RB, Vemulapalli S, Gadamsetty MV, Presingu D, Katuri R, Rachamsetty V, Kilaru NB | title=Chlorzoxazone reduced the paracetamol-induced toxicity via competitive inhibition of CYP2E1-mediated metabolism | journal=Future Journal of Pharmaceutical Sciences | volume=9 | issue=1 | date=2023-04-17 | issn=2314-7253 | doi=10.1186/s43094-023-00484-2 | doi-access=free}}</ref> |
|
For a more in depth look at Chlorzoxazone visit http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=15262. |
|
|
|
|
|
==Chemistry== |
|
|
|
It is available as a ].<ref>{{cite web | title=Competitive Generic Therapy Approvals | website=U.S. ] (FDA) | date=29 June 2023 | url=https://www.fda.gov/drugs/generic-drugs/competitive-generic-therapy-approvals | access-date=29 June 2023 | archive-date=29 June 2023 | archive-url=https://web.archive.org/web/20230629233651/https://www.fda.gov/drugs/generic-drugs/competitive-generic-therapy-approvals | url-status=live }}</ref> |
|
Chlorzoxazone, 5-chloro-2-benzoxazolione, is synthesized by a hetercyclization reaction of 2-amino-4-chlorphenol with ]. |
|
|
|
|
|
] |
|
|
|
Like ], its mechanism of action is still in question. It is believed that metaxalone works by altering ] levels and acting as a mild MAO inhibitor.{{medcn|date=April 2020}} The mechanism of action of chlorzoxazone is thought{{By whom?|date=April 2020}} to act on GABA<sub>A</sub> and GABA<sub>B</sub> receptors and voltage-gated calcium channels to a degree.{{medcn|date=April 2020}} General ] ] is the only currently accepted aspect to its medical benefits.{{medcn|date=April 2020}} Elucidation of the exact mechanism of action is ongoing but there is limited study due to the existence of more effective, safe muscle relaxants (e.g., ], ], ]), greatly limiting the potential benefit of identifying novel compounds which share chlorzoxazone's mechanism of action. |
| ⚫ |
*D.F. Marsh, {{US Patent|2895877}} (1959). |
|
|
|
|
| ⚫ |
==External links== |
|
|
|
== See also == |
| ⚫ |
* {{cite journal | author = Dong DL, Luan Y, Feng TM, Fan CL, Yue P, Sun ZJ, Gu RM, Yang BF. | title = Chlorzoxazone inhibit contraction of rat thoracic aorta | journal = Eur J Pharmacol | volume = 545| issue = 2–3| pages = 161–6| year = 2006 | pmid = 16859676 | doi = 10.1016/j.ejphar.2006.06.063}} |
|
|
|
* ] |
| ⚫ |
* {{cite journal | author = Park J, Kim K, Park P, Ha J | title = Effect of high-dose aspirin on CYP2E1 activity in healthy subjects measured using chlorzoxazone as a probe | journal = J Clin Pharmacol | volume = 46 | issue = 1 | pages = 109–14 | year = 2006 | pmid = 16397290 | doi = 10.1177/0091270005282635}} |
|
|
|
|
| ⚫ |
* {{cite journal | author = Wan J, Ernstgård L, Song B, Shoaf S | title = Chlorzoxazone metabolism is increased in fasted Sprague-Dawley rats | journal = J Pharm Pharmacol | volume = 58 | issue = 1 | pages = 51–61 | year = 2006 | pmid = 16393464 | doi = 10.1211/jpp.58.1.0007 | pmc = 1388188}} |
|
|
|
== References == |
|
|
{{Reflist}} |
|
|
|
|
|
== Further reading == |
|
|
{{refbegin}} |
|
⚫ |
* {{cite journal | vauthors = Dong DL, Luan Y, Feng TM, Fan CL, Yue P, Sun ZJ, Gu RM, Yang BF | title = Chlorzoxazone inhibits contraction of rat thoracic aorta | journal = European Journal of Pharmacology | volume = 545 | issue = 2–3 | pages = 161–166 | date = September 2006 | pmid = 16859676 | doi = 10.1016/j.ejphar.2006.06.063 }} |
|
⚫ |
* {{cite journal | vauthors = Park JY, Kim KA, Park PW, Ha JM | title = Effect of high-dose aspirin on CYP2E1 activity in healthy subjects measured using chlorzoxazone as a probe | journal = Journal of Clinical Pharmacology | volume = 46 | issue = 1 | pages = 109–114 | date = January 2006 | pmid = 16397290 | doi = 10.1177/0091270005282635 | s2cid = 20092326 }} |
|
⚫ |
* {{cite journal | vauthors = Wan J, Ernstgård L, Song BJ, Shoaf SE | title = Chlorzoxazone metabolism is increased in fasted Sprague-Dawley rats | journal = The Journal of Pharmacy and Pharmacology | volume = 58 | issue = 1 | pages = 51–61 | date = January 2006 | pmid = 16393464 | pmc = 1388188 | doi = 10.1211/jpp.58.1.0007 }} |
|
|
{{refend}} |
|
|
|
|
⚫ |
== External links == |
|
|
* {{cite web | url = https://druginfo.nlm.nih.gov/drugportal/name/chlorzoxazone | publisher = U.S. National Library of Medicine | work = Drug Information Portal | title = Chlorzoxazone }} |
|
|
* {{Webarchive|url=https://web.archive.org/web/20190712161010/https://chemicalbull.com/attachment-documents/Chlorzoxazone-msds.pdf |date=2019-07-12 }} |
|
⚫ |
* D.F. Marsh, {{US Patent|2895877}} (1959) |
|
|
|
|
|
{{Muscle relaxants}} |
|
{{Muscle relaxants}} |
|
|
{{Ion channel modulators}} |
|
|
{{Portal bar | Medicine}} |
|
|
|
|
|
] |
|
] |
|
] |
|
] |
|
] |
|
] |
|
|
] |
|
] |
|
] |
|
|
] |
|
|
|
|
|
{{Musculoskeletal-drug-stub}} |
|
{{Musculoskeletal-drug-stub}} |
|
|
|
|
] |
|
|
] |
|
|
] |
|