| Revision as of 08:06, 8 April 2020 view sourceAnomieBOT (talk | contribs)Bots6,590,192 editsm Dating maintenance tags: {{Update-inline}}← Previous edit | Latest revision as of 21:06, 16 January 2025 view source Tylermack999 (talk | contribs)Extended confirmed users597 editsm Clarified vague phrasingTags: Mobile edit Mobile app edit iOS app edit App section source | ||
| Line 1: | Line 1: | ||
| {{Short description|Contagious disease caused by SARS-CoV-2}} | |||
| {{About|the disease|the virus|Severe acute respiratory syndrome coronavirus 2|the pandemic|2019–20 coronavirus pandemic}} | |||
| {{About|the disease itself|the global pandemic caused by the disease|COVID-19 pandemic|other diseases caused by coronaviruses|Coronavirus diseases}} | |||
| {{pp-protected|small=yes}} | |||
| {{pp-extended|small=yes}} | |||
| {{Short description|Viral respiratory disease first detected in 2019}} | |||
| {{pp-move}} | |||
| {{Use Commonwealth English|date=March 2020}} | |||
| {{ |
{{EngvarB|date=April 2023}} | ||
| {{Use dmy dates|date=April 2023}} | |||
| {{cs1 config|name-list-style=vanc|display-authors=6}} | |||
| {{Infobox medical condition | {{Infobox medical condition | ||
| | name = Coronavirus disease 2019 (COVID-19) | | name = Coronavirus disease 2019<br />(COVID-19) | ||
| | synonyms = |
| synonyms = COVID, (the) coronavirus | ||
| | pronounce = {{ubl|{{IPAc-en|k|ə|ˈ|r|oʊ|n|ə|v|aɪ|r|ə|s}}|{{IPAc-en|ˌ|k|oʊ|v|ᵻ|d|n|aɪ|n|ˈ|t|iː|n|,_|ˌ|k|ɒ|v|ᵻ|d|-}}<ref>{{cite OED |Covid-19 |id=88575495 |date=April 2020 |access-date=15 April 2020}}</ref>}} | |||
| Early names: | |||
| | image = Fphar-11-00937-g001.jpg | |||
| * 2019-nCoV acute respiratory disease | |||
| | image_size = 310px | |||
| * Novel coronavirus pneumonia<ref>{{cite web|url=https://www.thelancet.com/pdfs/journals/lancet/PIIS0140-6736(20)30211-7.pdf|title=Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study|last=|first=|date=14 February 2020|website=The Lancet|url-status=live|archive-url=|archive-date=|access-date=}}</ref><ref>{{Cite journal|last=Han|first=Xiaoyu|last2=Cao|first2=Yukun|last3=Jiang|first3=Nanchuan|last4=Chen|first4=Yan|last5=Alwalid|first5=Osamah|last6=Zhang|first6=Xin|last7=Gu|first7=Jin|last8=Dai|first8=Meng|last9=Liu|first9=Jie|last10=Zhu|first10=Wanyue|last11=Zheng|first11=Chuansheng|title=Novel Coronavirus Pneumonia (COVID-19) Progression Course in 17 Discharged Patients: Comparison of Clinical and Thin-Section CT Features During Recovery|journal=Clinical Infectious Diseases|year=2020|language=en|doi=10.1093/cid/ciaa271|pmid=32227091}}</ref> | |||
| | caption = {{longitem|Transmission and life-cycle of ], which causes COVID-19}} | |||
| * Wuhan pneumonia<ref name=TIMEinfo>{{cite news | first = Charlie | last = Campbell | name-list-format = vanc | title = The Wuhan Pneumonia Crisis Highlights the Danger in China's Opaque Way of Doing Things | date = 20 January 2020 | accessdate = 13 March 2020 | url = https://time.com/5768230/wuhan-pneumonia-flu-crisis-china-government/ | work = ] | archive-url = https://web.archive.org/web/20200313053341/https://time.com/5768230/wuhan-pneumonia-flu-crisis-china-government/ | archive-date = 13 March 2020 | url-status = live }}</ref><ref name=FPinfo>{{cite web | first1 = Daniel | last1 = Lucey | first2 = Annie | last2 = Sparrow | name-list-format = vanc | title = China Deserves Some Credit for Its Handling of the Wuhan Pneumonia | date = 14 January 2020 | accessdate = 13 March 2020 | url = https://foreignpolicy.com/2020/01/14/china-response-wuhan-pneumonia-better-sars/ | work = ] | archive-url = https://web.archive.org/web/20200115042408/https://foreignpolicy.com/2020/01/14/china-response-wuhan-pneumonia-better-sars/ | archive-date = 15 January 2020 | url-status = live }}</ref> | |||
| | |
| specialty = ] | ||
| | symptoms = ]<ref name="CDC2020Sym"><!-- KEEP THIS NAMED REFERENCE -->{{#invoke:Cite web||url=https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html |title=Symptoms of Coronavirus |date=13 May 2020|website=U.S. ] (CDC) |url-status=live|archive-url=https://web.archive.org/web/20200617081119/https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html|archive-date=17 June 2020|access-date=18 June 2020}}</ref><ref name="WHO2020QA">{{#invoke:Cite web||url=https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question-and-answers-hub/q-a-detail/q-a-coronaviruses |title=Q&A on coronaviruses (COVID-19) |date=17 April 2020 |publisher=] (WHO) |archive-url=https://web.archive.org/web/20200514224315/https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question-and-answers-hub/q-a-detail/q-a-coronaviruses |archive-date=14 May 2020 |url-status=live |access-date=14 May 2020}}</ref> | |||
| | width = | |||
| | complications = ], ], ], ], ], ], ], ], ] | |||
| | alt = COVID-19 symptoms | |||
| | |
| onset = 2–14 days (typically 5)<br />after infection | ||
| | duration = 5 days to ] | |||
| | pronounce = {{IPAc-en|k|ə|ˈ|r|oʊ|n|ə|ˌ|v|aɪ|r|ə|s|_|d|ɪ|ˈ|z|i:|z}}, {{IPAc-en|ˈ|k|oʊ|v|ɪ|d}} | |||
| | types = | |||
| | specialty = ] | |||
| | cause = ] | |||
| | symptoms = Fever, cough, shortness of breath, none<ref name=CDC2020Sym/><ref name=WHO2020QA/> | |||
| | risks = | |||
| | complications = ], ], ], ] | |||
| | diagnosis = ], ], ] | |||
| | onset = 2–14 days (typically 5) from exposure | |||
| | |
| differential = | ||
| | prevention = ], face coverings, ], ], ventilation, hand washing | |||
| | types = | |||
| | treatment = ] | |||
| | cause = ] (SARS-CoV-2) | |||
| | medication = | |||
| | risks = Travel, viral exposure | |||
| | prognosis = | |||
| | diagnosis = ], ] | |||
| | frequency = {{COVID-19 data/Text|XW|cases}} confirmed cases (true case count is expected to be much higher<ref>{{#invoke:cite journal ||last1=Mathieu |first1=Edouard |last2=Ritchie |first2=Hannah |last3=Rodés-Guirao |first3=Lucas |last4=Appel |first4=Cameron |last5=Giattino |first5=Charlie |last6=Hasell |first6=Joe |last7=Macdonald |first7=Bobbie |last8=Dattani |first8=Saloni |last9=Beltekian |first9=Diana |last10=Ortiz-Ospina |first10=Esteban |last11=Roser |first11=Max |title=Coronavirus Pandemic (COVID-19) |url=https://ourworldindata.org/covid-cases |journal=Our World in Data |access-date=24 February 2024 |date=5 March 2020 |archive-date=24 February 2024 |archive-url=https://web.archive.org/web/20240224002105/https://ourworldindata.org/covid-cases |url-status=live }}</ref>) | |||
| | differential = | |||
| | deaths = {{ubl|{{COVID-19 data/Text|XW|deaths}} (reported)|18.2–33.5 million<ref>{{#invoke:cite news ||title=The pandemic's true death toll |newspaper=The Economist |url=https://www.economist.com/graphic-detail/coronavirus-excess-deaths-estimates |orig-date=2 November 2021 |date=28 August 2023 |access-date=28 August 2023}}</ref> (estimated)}} | |||
| | prevention = ], ], ]<br />{{small|] are recommended by the WHO when taking care of an infected person, or if symptoms occur, to prevent the spread of disease. Other health authorities have different guidelines surrounding the use of masks to prevent COVID-19 infection.}} | |||
| | alt = | |||
| | treatment = ] and ] | |||
| | medication = | |||
| | prognosis = | |||
| | frequency = {{Cases in 2019–20 coronavirus pandemic|confirmed|editlink=|ref=yes}} confirmed cases | |||
| | deaths = {{Cases in 2019–20 coronavirus pandemic|deaths|editlink=|ref=no}} ({{Cases in 2019–20 coronavirus pandemic|ratio|editlink=|ref=no}} of confirmed cases){{Cases in 2019–20 coronavirus pandemic|ref=yes}} | |||
| }} | }} | ||
| '''Coronavirus disease 2019''' ('''COVID-19''') is a ] caused by the ] ]. In January 2020 the disease spread worldwide, resulting in the ]. | |||
| {{2019–20 coronavirus pandemic sidebar}} | |||
| <!--Definition and symptoms--> | |||
| '''Coronavirus disease 2019''' ('''COVID-19''') is an ] caused by ] (SARS-CoV-2).<ref>{{cite web|url=https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it |title=Naming the coronavirus disease (COVID-19) and the virus that causes it |publisher=] (WHO) |url-status=live |archive-url=https://web.archive.org/web/20200228035651/https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it |archive-date=28 February 2020 |accessdate=28 February 2020| name-list-format = vanc}}</ref> The disease was first identified in December 2019 in ], the capital of China's ] province, and has since spread globally, resulting in the ongoing ].<ref name="Hui14Jan2020">{{cite journal |author-last1=Hui |author-first1=D. S. |author2=I. Azhar E. |author-last3=Madani |author-first3=T. A. |author-last4=Ntoumi |author-first4=F. |author-last5=Kock |author-first5=R. |author-last6=Dar |author-first6=O. |author-last7=Ippolito |author-first7=G. |author-last8=Mchugh |author-first8=T. D. |author-last9=Memish |author-first9=Z. A. |author-last10=Drosten |author-first10=Christian |author-link10=Christian Drosten |author-last11=Zumla |author-first11=A. |author-last12=Petersen |author-first12=E. | title=The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health—The latest 2019 novel coronavirus outbreak in Wuhan, China | journal=Int J Infect Dis | date=February 2020 | volume=91 | issue= | pages=264–66 | pmid=31953166 | doi=10.1016/j.ijid.2020.01.009 | doi-access=free }}</ref><ref name="WHOPandemic">{{cite press release | title=WHO Director-General's opening remarks at the media briefing on COVID-19 | website=] (WHO) | date=11 March 2020 | url=https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 |accessdate=12 March 2020 | url-status=live | archive-url=https://web.archive.org/web/20200311212521/https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 | archive-date=11 March 2020 }}</ref> Common ]s include ], ] and ].<ref name="CDCSym" /> Other symptoms may include fatigue, ], ], ], ] and abdominal pain.<ref name="CDC2020Sym" /><ref name="whoqa">{{cite web|url=https://www.who.int/news-room/q-a-detail/q-a-coronaviruses|title=Q&A on coronaviruses (COVID-19)|url-status=live|accessdate=11 March 2020|publisher=] (WHO)|archive-url=https://web.archive.org/web/20200120174649/https://www.who.int/news-room/q-a-detail/q-a-coronaviruses|archive-date=20 January 2020}}</ref><ref name="entuk-anosmia" /> While the majority of cases result in mild symptoms, some progress to viral ] and ].<ref name="Hui14Jan2020" /><ref name="WHO-q-a">{{cite web |url=https://www.who.int/news-room/q-a-detail/q-a-coronaviruses |title=Q&A on coronaviruses |website=] (WHO) |url-status=live |archive-url=https://web.archive.org/web/20200120174649/https://www.who.int/news-room/q-a-detail/q-a-coronaviruses |archive-date=20 January 2020 |accessdate=27 January 2020| name-list-format = vanc}}</ref> As of {{Cases in 2019–20 coronavirus pandemic|date|editlink=|ref=no}}, more than {{Cases in 2019–20 coronavirus pandemic|conround|editlink=|ref=yes}} ] have been reported in more than 200 countries and territories,<ref name="WOM">{{cite web |title=Coronavirus Update (Live): 1,001,069 Cases and 51,378 Deaths from COVID-19 Virus Outbreak—Worldometer |url=https://www.worldometers.info/coronavirus/ |website=www.worldometers.info |accessdate=2 April 2020 |language=en}}</ref> resulting in more than {{Cases in 2019–20 coronavirus pandemic|dround|editlink=|ref=no}} ].{{Cases in 2019–20 coronavirus pandemic|ref=yes}} More than {{Cases in 2019–20 coronavirus pandemic|recround|editlink=|ref=no}} people have recovered.{{Cases in 2019–20 coronavirus pandemic|ref=yes}} | |||
| The ] can vary but often include fever,<ref>{{#invoke:cite journal ||vauthors=Islam MA |date=April 2021 |title=Prevalence and characteristics of fever in adult and paediatric patients with coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis of 17515 patients |journal=PLOS ONE |volume=16 |issue=4 |pages=e0249788 |bibcode=2021PLoSO..1649788I |doi=10.1371/journal.pone.0249788 |pmc=8023501 |pmid=33822812 |doi-access=free |title-link=doi}}</ref> fatigue, cough, ], ], and ].<ref>{{#invoke:cite journal ||vauthors=Saniasiaya J, Islam MA |date=April 2021 |title=Prevalence of Olfactory Dysfunction in Coronavirus Disease 2019 (COVID-19): A Meta-analysis of 27,492 Patients |journal=The Laryngoscope |volume=131 |issue=4 |pages=865–878 |doi=10.1002/lary.29286 |issn=0023-852X |pmc=7753439 |pmid=33219539}}</ref><ref>{{#invoke:cite journal ||vauthors=Saniasiaya J, Islam MA |date=November 2020 |title=Prevalence and Characteristics of Taste Disorders in Cases of COVID-19: A Meta-analysis of 29,349 Patients |journal=Otolaryngology–Head and Neck Surgery |volume=165 |issue=1 |pages=33–42 |doi=10.1177/0194599820981018 |pmid=33320033 |s2cid=229174644|url=http://pure-oai.bham.ac.uk/ws/files/185167818/COVID_19_and_taste_disorders_V1.pdf }}</ref><ref>{{#invoke:cite journal ||vauthors=Agyeman AA, Chin KL, Landersdorfer CB, Liew D, Ofori-Asenso R |date=August 2020 |title=Smell and Taste Dysfunction in Patients With COVID-19: A Systematic Review and Meta-analysis |journal=Mayo Clin. Proc. |volume=95 |issue=8 |pages=1621–1631 |doi=10.1016/j.mayocp.2020.05.030 |pmc=7275152 |pmid=32753137}}</ref> Symptoms may begin one to fourteen days ] to the virus. At least a third of people who are infected ].<ref>{{#invoke:cite journal ||vauthors=Wang B, Andraweera P, Elliott S, Mohammed H, Lassi Z, Twigger A, Borgas C, Gunasekera S, Ladhani S, Marshall HS |title=Asymptomatic SARS-CoV-2 Infection by Age: A Global Systematic Review and Meta-analysis |journal=The Pediatric Infectious Disease Journal |date=March 2023 |volume=42 |issue=3 |pages=232–239 |doi=10.1097/INF.0000000000003791 |pmid=36730054 |pmc=9935239 }}</ref><ref>{{#invoke:cite journal ||vauthors=Oran DP, Topol EJ |date=January 2021 |title=The Proportion of SARS-CoV-2 Infections That Are Asymptomatic: A Systematic Review |journal=Annals of Internal Medicine |volume=174 |issue=5 |pages=M20-6976 |doi=10.7326/M20-6976 |pmc=7839426 |pmid=33481642}}</ref> Of those who develop symptoms noticeable enough to be classified as patients, most (81%) develop mild to moderate symptoms (up to mild ]), while 14% develop severe symptoms (], ], or more than 50% lung involvement on imaging), and 5% develop critical symptoms (], ], or ]).<ref name="CDC Interim Guidance">{{#invoke:cite web||date=6 April 2020|title=Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID-19)|url=https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html|url-status=live|archive-url=https://web.archive.org/web/20200302201644/https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html|archive-date=2 March 2020|access-date=19 April 2020|website=U.S. ] (CDC)}}</ref> Older people have a higher risk of developing severe symptoms. Some complications result in death. Some people continue to experience a range of effects (]) for months or years after infection, and damage to organs has been observed.<ref name="davis" /> Multi-year studies on the long-term effects are ongoing.<ref name="CDC-2020">{{#invoke:Cite web||last=CDC|date=11 February 2020|title=Post-COVID Conditions|url=https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects.html|access-date=12 July 2021|website=U.S. ] (CDC)}}</ref> | |||
| <!--Cause and diagnosis--> | |||
| <!--DO NOT INTERFERE WITH THE SECTION BEGIN/END TAGS, AS IT WILL BREAK THE PANDEMIC ARTICLE--><section begin="Spread"/>The virus is mainly ] during close contact{{efn|Close contact is defined as one metre (three feet) by the WHO<ref name="WHO2020QA" /> and two metres (six feet) by the CDC.<ref name="CDCTrans" />}} and by ] produced when those infected cough, sneeze or talk.<ref name=WHO2020QA/><!--Quote: "The main way the disease spreads is through respiratory droplets expelled by someone who is coughing."--><ref name=CDCTrans/><!--The virus is thought to spread mainly from person-to-person Between people who are in close contact with one another Through respiratory droplets produced when an infected person coughs, sneezes or talks.--><ref name="ECDCQA" /><!--The virus seems to be transmitted mainly via small respiratory droplets through sneezing, coughing, or when people interact with each other for some time in close proximity (usually less than one metre).--> These droplets may also be produced during breathing; however, they rapidly fall to the ground or surfaces and are not generally ].<ref name=WHO2020QA/><!--The disease can spread from person to person through small droplets from the nose or mouth which are spread when a person with COVID-19 coughs or exhales. These droplets land on objects and surfaces around the person.--><ref name=Modes>{{cite web |title=Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations |url=https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations |website=World Health Organization |accessdate=3 April 2020 |language=en |date=29 March 2020 |quote=According to current evidence, COVID-19 virus is primarily transmitted between people through respiratory droplets and contact routes.}}</ref><!--airborne transmission was not reported... Airborne transmission is different from droplet transmission as it refers to the presence of microbes within droplet nuclei, which are generally considered to be particles <5μm in diameter, can remain in the air for long periods of time and be transmitted to others over distances greater than a meter.--><ref>{{cite web |last1=Organization (WHO) |first1=World Health |title=FACT: #COVID19 is NOT airborne. The #coronavirus is mainly transmitted through droplets generated when an infected person coughs, sneezes or speaks.To protect yourself:-keep 1m distance from others-disinfect surfaces frequently-wash/rub your -avoid touching your pic.twitter.com/fpkcpHAJx7 |url=https://twitter.com/WHO/status/1243972193169616898/photo/1 |website=@WHO |accessdate=3 April 2020 |language=en |date=28 March 2020 |quote=These droplets are too heavy to hang in the air. They quickly fall on floors or sufaces.}}</ref> People may also become infected by touching a contaminated surface and then their face.<ref name=WHO2020QA/><!--These droplets land on objects and surfaces around the person. Other people then catch COVID-19 by touching these objects or surfaces, then touching their eyes, nose or mouth.--><ref name=CDCTrans/><!--The virus is thought to spread mainly from person-to-person Between people who are in close contact with one another--> The virus can survive on surfaces for up to 72 hours.<ref name="StableNIH"/><!--Quote: The scientists found that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was detectable in aerosols for up to three hours, up to four hours on copper, up to 24 hours on cardboard and up to two to three days on plastic and stainless steel.--> It is most contagious during the first three days after onset of symptoms, although spread may be possible before symptoms appear and in later stages of the disease.<!--Quote: "People are thought to be most contagious when they are most symptomatic (the sickest) Some spread might be possible before people show symptoms"--><ref name=":22">{{cite web|url=https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200402-sitrep-73-covid-19.pdf?sfvrsn=5ae25bc7_4|title=Coronavirus disease 2019 (COVID-19) Situation Report—73|last=|first=|date=2 April 2020|website=World Health Organization|url-status=live|archive-url=|archive-date=|access-date=3 April 2020}}</ref><!--Quote: shedding of the COVID-19 virus is highest in upper respiratory tract (nose and throat) early in the course of the disease.8-11 That is, within the first three days from onset of symptoms.10-11 Preliminary data suggests that people may be more contagious around the time of symptom onset as compared to later on in the disease.--><!--DO NOT REMOVE THE FOLLOWING TAG--><section end="Spread"/> The ] is typically around five days, but may range from two to 14 days.<ref name=CDCSym>{{cite web|url=https://www.cdc.gov/coronavirus/2019-ncov/about/symptoms.html|title=Symptoms of Novel Coronavirus (2019-nCoV) |date=10 February 2020|website=www.cdc.gov|access-date=11 February 2020|archive-url=https://web.archive.org/web/20200130202038/https://www.cdc.gov/coronavirus/2019-ncov/about/symptoms.html|archive-date=30 January 2020|url-status=live }}</ref><ref>{{Cite journal|author-last1=Velavan |author-first1=T. P. |author-last2=Meyer |author-first2=C. G. |title=The COVID-19 epidemic|journal=Tropical Medicine & International Health|volume=n/a|issue=n/a|pages=278–80|doi=10.1111/tmi.13383 |doi-access=free |pmid=32052514|date=March 2020 }}</ref> The standard method of ] is by ] (rRT-PCR) from a ].<ref name=CDC2020Testing>{{cite web |title=Coronavirus Disease 2019 (COVID-19) |url=https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html |website=Centers for Disease Control and Prevention |accessdate=26 March 2020 |language=en-us |date=11 February 2020 |archive-url=https://web.archive.org/web/20200304165907/https://www.cdc.gov/coronavirus/2019-nCoV/lab/guidelines-clinical-specimens.html |archive-date=4 March 2020 |url-status=live }}</ref> The infection can also be diagnosed from a combination of symptoms, ]s and a chest ] showing features of pneumonia.<ref name=Jin2020 /><ref name=":4">{{cite web|url=https://www.sciencedaily.com/releases/2020/02/200226151951.htm|title=CT provides best diagnosis for COVID-19|date=26 February 2020|website=ScienceDaily|url-status=live|access-date=2 March 2020|archive-url=https://web.archive.org/web/20200318210532/https://www.sciencedaily.com/releases/2020/02/200226151951.htm|archive-date=18 March 2020}}</ref> | |||
| ] occurs when infectious particles are breathed in or come into contact with the eyes, nose, or mouth. The risk is highest when people are in close proximity, but small ] particles containing the virus can remain suspended in the air and travel over longer distances, particularly indoors. Transmission can also occur when people touch their eyes, nose or mouth after touching surfaces or objects that have been contaminated by the virus. People remain contagious for up to 20 days and can spread the virus even if they do not develop symptoms.<ref>{{#invoke:cite web ||title=Coronavirus disease (COVID-19): How is it transmitted? |url=https://www.who.int/news-room/questions-and-answers/item/coronavirus-disease-covid-19-how-is-it-transmitted |access-date=13 April 2023 |website=] (WHO) }}</ref> | |||
| <!--Prevention and management--> | |||
| Recommended measures to prevent infection include frequent ], ] (maintaining physical distance from others, especially from those with symptoms), covering coughs and sneezes with a tissue or inner elbow and keeping unwashed hands away from the face.<ref name="Advice for public">{{cite web|url=https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public|title=Advice for public|website=] (WHO)|access-date=25 February 2020|archive-url=https://web.archive.org/web/20200126025750/https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public|archive-date=26 January 2020|url-status=live| name-list-format = vanc}}</ref><ref>{{cite web|url=https://www.gov.uk/government/publications/covid-19-guidance-on-social-distancing-and-for-vulnerable-people/guidance-on-social-distancing-for-everyone-in-the-uk-and-protecting-older-people-and-vulnerable-adults|title=Guidance on social distancing for everyone in the UK|website=GOV.UK|language=en|access-date=25 March 2020|archive-url=https://web.archive.org/web/20200324214400/https://www.gov.uk/government/publications/covid-19-guidance-on-social-distancing-and-for-vulnerable-people/guidance-on-social-distancing-for-everyone-in-the-uk-and-protecting-older-people-and-vulnerable-adults|archive-date=24 March 2020|url-status=live}}</ref> The use of ] is recommended for those who suspect they have the virus and their caregivers.<ref name="CDC2020IfSick">{{cite web|url=https://www.cdc.gov/coronavirus/2019-ncov/about/steps-when-sick.html|title=2019 Novel Coronavirus (2019-nCoV)|author=CDC|date=11 February 2020|website=Centers for Disease Control and Prevention|url-status=live|archive-url=https://web.archive.org/web/20200214153016/https://www.cdc.gov/coronavirus/2019-ncov/about/steps-when-sick.html|archive-date=14 February 2020|access-date=15 February 2020| name-list-format = vanc}}</ref> Recommendations for mask use by the general public vary, with some authorities recommending against their use, some recommending their use and others requiring their use.<ref>{{Cite journal|last=Feng|first=Shuo |last2=Shen|first2=Chen |last3=Xia|first3=Nan |last4=Song|first4=Wei |last5=Fan|first5=Mengzhen |last6=Cowling|first6=Benjamin J.|date=2020-03-20|title=Rational use of face masks in the COVID-19 pandemic|url=https://www.thelancet.com/journals/lanres/article/PIIS2213-2600(20)30134-X/abstract|journal=The Lancet Respiratory Medicine|language=English|volume=0|doi=10.1016/S2213-2600(20)30134-X|issn=2213-2600|pmid=32203710|pmc=7118603 }}</ref><ref>{{cite web |title=When and how to use masks |url=https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public/when-and-how-to-use-masks |website=www.who.int |accessdate=31 March 2020 |language=en |archive-url=https://web.archive.org/web/20200307013848/https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public/when-and-how-to-use-masks |archive-date=7 March 2020 |url-status=live }}</ref><!--Quote: "If you are healthy, you only need to wear a mask if you are taking care of a person with suspected 2019-nCoV infection."--><ref>{{Cite news|last=Tait|first=Robert|url=https://www.theguardian.com/world/2020/mar/30/czechs-get-to-work-making-masks-after-government-decree-coronavirus|title=Czechs get to work making masks after government decree|date=2020-03-30|work=The Guardian|access-date=2020-03-31|language=en-GB|issn=0261-3077|archive-url=https://web.archive.org/web/20200330235911/https://www.theguardian.com/world/2020/mar/30/czechs-get-to-work-making-masks-after-government-decree-coronavirus|archive-date=30 March 2020|url-status=live}}</ref> Currently, there is no ] or specific ] for COVID-19.<ref name=WHO2020QA/><!--To date, there is no vaccine and no specific antiviral medicine to prevent or treat COVID-2019.--> Management involves ], ], ] and ].<ref name="cdc21Jan20202">{{cite web|url=https://www.cdc.gov/coronavirus/2019-ncov/about/prevention-treatment.html|title=Coronavirus Disease 2019 (COVID-19)|date=15 February 2020|publisher=] (CDC)|url-status=live|archive-url=https://web.archive.org/web/20200226145347/https://www.cdc.gov/coronavirus/2019-ncov/about/prevention-treatment.html|archive-date=26 February 2020|access-date=20 February 2020| name-list-format = vanc}}</ref> | |||
| ] to detect the virus's ] include real-time ] (RT{{nbhyph}}PCR),<ref name="CDC testing">{{#invoke:cite web||title=Overview of Testing for SARS-CoV-2, the virus that causes COVID-19|publisher=U.S. ] (CDC)|date=11 February 2020|url=https://www.cdc.gov/coronavirus/2019-ncov/hcp/testing-overview.html|access-date=31 July 2022}}</ref><ref name="CDC NAATs">{{#invoke:cite web||title=Nucleic Acid Amplification Tests (NAATs)|publisher=U.S. ] (CDC)|date=11 February 2020|url=https://www.cdc.gov/coronavirus/2019-ncov/lab/naats.html|access-date=31 July 2022}}</ref> ],<ref name="CDC testing" /><ref name="CDC NAATs" /><ref>{{#invoke:cite journal ||vauthors=Gorzalski AJ, Tian H, Laverdure C, Morzunov S, Verma SC, VanHooser S, Pandori MW | date=August 2020 |title=High-Throughput Transcription-mediated amplification on the Hologic Panther is a highly sensitive method of detection for SARS-CoV-2 |journal=Journal of Clinical Virology |volume=129 |pages=104501 |doi=10.1016/j.jcv.2020.104501 |pmc=7286273 |pmid=32619959}}</ref> and ] (RT{{nbhyph}}LAMP)<ref name="CDC testing" /><ref name="CDC NAATs" /> from a ].<ref name="pmid32621814" /> | |||
| Several ]s have been approved and distributed in various countries, many of which have initiated ]s. Other ]s include physical or ], ], ventilation of indoor spaces, ] in public, covering coughs and sneezes, ], and keeping unwashed hands away from the face. While ] to inhibit the virus, the primary ] is still ], managing the disease through ], ], and ]s. | |||
| The first known case was ],<!-- Wuhan is the capital of Hubei --> China, in December 2019.<ref name="WSJ-20210226">{{#invoke:cite news||vauthors=Page J, Hinshaw D, McKay B|title=In Hunt for Covid-19 Origin, Patient Zero Points to Second Wuhan Market – The man with the first confirmed infection of the new coronavirus told the WHO team that his parents had shopped there|url=https://www.wsj.com/articles/in-hunt-for-covid-19-origin-patient-zero-points-to-second-wuhan-market-11614335404|date=26 February 2021|work=]|access-date=27 February 2021}}</ref> Most scientists believe the SARS-CoV-2 virus entered into human populations through natural ], similar to the ] and ] outbreaks, and consistent with other pandemics in human history.<ref name="pekar">{{#invoke:cite journal||last1=Pekar|first1=Jonathan|title=The molecular epidemiology of multiple zoonotic origins of SARS-CoV-2|journal=Science|date=26 July 2022|volume=377|issue=6609|pages=960–966|doi=10.1126/science.abp8337|pmid=35881005|pmc=9348752|bibcode=2022Sci...377..960P}}</ref><ref name="jiang_wang">{{#invoke:cite journal||last1=Jiang|first1=Xiaowei|last2=Wang|first2=Ruoqi|title=Wildlife trade is likely the source of SARS-CoV-2|journal=Science|date=25 August 2022|volume=377|issue=6609|pages=925–926|doi=10.1126/science.add8384|pmid=36007033|bibcode=2022Sci...377..925J|s2cid=251843410|url=https://www.science.org/doi/10.1126/science.add8384|access-date=20 November 2022}}</ref> Social and environmental factors including ], ] and ] increased the likelihood of such ].<ref name="IPCC-2022a">{{#invoke:cite book||title=Terrestrial and Freshwater Ecosystems and Their Services. In: Climate Change 2022: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change|date=2022|publisher=IPCC|pages=233–235|url=https://www.ipcc.ch/report/ar6/wg2/downloads/report/IPCC_AR6_WGII_Chapter02.pdf|access-date=14 March 2023}}</ref><ref name="IPCC-2022b">{{#invoke:cite book||title=Health, Wellbeing, and the Changing Structure of Communities. In: Climate Change 2022: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change|date=2022|publisher=IPCC|pages=1067–1070|url=https://www.ipcc.ch/report/ar6/wg2/downloads/report/IPCC_AR6_WGII_Chapter07.pdf|access-date=14 March 2023}}</ref><ref name="University-of-Cambridge-2021">{{#invoke:cite web||title=Climate change may have driven the emergence of SARS-CoV-2|url=https://www.cam.ac.uk/research/news/climate-change-may-have-driven-the-emergence-of-sars-cov-2|website=University of Cambridge|date=5 February 2021|publisher=Science of the Total Environment|access-date=14 March 2023}}</ref><ref name="European-Commission">{{#invoke:cite web||title=Climate change the culprit in the COVID-19 pandemic|url=https://cordis.europa.eu/article/id/430229-climate-change-the-culprit-in-the-covid-19-pandemic|website=European Commission|access-date=24 March 2023}}</ref> | |||
| <!--Epidemiology and history--> | |||
| The ] (WHO) declared the 2019–20 coronavirus ] a ] (PHEIC)<ref>{{cite web|url=https://www.who.int/news-room/detail/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov)|title=Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV)|website=] (WHO)|access-date=11 February 2020|archive-url=https://web.archive.org/web/20200131005904/https://www.who.int/news-room/detail/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov)|archive-date=31 January 2020|url-status=live| name-list-format = vanc}}</ref><ref>{{Cite news | author-last1=Mahtani |author-first1=S. |author-last2=Berger |author-first2=M. |author-last3=O'Grady |author-first3=S. |author-last4=Iati |author-first4=M. | url = https://www.washingtonpost.com/world/asia_pacific/coronavirus-china-live-updates/2020/02/05/114ced8a-479c-11ea-bc78-8a18f7afcee7_story.html |title = Hundreds of evacuees to be held on bases in California; Hong Kong and Taiwan restrict travel from mainland China | work = ] | date = 6 February 2020 | access-date = 11 February 2020 | archive-url = https://web.archive.org/web/20200207134650/https://www.washingtonpost.com/world/asia_pacific/coronavirus-china-live-updates/2020/02/05/114ced8a-479c-11ea-bc78-8a18f7afcee7_story.html | archive-date = 7 February 2020 | url-status = live }}</ref> on 30 January 2020 and a ] on 11 March 2020.<ref name="WHOPandemic" /> ] of the disease has been recorded in many countries across all six ].<ref>{{cite web|url=http://who.int/docs/default-source/coronaviruse/situation-reports/20200325-sitrep-65-covid-19.pdf|title=WHO Situation Report #65|last=|first=|date=25 March 2020|website=WHO|url-status=live|archive-url=|archive-date=|access-date=}}</ref> | |||
| ])]] | |||
| {{TOC limit}} | {{TOC limit}} | ||
| == Nomenclature == | |||
| ==Signs and symptoms== | |||
| {{Main|COVID-19 naming}} | |||
| During the initial outbreak in ], the virus and disease were commonly referred to as "coronavirus" and "Wuhan coronavirus",<ref>{{#invoke:cite news ||url=https://www.npr.org/sections/health-shots/2020/01/24/799208865/a-second-u-s-case-of-wuhan-coronavirus-is-confirmed|title=2nd U.S. Case Of Wuhan Coronavirus Confirmed|publisher=NPR|access-date=4 April 2020}}</ref><ref>{{#invoke:cite news ||vauthors=McNeil Jr DG |author-link=Donald McNeil Jr. |url=https://www.nytimes.com/2020/02/02/health/coronavirus-pandemic-china.html |archive-url=https://web.archive.org/web/20200202194034/https://www.nytimes.com/2020/02/02/health/coronavirus-pandemic-china.html |archive-date=2 February 2020 |url-access=subscription |url-status=live|title=Wuhan Coronavirus Looks Increasingly Like a Pandemic, Experts Say|date=2 February 2020|work=]|access-date=4 April 2020 |issn=0362-4331}}</ref><ref>{{#invoke:cite news||url=https://www.cnn.com/2020/02/05/asia/wuhan-coronavirus-update-death-toll-spike-intl-hnk/index.html|title=Wuhan coronavirus deaths spike again as outbreak shows no signs of slowing| vauthors = Griffiths J | publisher=CNN|access-date=4 April 2020 }}</ref> with the disease sometimes called "Wuhan pneumonia".<ref>{{#invoke:cite journal ||vauthors=Jiang S, Xia S, Ying T, Lu L |date=May 2020 |title=A novel coronavirus (2019-nCoV) causing pneumonia-associated respiratory syndrome |journal=Cellular & Molecular Immunology |volume=17 |issue=5 |pages=554 |doi=10.1038/s41423-020-0372-4 |pmc=7091741 |pmid=32024976 |doi-access=free |title-link=doi}}</ref><ref>{{#invoke:cite journal ||vauthors=Chan JF, Yuan S, Kok KH, To KK, Chu H, Yang J, Xing F, Liu J, Yip CC, Poon RW, Tsoi HW, Lo SK, Chan KH, Poon VK, Chan WM, Ip JD, Cai JP, Cheng VC, Chen H, Hui CK, Yuen KY |date=February 2020 |title=A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster |journal=Lancet |volume=395 |issue=10223 |pages=514–523 |doi=10.1016/S0140-6736(20)30154-9 |pmc=7159286 |pmid=31986261 |doi-access=free |title-link=doi}}</ref> In the past, many diseases have been named after geographical locations, such as the ],<ref>{{#invoke:cite journal ||vauthors=Shablovsky S |date=September 2017 |title=The legacy of the Spanish flu |journal=Science |volume=357 |issue=6357 |pages=1245 |bibcode=2017Sci...357.1245S |doi=10.1126/science.aao4093 |issn=0036-8075 |doi-access=free |s2cid=44116811 |title-link=doi}}</ref> ], and ].<ref name="Nature Stop">{{#invoke:cite journal ||title=Stop the coronavirus stigma now |url=https://www.nature.com/articles/d41586-020-01009-0 |access-date=16 April 2020 |journal=Nature |date=7 April 2020 |volume=580 |issue=7802 |pages=165 |doi=10.1038/d41586-020-01009-0|pmid=32265571 |bibcode=2020Natur.580..165. |s2cid=214809950}}</ref> In January 2020, the ] (WHO) recommended 2019-nCoV<ref>{{#invoke:cite web||url=https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200121-sitrep-1-2019-ncov.pdf|title=Novel Coronavirus (2019-nCoV) Situation Report – 1|date=21 January 2020|website=] (WHO)}}</ref> and 2019-nCoV acute respiratory disease<ref>{{#invoke:cite web||url=https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200130-sitrep-10-ncov.pdf|title=Novel Coronavirus(2019-nCoV) Situation Report – 10 |date=30 January 2020|website=] (WHO)}}</ref> as interim names for the virus and disease per 2015 guidance and international guidelines against using geographical locations or groups of people in disease and virus names to prevent ].<ref>{{#invoke:cite news ||title=Novel coronavirus named 'Covid-19': WHO |url=https://www.todayonline.com/world/wuhan-novel-coronavirus-named-covid-19-who |access-date=11 February 2020 |work=Today|location=Singapore |archive-url=https://archive.today/20200321085608/https://www.todayonline.com/world/wuhan-novel-coronavirus-named-covid-19-who |archive-date=21 March 2020 |url-status=live}}</ref><ref name="veconomist">{{#invoke:cite news ||title=The coronavirus spreads racism against – and among – ethnic Chinese |url=https://www.economist.com/china/2020/02/17/the-coronavirus-spreads-racism-against-and-among-ethnic-chinese |newspaper=] |date=17 February 2020 |access-date=17 February 2020 |archive-url=https://web.archive.org/web/20200217223902/https://www.economist.com/china/2020/02/17/the-coronavirus-spreads-racism-against-and-among-ethnic-chinese |archive-date=17 February 2020 |url-status=live}}</ref><ref>{{#invoke:cite report||url=https://apps.who.int/iris/bitstream/handle/10665/163636/WHO_HSE_FOS_15.1_eng.pdf|title=World Health Organization Best Practices for the Naming of New Human Infectious Diseases |date=May 2015|publisher=] (WHO) |hdl=10665/163636 |hdl-access=free}}</ref> The official names COVID‑19 and SARS-CoV-2 were issued by the WHO on 11 February 2020 with COVID-19 being shorthand for "coronavirus disease 2019".<ref name="WHO-naming">{{#invoke:cite web||url=https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it|title=Naming the coronavirus disease (COVID-19) and the virus that causes it|website=] (WHO)|access-date=13 March 2020|archive-url=https://web.archive.org/web/20200228035651/https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it|archive-date=28 February 2020|url-status=live}}</ref><ref>{{#invoke:cite web ||title=Novel Coronavirus(2019-nCoV) Situation Report – 22 |url=https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200211-sitrep-22-ncov.pdf |publisher=WHO |date=11 February 2020}}</ref> The WHO additionally uses "the COVID‑19 virus" and "the virus responsible for COVID‑19" in public communications.<ref name="WHO-naming" /><ref>{{#invoke:cite journal ||vauthors=Gover AR, Harper SB, Langton L |date=July 2020 |title=Anti-Asian Hate Crime During the COVID-19 Pandemic: Exploring the Reproduction of Inequality |journal=American Journal of Criminal Justice |volume=45 |issue=4 |pages=647–667 |doi=10.1007/s12103-020-09545-1 |pmc=7364747 |pmid=32837171}}</ref> | |||
| == Symptoms and signs == | |||
| {{clear}} | |||
| {{Main|Symptoms of COVID-19}} | |||
| {| class="wikitable" style = "float:right; margin-left:1em; text-align:center" | |||
| <!-- TO EDIT THIS SECTION, GO TO ]. --> | |||
| !Symptom<ref name="WHOReport24Feb2020">{{cite report | title = Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19) | date = 16–24 February 2020 | url = https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf | publisher = ] (WHO) | access-date = 21 March 2020 | archive-url = https://web.archive.org/web/20200229221222/https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf | archivedate = 29 February 2020 | url-status = live }}</ref> | |||
| {{Excerpt|Symptoms of COVID-19|hat=no}} | |||
| !% | |||
| |- | |||
| |Fever | |||
| |88 | |||
| |- | |||
| |Dry cough | |||
| |68 | |||
| |- | |||
| |Fatigue | |||
| |38 | |||
| |- | |||
| |] production | |||
| |33 | |||
| |- | |||
| | ] | |||
| | 15<ref name=Palus/> to 30<ref name="entuk-anosmia"/><ref name="Iacobucci2020"/> | |||
| |- | |||
| |Shortness of breath | |||
| |19 | |||
| |- | |||
| |] or ] | |||
| |15 | |||
| |- | |||
| |Sore throat | |||
| |14 | |||
| |- | |||
| |Headache | |||
| |14 | |||
| |- | |||
| |Chills | |||
| |11 | |||
| |- | |||
| |Nausea or vomiting | |||
| |5 | |||
| |- | |||
| |Nasal congestion | |||
| |5 | |||
| |- | |||
| |] | |||
| |4 to 31<ref name=":10" /> | |||
| |- | |||
| |] | |||
| |0.9 | |||
| |- | |||
| |]s | |||
| |0.8 | |||
| |} | |||
| === Complications === | |||
| Those infected with the virus may be ] or develop ] including fever, cough, fatigue and shortness of breath.<ref name="CDC2020Sym"><!--KEEP THIS NAMED REFERENCE-->{{cite web|url=https://www.cdc.gov/coronavirus/2019-ncov/about/symptoms.html|title=Coronavirus Disease 2019 (COVID-19) Symptoms|date=10 February 2020|website=]|location=United States|url-status=live|archive-url=https://web.archive.org/web/20200130202038/https://www.cdc.gov/coronavirus/2019-ncov/about/symptoms.html|archive-date=30 January 2020|access-date=| name-list-format = vanc}}</ref><ref name=":2">{{cite journal |author-last1= Chen |author-first1=N. |author-last2=Zhou |author-first2=M. |author-last3=Dong |author-first3=X. |author-last4=Qu |author-first4=J. |author-last5=Gong |author-first5=F. |author-last6=Han |author-first6=Y. |author-last7=Qiu |author-first7=Y. |author-last8=Wang |author-first8=J. |author-last9=Liu |author-first9=Y. |author-last10=Wei |author-first10=Y. |author-last11=Xia |author-first11=J. |author-last12=Yu |author-first12=T. |author-last13=Zhang |author-first13=X. |author-last14=Zhang |author-first14=L. | title = Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study | language = English | journal = Lancet | volume = 395 | issue = 10223 | pages = 507–513 | date = February 2020 | pmid = 32007143 | doi = 10.1016/S0140-6736(20)30211-7 | doi-access = free }}</ref><ref name="Hessen27Jan2020">{{cite web |url=https://www.elsevier.com/connect/coronavirus-information-center |title=Novel Coronavirus Information Center: Expert guidance and commentary |last=Hessen |first=Margaret Trexler | name-list-format = vanc |date=27 January 2020 |website=Elsevier Connect |url-status=live |access-date=31 January 2020 |archive-url=https://web.archive.org/web/20200130171622/https://www.elsevier.com/connect/coronavirus-information-center |archive-date=30 January 2020 }}</ref> Emergency symptoms include difficulty breathing, persistent chest pain or pressure, confusion, difficulty waking and bluish face or lips; immediate medical attention is advised if these symptoms are present.<ref>{{cite web |title=Coronavirus Disease 2019 (COVID-19)—Symptoms |url=https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html |website=Centers for Disease Control and Prevention |access-date=21 March 2020 |language=en-us |date=20 March 2020 |archive-url=https://web.archive.org/web/20200320231801/https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html |archive-date=20 March 2020 |url-status=live }}</ref> Less commonly, ] symptoms, such as ], ] or ] may be seen. Symptoms such as ], ] and ] have been observed in varying percentages.<ref name=":10">{{Cite news|title=Clinical Characteristics of SARS-CoV-2 Infected Pneumonia with Diarrhea|first1=Xiao-Shan|last1=Wei|first2=Xuan|last2=Wang|first3=Yi-Ran|last3=Niu|first4=Lin-Lin|last4=Ye|first5=Wen-Bei|last5=Peng|first6=Zi-Hao|last6=Wang|first7=Wei-Bing|last7=Yang|first8=Bo-Han|last8=Yang|first9=Jian-Chu|last9=Zhang|first10=Wan-Li|last10=Ma|first11=Xiao-Rong|last11=Wang|first12=Qiong|last12=Zhou|date=26 February 2020|doi=10.2139/ssrn.3546120|ssrn = }}</ref><ref name="Huang24Jan2020">{{cite journal | vauthors = Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B | display-authors = 6 | title = Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China | journal = Lancet | volume = 395 | issue = 10223 | pages = 497–506 | date = February 2020 | pmid = 31986264 | doi = 10.1016/S0140-6736(20)30183-5 | doi-access = free }}</ref><ref>{{Cite journal|last1=Lai|first1=Chih-Cheng|last2=Shih|first2=Tzu-Ping|last3=Ko|first3=Wen-Chien|last4=Tang|first4=Hung-Jen|last5=Hsueh|first5=Po-Ren|date=1 March 2020|title=Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges|journal=International Journal of Antimicrobial Agents|language=en|volume=55|issue=3|page=105924|doi=10.1016/j.ijantimicag.2020.105924|pmid=32081636|issn=0924-8579}}</ref> Some cases in China initially presented only with ] and ].<ref name="Zheng Ma Zhang Xie p.">{{cite journal | vauthors = Zheng YY, Ma YT, Zhang JY, Xie X | title = COVID-19 and the cardiovascular system | journal = Nature Reviews. Cardiology | date = March 2020 | pmid = 32139904 | doi = 10.1038/s41569-020-0360-5 | doi-access = free }}</ref> In March 2020 there were reports indicating that ] (anosmia) may be a common symptom among those who have mild disease,<ref name="entuk-anosmia">{{cite web|url=https://www.entuk.org/loss-sense-smell-marker-covid-19-infection|title=Loss of sense of smell as marker of COVID-19 infection|last=Hopkins|first=Claire|date=|website=Ear, Nose and Throat surgery body of United Kingdom|url-status=live|archive-url=|archive-date=|access-date=|accessdate=2020-03-28}}</ref><ref name="Iacobucci2020">{{cite journal|last1=Iacobucci|first1=Gareth|title=Sixty seconds on . . . anosmia|journal=BMJ|year=2020|volume=368|pages=m1202|issn=1756-1833|doi=10.1136/bmj.m1202|pmid=32209546}}</ref> although not as common as initially reported.<ref name=Palus>{{cite web|url=https://slate.com/technology/2020/03/coronavirus-sense-of-smell-nytimes-fact-check.html|title=The Key Stat in the NYTimes' Piece About Losing Your Sense of Smell Was Wrong|last=Palus|first=Shannon|date=2020-03-27|website=Slate Magazine|language=en|access-date=2020-03-28|archive-url=https://web.archive.org/web/20200328163737/https://slate.com/technology/2020/03/coronavirus-sense-of-smell-nytimes-fact-check.html|archive-date=28 March 2020|url-status=live}}</ref> In some, the disease may progress to ], ] and ].<ref name="Hui14Jan2020" /><ref name="WHO-q-a" /> In those who develop severe symptoms, time from symptom onset to needing ] is typically eight days.<ref>{{cite web |title=Coronavirus Disease 2019 (COVID-19) |url=https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html |website=Centers for Disease Control and Prevention |language=en-us |date=11 February 2020 |access-date=26 March 2020 |archive-url=https://web.archive.org/web/20200302201644/https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html |archive-date=2 March 2020 |url-status=live }}</ref> | |||
| ] and complications]] | |||
| Complications may include ], ] (ARDS), ], ], and death.<ref name="Hui14Jan2020">{{#invoke:cite journal || vauthors = Hui DS, I Azhar E, Madani TA, Ntoumi F, Kock R, Dar O, Ippolito G, Mchugh TD, Memish ZA, Drosten C, Zumla A, Petersen E | title = The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health – The latest 2019 novel coronavirus outbreak in Wuhan, China | journal = International Journal of Infectious Diseases | volume = 91 | pages = 264–266 | date = February 2020 | pmid = 31953166 | pmc = 7128332 | doi = 10.1016/j.ijid.2020.01.009 | title-link = doi | doi-access = free }}</ref><ref>{{#invoke:cite journal || vauthors = Murthy S, Gomersall CD, Fowler RA | title = Care for Critically Ill Patients With COVID-19 | journal = JAMA | volume = 323 | issue = 15 | pages = 1499–1500 | date = April 2020 | pmid = 32159735 | doi = 10.1001/jama.2020.3633 | doi-access = free | title-link = doi }}</ref><ref name="StatPearls">{{#invoke:cite book||title=StatPearls|vauthors=Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R|date=2020|publisher=StatPearls Publishing|location=Treasure Island (FL)|chapter=Features, Evaluation and Treatment Coronavirus (COVID-19)|pmid=32150360|access-date=18 March 2020|chapter-url=https://www.ncbi.nlm.nih.gov/books/NBK554776/}}</ref><ref name="Heymann Shindo 2020 pp. 542–545222">{{#invoke:cite journal || vauthors = Heymann DL, Shindo N | title = COVID-19: what is next for public health? | journal = Lancet | volume = 395 | issue = 10224 | pages = 542–545 | date = February 2020 | pmid = 32061313 | pmc = 7138015 | doi = 10.1016/s0140-6736(20)30374-3 | collaboration = WHO Scientific and Technical Advisory Group for Infectious Hazards }}</ref> Cardiovascular complications may include heart failure, ]s (including ]), ], ], particularly ],<ref>{{#invoke:cite journal || vauthors = Romiti GF, Corica B, Lip GY, Proietti M | title = Prevalence and Impact of Atrial Fibrillation in Hospitalized Patients with COVID-19: A Systematic Review and Meta-Analysis | journal = Journal of Clinical Medicine | volume = 10 | issue = 11 | pages = 2490 | date = June 2021 | pmid = 34199857 | doi = 10.3390/jcm10112490 | pmc = 8200114 | doi-access = free | title-link = doi }}</ref><ref>{{#invoke:cite journal || vauthors = Wen W, Zhang H, Zhou M, Cheng Y, Ye L, Chen J, Wang M, Feng Z | title = Arrhythmia in patients with severe coronavirus disease (COVID-19): a meta-analysis | journal = European Review for Medical and Pharmacological Sciences | volume = 24 | issue = 21 | pages = 11395–11401 | date = November 2020 | pmid = 33215461 | doi = 10.26355/eurrev_202011_23632 | s2cid = 227077132 }}</ref><ref name="Long-2020">{{#invoke:cite journal || vauthors = Long B, Brady WJ, Koyfman A, Gottlieb M | title = Cardiovascular complications in COVID-19 | journal = The American Journal of Emergency Medicine | volume = 38 | issue = 7 | pages = 1504–1507 | date = July 2020 | pmid = 32317203 | pmc = 7165109 | doi = 10.1016/j.ajem.2020.04.048 }}</ref><ref>{{#invoke:cite journal || vauthors = Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, Shchendrygina A, Escher F, Vasa-Nicotera M, Zeiher AM, Vehreschild M, Nagel E | title = Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID-19) | journal = JAMA Cardiology | volume = 5 | issue = 11 | pages = 1265–1273 | date = November 2020 | pmid = 32730619 | pmc = 7385689 | doi = 10.1001/jamacardio.2020.3557 | title-link = doi | doi-access = free }}</ref><ref>{{#invoke:cite journal || vauthors = Lindner D, Fitzek A, Bräuninger H, Aleshcheva G, Edler C, Meissner K, Scherschel K, Kirchhof P, Escher F, Schultheiss HP, Blankenberg S, Püschel K, Westermann D | title = Association of Cardiac Infection With SARS-CoV-2 in Confirmed COVID-19 Autopsy Cases | journal = JAMA Cardiology | volume = 5 | issue = 11 | pages = 1281–1285 | date = November 2020 | pmid = 32730555 | pmc = 7385672 | doi = 10.1001/jamacardio.2020.3551 | title-link = doi | doi-access = free }}</ref><ref>{{#invoke:cite journal || vauthors = Siripanthong B, Nazarian S, Muser D, Deo R, Santangeli P, Khanji MY, Cooper LT, Chahal CA | title = Recognizing COVID-19-related myocarditis: The possible pathophysiology and proposed guideline for diagnosis and management | journal = Heart Rhythm | volume = 17 | issue = 9 | pages = 1463–1471 | date = September 2020 | pmid = 32387246 | pmc = 7199677 | doi = 10.1016/j.hrthm.2020.05.001 }}</ref> and endothelial cell injury and dysfunction.<ref>{{Cite journal |last1=Perico |first1=Luca |last2=Benigni |first2=Ariela |last3=Remuzzi |first3=Giuseppe |date=January 2024 |title=SARS-CoV-2 and the spike protein in endotheliopathy |journal=Trends in Microbiology |volume=32 |issue=1 |pages=53–67 |doi=10.1016/j.tim.2023.06.004|pmid=37393180 |pmc=10258582 }}</ref> Approximately 20–30% of people who present with COVID‑19 have ], reflecting liver injury.<ref>{{#invoke:cite journal || vauthors = Xu L, Liu J, Lu M, Yang D, Zheng X | title = Liver injury during highly pathogenic human coronavirus infections | journal = Liver International | volume = 40 | issue = 5 | pages = 998–1004 | date = May 2020 | pmid = 32170806 | pmc = 7228361 | doi = 10.1111/liv.14435 | title-link = doi | doi-access = free }}</ref><ref name="Sanders202022">{{#invoke:cite journal || vauthors = Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB | title = Pharmacologic Treatments for Coronavirus Disease 2019 (COVID-19): A Review | journal = JAMA | volume = 323 | issue = 18 | pages = 1824–1836 | date = May 2020 | pmid = 32282022 | doi = 10.1001/jama.2020.6019 | doi-access = free | title-link = doi }}</ref> | |||
| Neurologic manifestations include ], stroke, ], and ] (which includes ]s).<ref name="Carod-Artal-2020">{{#invoke:cite journal || vauthors = Carod-Artal FJ | title = Neurological complications of coronavirus and COVID-19 | journal = Revista de Neurología | volume = 70 | issue = 9 | pages = 311–322 | date = May 2020 | pmid = 32329044 | doi = 10.33588/rn.7009.2020179 | s2cid = 226200547 }}</ref><ref>{{#invoke:cite journal || vauthors = Toscano G, Palmerini F, Ravaglia S, Ruiz L, Invernizzi P, Cuzzoni MG, Franciotta D, Baldanti F, Daturi R, Postorino P, Cavallini A, Micieli G | title = Guillain-Barré Syndrome Associated with SARS-CoV-2 | journal = The New England Journal of Medicine | volume = 382 | issue = 26 | pages = 2574–2576 | date = June 2020 | pmid = 32302082 | pmc = 7182017 | doi = 10.1056/NEJMc2009191 }}</ref> Following the infection, children may develop ], which has symptoms similar to ], which can be fatal.<ref>{{#invoke:Cite web||title=Multisystem inflammatory syndrome in children and adolescents temporally related to COVID-19 |url=https://www.who.int/news-room/commentaries/detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19 |website=] (WHO) |date=15 May 2020|access-date=20 May 2020}}</ref><ref>{{#invoke:cite report ||title=HAN Archive – 00432 |url=https://emergency.cdc.gov/han/2020/han00432.asp |website=U.S. ] (CDC) |access-date=20 May 2020 |date=15 May 2020}}</ref> In very rare cases, acute ] can occur, and it can be considered in those who have been diagnosed with COVID‑19 and have an altered mental status.<ref>{{#invoke:cite journal || vauthors = Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B | title = COVID-19-associated Acute Hemorrhagic Necrotizing Encephalopathy: Imaging Features | journal = Radiology | volume = 296 | issue = 2 | pages = E119–E120 | date = August 2020 | pmid = 32228363 | pmc = 7233386 | doi = 10.1148/radiol.2020201187 }}</ref> | |||
| As is common with infections, there is a delay between the moment when a person is infected with the virus and the time when they develop symptoms. This is called the ]. The incubation period for COVID-19 is typically five to six days but may range from two to 14 days.<ref>{{Cite document | vauthors=((World Health Organization)) |title=Coronavirus disease 2019 (COVID-19): situation report, 29 |date=19 February 2020|website=] (WHO) | hdl=10665/331118 | hdl-access=free }}</ref><ref>{{cite web|url=https://www.who.int/news-room/q-a-detail/q-a-coronaviruses|title=Q&A on coronaviruses (COVID-19): How long is the incubation period for COVID-19?|date=|website=] (WHO)|url-status=live|archive-url=https://web.archive.org/web/20200120174649/https://www.who.int/news-room/q-a-detail/q-a-coronaviruses|archive-date=20 January 2020|access-date=26 February 2020| name-list-format = vanc}}</ref> 97.5% of people who develop symptoms will do so within 11.5 days of infection.<ref>{{cite journal|last1=Lauer|first1=Stephen A.|last2=Grantz|first2=Kyra H.|last3=Bi|first3=Qifang|last4=Jones|first4=Forrest K.|last5=Zheng|first5=Qulu|last6=Meredith|first6=Hannah R.|last7=Azman|first7=Andrew S.|last8=Reich|first8=Nicholas G.|last9=Lessler|first9=Justin|date=10 March 2020|title=The Incubation Period of Coronavirus Disease 2019 (COVID-19) From Publicly Reported Confirmed Cases: Estimation and Application|url=https://annals.org/aim/fullarticle/2762808/incubation-period-coronavirus-disease-2019-covid-19-from-publicly-reported|journal=Annals of Internal Medicine|language=en|doi=10.7326/M20-0504|pmid=32150748|pmc=7081172|issn=0003-4819|access-date=24 March 2020|archive-url=https://web.archive.org/web/20200324032020/https://annals.org/aim/fullarticle/2762808/incubation-period-coronavirus-disease-2019-covid-19-from-publicly-reported|archive-date=24 March 2020|url-status=live}}</ref> | |||
| According to the US ], pregnant women are at increased risk of becoming seriously ill from COVID‑19.<ref name="Cordoba-Vives-2020">{{#invoke:cite journal||vauthors=Córdoba-Vives S, Peñaranda G|date=April 2020|title=COVID-19 y Embarazo|url=https://revistamedicacr.com/index.php/rmcr/article/viewFile/288/265|journal=Medical Journal of Costa Rica |pages=629 |language=es|access-date=14 February 2022|archive-date=18 June 2021|archive-url=https://web.archive.org/web/20210618082133/http://revistamedicacr.com/index.php/rmcr/article/viewFile/288/265|url-status=usurped}}</ref> This is because pregnant women with COVID‑19 appear to be more likely to develop respiratory and obstetric complications that can lead to ], ] and ].<ref name="Cordoba-Vives-2020" /> | |||
| Reports indicate that not all who are infected develop symptoms, but their role in transmission is unknown.<ref>{{cite web|url=https://www.cdc.gov/coronavirus/2019-ncov/hcp/faq.html|title=Coronavirus Disease 2019 (COVID-19)|last=|date=2020-02-11|website=Centers for Disease Control and Prevention|language=en-us|access-date=2020-03-31|archive-url=https://web.archive.org/web/20200214023335/https://www.cdc.gov/coronavirus/2019-ncov/hcp/faq.html|archive-date=14 February 2020|url-status=live}}</ref> Preliminary evidence suggests asymptomatic cases may contribute to the spread of the disease.<ref>{{Cite journal|last=Bai|first=Yan|last2=Yao|first2=Lingsheng|last3=Wei|first3=Tao|last4=Tian|first4=Fei|last5=Jin|first5=Dong-Yan|last6=Chen|first6=Lijuan|last7=Wang|first7=Meiyun|date=2020-02-21|title=Presumed Asymptomatic Carrier Transmission of COVID-19|url=https://jamanetwork.com/journals/jama/fullarticle/2762028|journal=JAMA|language=en|doi=10.1001/jama.2020.2565|issn=0098-7484|pmc=7042844|pmid=32083643|access-date=8 March 2020|archive-url=https://web.archive.org/web/20200304210815/https://jamanetwork.com/journals/jama/fullarticle/2762028|archive-date=4 March 2020|url-status=live}}</ref><ref name=":1">{{cite web|url=https://www.bloomberg.com/news/articles/2020-03-31/china-reveals-1-541-symptom-free-virus-cases-under-pressure|title=China Reveals 1,541 Symptom-Free Virus Cases Under Pressure|last=|first=|date=31 March 2020|website=www.bloomberg.com|url-status=live|archive-url=|archive-date=|access-date=2020-03-31}}</ref> The proportion of infected people who do not display symptoms is currently unknown and being studied, with the ] (KCDC) reporting that 20% of all confirmed cases remained asymptomatic during their hospital stay.<ref name=":1" /><ref>{{cite web|url=http://www.ktv.go.kr/program/home/PG1110921D/content/595426|title=코로나19 국내 발생현황 브리핑 (20. 03. 16. 14시)|website=ktv.go.kr|language=ko|access-date=2020-03-31}}</ref> China’s ] began including asymptomatic cases in its daily cases on 1 April, of the 166 infections on that day, 130 (78%) were asymptomatic.<ref>{{Cite journal|last=Day|first=Michael|date=2020-04-02|title=Covid-19: four fifths of cases are asymptomatic, China figures indicate|url=http://www.bmj.com/lookup/doi/10.1136/bmj.m1375|journal=BMJ|language=en|pages=m1375|doi=10.1136/bmj.m1375|issn=1756-1833}}</ref> | |||
| Fungal infections such as ], ], ] and ] have been recorded in people recovering from COVID‑19.<ref>{{#invoke:cite journal ||vauthors=Das S, Dhar S |title=Mucormycosis Following COVID-19 Infections: an Insight |journal=The Indian Journal of Surgery |volume=84| pages=585–586 |date=July 2021 |issue=3 |pmid=34276145 |pmc=8270771 |doi=10.1007/s12262-021-03028-1 |s2cid=235782159}}</ref><ref>{{#invoke:cite journal ||vauthors=Baruah C, Devi P, Deka B, Sharma DK |date=June 2021|title=Mucormycosis and Aspergillosis have been Linked to Covid-19-Related Fungal Infections in India |url=https://www.researchgate.net/publication/352554687 |journal=Advancements in Case Studies|volume=3|issue=1|doi=10.31031/AICS.2021.03.000555|s2cid=244678882|issn=2639-0531|via=]}}</ref> | |||
| ==Cause== | |||
| == Cause == | |||
| {{See also|Severe acute respiratory syndrome coronavirus 2}} | |||
| COVID‑19 is caused by infection with a ] of ] known as "severe acute respiratory syndrome coronavirus 2" (]).<ref>{{#invoke:cite journal ||vauthors=Hu B, Guo H, Zhou P, Shi ZL |date=March 2021 |title=Characteristics of SARS-CoV-2 and COVID-19 |journal=Nature Reviews. Microbiology |volume=19 |issue=3 |pages=141–154 |doi=10.1038/s41579-020-00459-7 |pmc=7537588 |pmid=33024307}}</ref> | |||
| ===Transmission=== | === Transmission === | ||
| {{Main|Transmission of COVID-19}} | |||
| <!-- TO EDIT THIS SECTION, GO TO ]. --> | |||
| ] of COVID‑19]] | |||
| {{Excerpt|Transmission of COVID-19|paragraphs=1–4|hat=no}} | |||
| === Virology === | |||
| ]]] | |||
| {{Main|SARS-CoV-2}} | |||
| ] and ] in the context of the pandemic]] | |||
| ] ]]] | |||
| Severe acute respiratory syndrome coronavirus{{spaces}}2 (SARS-CoV-2) is a ] severe acute respiratory syndrome coronavirus. It was first isolated from three people with pneumonia connected to the ] of acute respiratory illness cases in Wuhan.<ref name="ECDC risk assessment">{{#invoke:Cite web||url=https://www.ecdc.europa.eu/sites/default/files/documents/SARS-CoV-2-risk-assessment-14-feb-2020.pdf |title=Outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): increased transmission beyond China – fourth update |publisher=European Centre for Disease Prevention and Control |date=14 February 2020 |access-date=8 March 2020}}</ref> All structural features of the novel SARS-CoV-2 virus particle occur in related ]es in nature,<ref name="NM-20200317" /> particularly in '']'' (Chinese horseshoe bats).<ref name="zhou20">{{#invoke:cite journal ||doi=10.1038/s41586-020-2012-7|title=A pneumonia outbreak associated with a new coronavirus of probable bat origin |year=2020 |last1=Zhou |first1=Peng |last2=Yang |first2=Xing-Lou |last3=Wang |first3=Xian-Guang |last4=Hu |first4=Ben |last5=Zhang |first5=Lei |last6=Zhang |first6=Wei |last7=Si |first7=Hao-Rui |last8=Zhu |first8=Yan |last9=Li |first9=Bei |last10=Huang |first10=Chao-Lin |last11=Chen |first11=Hui-Dong |last12=Chen |first12=Jing |last13=Luo |first13=Yun |last14=Guo |first14=Hua |last15=Jiang |first15=Ren-Di |last16=Liu |first16=Mei-Qin |last17=Chen |first17=Ying |last18=Shen |first18=Xu-Rui |last19=Wang |first19=Xi |last20=Zheng |first20=Xiao-Shuang |last21=Zhao |first21=Kai |last22=Chen |first22=Quan-Jiao |last23=Deng |first23=Fei |last24=Liu |first24=Lin-Lin |last25=Yan |first25=Bing |last26=Zhan |first26=Fa-Xian |last27=Wang |first27=Yan-Yi |last28=Xiao |first28=Geng-Fu |last29=Shi |first29=Zheng-Li |journal=Nature |volume=579 |issue=7798 |pages=270–273 |pmid=32015507 |pmc=7095418 |bibcode=2020Natur.579..270Z }}</ref> | |||
| Outside the human body, the virus is destroyed by household soap which bursts its ].<ref name="NatGeoSoap">{{#invoke:Cite web||url=https://www.nationalgeographic.com/science/2020/03/why-soap-preferable-bleach-fight-against-coronavirus/ |vauthors=Gibbens S |title=Why soap is preferable to bleach in the fight against coronavirus |date=18 March 2020 |website=] |url-status=live |archive-url=https://web.archive.org/web/20200402001042/https://www.nationalgeographic.com/science/2020/03/why-soap-preferable-bleach-fight-against-coronavirus/ |archive-date=2 April 2020 |access-date=2 April 2020}}</ref> Hospital disinfectants, alcohols, heat, ], and ]-C (UV-C) irradiation are also effective disinfection methods for surfaces.<ref>{{#invoke:cite journal ||last1=Viana Martins |first1=C. P. |last2=Xavier |first2=C. S. F. |last3=Cobrado |first3=L. |date=2022 |title=Disinfection methods against SARS-CoV-2: a systematic review |url= |journal=The Journal of Hospital Infection |volume=119 |pages=84–117 |doi=10.1016/j.jhin.2021.07.014 |issn=1532-2939 |pmc=8522489 |pmid=34673114}}</ref> | |||
| Some details about how the disease is ] are still being determined.<ref name="CDCTrans">{{cite web|url=https://www.cdc.gov/coronavirus/2019-ncov/prepare/transmission.html|title=Coronavirus Disease 2019 (COVID-19)—Transmission|last=|first=|date=April 2, 2020|website=Centers for Disease Control and Prevention|language=en-us|url-status=live|archive-url=https://web.archive.org/web/20200403001235/https://www.cdc.gov/coronavirus/2019-ncov/prepare/transmission.html|archive-date=April 3, 2020|accessdate=April 3, 2020}}</ref><!--Quote: "We are still learning how it spreads"--><ref name="ECDCQA">{{cite web|url=https://www.ecdc.europa.eu/en/novel-coronavirus-china/questions-answers|title=Q & A on COVID-19|website=European Centre for Disease Prevention and Control|language=en|url-status=live|archive-url=https://web.archive.org/web/20200205054338/https://www.ecdc.europa.eu/en/novel-coronavirus-china/questions-answers|archive-date=5 February 2020|accessdate=23 March 2020}}</ref> The WHO and the US ] (CDC) say it is primarily spread during close contact and by ] produced when people cough, sneeze or talk;<ref name="WHO2020QA">{{cite web|url=https://www.who.int/news-room/q-a-detail/q-a-coronaviruses|title=Q&A on coronaviruses|date=11 February 2020|work=]|url-status=live|archive-url=https://web.archive.org/web/20200120174649/https://www.who.int/news-room/q-a-detail/q-a-coronaviruses|archive-date=20 January 2020|accessdate=24 February 2020}}</ref><ref name=CDCTrans/><!--The virus is thought to spread mainly from person-to-person ... Between people who are in close contact with one another ... Via respiratory droplets produced when an infected person coughs or sneezes.--><!--Quote: "The main way the disease spreads is through respiratory droplets expelled by someone who is coughing."--> with close contact being within {{cvt|1|–|3|m}}.<ref name=WHO2020QA/><!--This is why it is important to stay more than a meter (3 feet) away from a person who is sick.--> A study in Singapore found that an uncovered cough can lead to droplets travelling up to {{convert|4.5|m|ft|abbr=off|sp=us}}.<ref>{{cite journal |display-authors=etal |last1=Loh |first1=Ne-Hooi Will |last2=Tan |first2=Yanni |last3=Taculod |first3=Juvel H. |title=The Impact of High-Flow Nasal Cannula (HFNC) on Coughing Distance: Implications on Its Use During the Novel Coronavirus Disease Outbreak |journal=Canadian Journal of Anesthesia |date=18 March 2020 |doi=10.1007/s12630-020-01634-3 |pmid=32189218 |pmc=7090637}}</ref><ref>{{cite journal |last1=Bourouiba|first1=Lydia|title=Turbulent Gas Clouds and Respiratory Pathogen Emissions: Potential Implications for Reducing Transmission of COVID-19|journal=JAMA|date=26 March 2020 |pmid = 32215590| doi= 10.1001/jama.2020.4756}}</ref> A second study, produced during the 2020 pandemic, found that advice on the distance droplets could travel might be based on old 1930s research which ignored the protective effect and speed of the warm moist outbreath surrounding the droplets; it advised that droplets can travel around 7–8 metres.<ref>{{Cite journal| doi = 10.1001/jama.2020.4756| last = Bourouiba| first = Lydia| title = Turbulent Gas Clouds and Respiratory Pathogen Emissions: Potential Implications for Reducing Transmission of COVID-19| journal = JAMA| accessdate = 2020-04-06| date = 2020-03-26| url = https://jamanetwork.com/journals/jama/fullarticle/2763852}}</ref> | |||
| SARS-CoV-2 is closely related to the original ].<ref name="Zhu24Jan2020">{{#invoke:cite journal ||vauthors=Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W |title=A Novel Coronavirus from Patients with Pneumonia in China, 2019 |journal=The New England Journal of Medicine |volume=382 |issue=8 |pages=727–733 |date=February 2020 |pmid=31978945 |pmc=7092803 |doi=10.1056/NEJMoa2001017}}</ref> It is thought to have an animal (]) origin. Genetic analysis has revealed that the coronavirus genetically clusters with the genus '']'', in subgenus '']'' (lineage B) together with two bat-derived strains. It is 96% identical at the whole ] level to other bat coronavirus samples (BatCov ]).<ref name="WHOReport24Feb2020">{{#invoke:cite report ||url=https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf |title=Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19) |date=February 2020 |publisher=] (WHO) |access-date=21 March 2020 |archive-url=https://web.archive.org/web/20200229221222/https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf |archive-date=29 February 2020 |url-status=live}}</ref><ref>{{#invoke:Cite web|| title=Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19) | publisher=] (WHO) | url=https://www.who.int/publications/i/item/report-of-the-who-china-joint-mission-on-coronavirus-disease-2019-(covid-19) | access-date=25 January 2022}}</ref><ref name="RathoreSingh">{{#invoke:cite journal || vauthors = Rathore JS, Ghosh C | title = Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), a newly emerged pathogen: an overview | journal = Pathogens and Disease | volume = 78 | issue = 6 | date = August 2020 | pmid = 32840560 | pmc = 7499575 | doi = 10.1093/femspd/ftaa042 | oclc = 823140442 | doi-access = free | title-link = doi }}</ref> The structural proteins of SARS-CoV-2 include ] (M), ] (E), ] (N), and the ] (S). The M protein of SARS-CoV-2 is about 98% similar to the M protein of bat SARS-CoV, maintains around 98% homology with pangolin SARS-CoV, and has 90% homology with the M protein of SARS-CoV; whereas, the similarity is only around 38% with the M protein of ].<ref>{{#invoke:cite journal || vauthors = Thomas S | title = The Structure of the Membrane Protein of SARS-CoV-2 Resembles the Sugar Transporter SemiSWEET | journal = Pathogens & Immunity | volume = 5 | issue = 1 | pages = 342–363 | date = October 2020 | pmid = 33154981 | pmc = 7608487 | doi = 10.20411/pai.v5i1.377 }}</ref> | |||
| Respiratory droplets may also be produced while breathing out, including when talking. Though the virus is not generally ],<ref name=WHO2020QA/><!--when a person with COVID-19 coughs or exhales ... Studies to date suggest that the virus that causes COVID-19 is mainly transmitted through contact with respiratory droplets rather than through the air.--><ref name=WHOMar27Airborne>{{cite web |title=Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations |url=https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations |website=www.who.int |accessdate=29 March 2020 |language=en}}</ref><!--Based on the available evidence, including the recent publications mentioned above, the WHO continues to recommend droplet and contact precautions for those people caring for COVID-19 patients and contact and airborne precautions for circumstances and settings in which aerosol generating procedures are performed.--> The National Academy of Science has suggested that ] transmission may be possible and air collectors positioned in the hallway outside of people's rooms yielded samples positive for viral RNA.<ref>{{cite web|title=Rapid Expert Consultation on the Possibility of Bioaerosol Spread of SARS-CoV-2 for the COVID-19 Pandemic|date=1 April 2020|url=https://www.nap.edu/catalog/25769/rapid-expert-consultation-on-the-possibility-of-bioaerosol-spread-of-sars-cov-2-for-the-covid-19-pandemic-april-1-2020|publisher=The National Academies Press|accessdate=1 April 2020}}</ref> The droplets can land in the mouths or noses of people who are nearby or possibly be inhaled into the lungs.<ref>{{cite web |title=Coronavirus Disease 2019 (COVID-19)—Transmission |url=https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/how-covid-spreads.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fprepare%2Ftransmission.html |website=Centers for Disease Control and Prevention |accessdate=29 March 2020 |language=en-us |date=17 March 2020}}</ref><!--Quote: "These droplets can land in the mouths or noses of people who are nearby or possibly be inhaled into the lungs."--> Some medical procedures such as intubation and ] (CPR) may cause respiratory secretions to be aerosolised and thus result in airborne spread.<ref name=WHOMar27Airborne/> It may also spread when one touches a contaminated surface, known as ] transmission, and then touches ones eyes, nose or mouth.<ref name=WHO2020QA/><!--These droplets land on objects and surfaces around the person. Other people then catch COVID-19 by touching these objects or surfaces, then touching their eyes, nose or mouth.--> While there are concerns it may spread by ], this risk is believed to be low.<ref name=WHO2020QA/><!--The risk of catching COVID-19 from the feces of an infected person appears to be low.--><ref name=CDCTrans/><!--within about six feet--> | |||
| === SARS-CoV-2 variants === | |||
| The virus is most contagious when people are symptomatic; while spread may be possible before symptoms appear, this risk is low.<ref name=WHO2020QA/><!--Quote: "The risk of catching COVID-19 from someone with no symptoms at all is very low."--><ref name=CDCTrans/><!--Quote: "People are thought to be most contagious when they are most symptomatic (the sickest) Some spread might be possible before people show symptoms"--> The ] (ECDC) says while it is not entirely clear how easily the disease spreads, one person generally infects two to three others.<ref name="ECDCQA" /> | |||
| {{Main|Variants of SARS-CoV-2}} | |||
| The many thousands of SARS-CoV-2 variants are grouped into either ]s or ]s.<ref>{{#invoke:cite journal ||vauthors = Koyama T, Platt D, Parida L |title=Variant analysis of SARS-CoV-2 genomes |journal=Bulletin of the World Health Organization |volume=98 |issue=7 |pages=495–504 |date=July 2020 |pmid=32742035 |pmc=7375210 |doi=10.2471/BLT.20.253591 |doi-broken-date = 5 December 2024 |quote=We detected in total 65776 variants with 5775 distinct variants.}}</ref><ref name="Rambaut-2020"/> The WHO, in collaboration with partners, expert networks, national authorities, institutions and researchers, have established nomenclature systems for naming and tracking SARS-CoV-2 genetic lineages by ], ] and ]. The expert group convened by the WHO recommended the labelling of variants using letters of the ], for example, ], ], ], and ], giving the justification that they "will be easier and more practical to discussed by non-scientific audiences".<ref>{{#invoke:Cite web||date=1 July 2021|title=Tracking SARS-CoV-2 variants|url=https://www.who.int/activities/tracking-SARS-CoV-2-variants|access-date=5 July 2021|website=] (WHO) }}</ref> ] divides the variants into five clades (19A, 19B, 20A, 20B, and 20C), while ] divides them into seven (L, O, V, S, G, GH, and GR).<ref name="Alm2020Aug">{{#invoke:cite journal ||vauthors=Alm E, Broberg EK, Connor T, Hodcroft EB, Komissarov AB, Maurer-Stroh S, Melidou A, Neher RA, O'Toole Á, Pereyaslov D |title=Geographical and temporal distribution of SARS-CoV-2 clades in the WHO European Region, January to June 2020 |journal=Euro Surveillance |volume=25 |issue=32 |date=August 2020 |pmid=32794443 |pmc=7427299 |doi=10.2807/1560-7917.ES.2020.25.32.2001410}}</ref> The Pango tool groups variants into ]s, with many circulating lineages being classed under the B.1 lineage.<ref name="Rambaut-2020">{{#invoke:cite journal ||vauthors=Rambaut A, Holmes EC, O'Toole Á, Hill V, McCrone JT, Ruis C, du Plessis L, Pybus OG |title=A dynamic nomenclature proposal for SARS-CoV-2 lineages to assist genomic epidemiology |journal=Nature Microbiology |volume=5 |issue=11 |pages=1403–1407 |date=November 2020 |pmid=32669681 |pmc=7610519 |doi=10.1038/s41564-020-0770-5}}</ref><ref>{{#invoke:Cite web||title=PANGO lineages|url=https://cov-lineages.org/pango_lineages.html|access-date=9 May 2021|url-status=dead |website=cov-lineages.org|archive-date=10 May 2021|archive-url=https://web.archive.org/web/20210510111318/https://cov-lineages.org/pango_lineages.html}}</ref> | |||
| Several notable variants of SARS-CoV-2 emerged throughout 2020.<ref name="Lauring Hodcroft 2021 pp. 529–531">{{#invoke:cite journal ||vauthors=Lauring AS, Hodcroft EB |title=Genetic Variants of SARS-CoV-2-What Do They Mean? |journal=JAMA |volume=325 |issue=6 |pages=529–531 |date=February 2021 |pmid=33404586 |s2cid=230783233 |doi=10.1001/jama.2020.27124 |doi-access=free |title-link=doi}}</ref><ref name="Abdool Karim de Oliveira pp. 1866–1868">{{#invoke:cite journal ||vauthors=Abdool Karim SS, de Oliveira T |title=New SARS-CoV-2 Variants – Clinical, Public Health, and Vaccine Implications |journal=The New England Journal of Medicine |volume=384 |issue=19 |pages=1866–1868 |date=May 2021 |pmid=33761203 |doi=10.1056/nejmc2100362| issn=0028-4793 |publisher=Massachusetts Medical Society |pmc=8008749}}</ref> ] emerged among ]s and mink farmers in ].<ref>{{#invoke:cite journal ||vauthors=Mallapaty S |title=COVID mink analysis shows mutations are not dangerous – yet |journal=Nature |volume=587 |issue=7834 |pages=340–341 |date=November 2020 |pmid=33188367 |doi=10.1038/d41586-020-03218-z |doi-access=free |s2cid=226947606 |bibcode=2020Natur.587..340M}}</ref> After ] and the ], the cluster was assessed to no longer be circulating among humans in Denmark as of 1 February 2021.<ref>{{#invoke:cite journal ||vauthors=Larsen HD, Fonager J, Lomholt FK, Dalby T, Benedetti G, Kristensen B, Urth TR, Rasmussen M, Lassaunière R, Rasmussen TB, Strandbygaard B, Lohse L, Chaine M, Møller KL, Berthelsen AN, Nørgaard SK, Sönksen UW, Boklund AE, Hammer AS, Belsham GJ, Krause TG, Mortensen S, Bøtner A, Fomsgaard A, Mølbak K |title=Preliminary report of an outbreak of SARS-CoV-2 in mink and mink farmers associated with community spread, Denmark, June to November 2020 |journal=Euro Surveillance |volume=26 |issue=5 |pages=2100009 |date=February 2021 |pmid=33541485 |pmc=7863232 |doi=10.2807/1560-7917.ES.2021.26.5.210009 |quote=As at 1 February 2021, we assess that the cluster 5 variant is no longer circulating among humans in Denmark.}}</ref> | |||
| The virus survives for hours to days on surfaces.<ref name=WHO2020QA/><!--Quote: "Current evidence suggests that novel coronavirus may remain viable for hours to days on surfaces made from a variety of materials"--><ref name="ECDCQA" /> Specifically, the virus was found to be detectable for one day on cardboard, for up to three days on plastic and stainless steel and for up to four hours on copper.<ref name="StableNIH">{{cite web|url=https://www.nih.gov/news-events/news-releases/new-coronavirus-stable-hours-surfaces|title=New coronavirus stable for hours on surfaces|date=17 March 2020|publisher=]|url-status=live|archive-url=https://web.archive.org/web/20200323032520/https://www.nih.gov/news-events/news-releases/new-coronavirus-stable-hours-surfaces|archive-date=23 March 2020|accessdate=23 March 2020}}</ref><ref>{{Cite journal|last=van Doremalen|first=Neeltje|last2=Bushmaker|first2=Trenton|last3=Morris|first3=Dylan H.|last4=Holbrook|first4=Myndi G.|last5=Gamble|first5=Amandine|last6=Williamson|first6=Brandi N.|last7=Tamin|first7=Azaibi|last8=Harcourt|first8=Jennifer L.|last9=Thornburg|first9=Natalie J.|last10=Gerber|first10=Susan I.|last11=Lloyd-Smith|first11=James O.|date=2020-03-17|title=Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1|url=http://www.nejm.org/doi/10.1056/NEJMc2004973|journal=New England Journal of Medicine|language=en|pages=NEJMc2004973|doi=10.1056/NEJMc2004973|issn=0028-4793|pmc=7121658|pmid=32182409}}</ref> This, however, varies based on the humidity and temperature.<ref>{{cite journal |last1=Moriyama |first1=M |last2=Hugentobler |first2=WJ |last3=Iwasaki |first3=A |title=Seasonality of Respiratory Viral Infections. |journal=Annual Review of Virology |date=20 March 2020 |volume=7 |doi=10.1146/annurev-virology-012420-022445 |pmid=32196426}}</ref><ref>Holden, Emily, '''', ], Thursday, April 2, 2020</ref> Surfaces may be decontaminated with a number of solutions (within one minute of exposure to the disinfectant to achieve a 4 or more ]), including 78–95% ] (alcohol used in spirits), 70–100% ] (isopropyl alcohol), the combination of 45% 2-propanol with 30% ], 0.21% ] (bleach), 0.5% ], or 0.23–7.5% ]. Ordinary ] and ] are also highly effective if correctly used; soap products attack the virus' fatty protective layer, deactivating it, as well as freeing them from skin and other surfaces.<ref name="cnn-soap">{{cite news|author= |url=https://edition.cnn.com/2020/03/24/health/soap-warm-water-hand-sanitizer-coronavirus-wellness-scn/index.html |title=COVID-19 prevention: Why soap, sanitizer and warm water work against coronavirus - CNN |publisher=Edition.cnn.com |date=2020-03-24 |accessdate=2020-04-07}}</ref> Other solutions, such as ] and ] (a surgical disinfectant), are less effective,<ref name="SurfacePersistence">{{cite journal |last1=Kampf |first1=G. |last2=Todt |first2=D. |last3=Pfaender |first3=S. |last4=Steinmann |first4=E. |title=Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents |url=https://www.journalofhospitalinfection.com/article/S0195-6701(20)30046-3/fulltext |journal=The Journal of Hospital Infection |volume=104 |issue=3 |pages=246–251 |date=March 2020 |pmid=32035997 |doi=10.1016/j.jhin.2020.01.022}} {{free access}}</ref> as are products advertised as killing bacteria which have little effect on a virus. | |||
| {{as of|2021|12}}, there are five dominant variants of SARS-CoV-2 spreading among global populations: the ] (B.1.1.7, formerly called the UK variant), first found in London and Kent, the ] (B.1.351, formerly called the South Africa variant), the ] (P.1, formerly called the Brazil variant), the ] (B.1.617.2, formerly called the India variant),<ref>{{#invoke:Cite web||title=New COVID-19 Variants|url=https://www.cdc.gov/coronavirus/2019-ncov/transmission/variant.html|access-date=15 July 2021|website=U.S. ] (CDC)|date=28 June 2021|orig-date=First published 11 February 2020}}</ref> and the ] (B.1.1.529), which had spread to 57 countries as of 7 December.<ref>{{#invoke:Cite web||url=https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---7-december-2021 |title=COVID-19 Weekly Epidemiological Update Edition 69 |website=] (WHO) |date=7 December 2021}}</ref><ref>{{#invoke:Cite web||title=Classification of Omicron (B.1.1.529): SARS-CoV-2 Variant of Concern |url=https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern |access-date=9 December 2021 |website=] (WHO)}}</ref> | |||
| ===Virology=== | |||
| On December 19, 2023, the WHO declared that another distinctive variant, JN.1, had emerged as a "variant of interest". Though the WHO expected an increase in cases globally, particularly for countries entering winter, the overall global health risk was considered low.<ref>{{#invoke:Cite web ||author-link=World Health Organization |date=December 19, 2023 |title=JN.1 |url=https://www.who.int/docs/default-source/coronaviruse/18122023_jn.1_ire_clean.pdf?sfvrsn=6103754a_3 |access-date=December 21, 2023}}</ref><ref>{{#invoke:Cite web ||last=Benadjaoud |first=Youri |date=2023-12-19 |title=COVID variant JN.1 listed as 'variant of interest' by World Health Organization |url=https://abcnews.go.com/Health/covid-variant-jn1-listed-variant-interest-world-health/story?id=105782742 |access-date=2023-12-22 |website=ABC News }}</ref> | |||
| {{Main|Severe acute respiratory syndrome coronavirus 2}} | |||
| ] | |||
| == Pathophysiology == | |||
| Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a ] severe acute respiratory syndrome coronavirus, first isolated from three people with pneumonia connected to the ] of acute respiratory illness cases in Wuhan.<ref name="ECDC risk assessment">{{cite web |url=https://www.ecdc.europa.eu/sites/default/files/documents/SARS-CoV-2-risk-assessment-14-feb-2020.pdf |title=Outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): increased transmission beyond China—fourth update |publisher=European Centre for Disease Prevention and Control |date=14 February 2020 |access-date=8 March 2020}}</ref> All features of the novel SARS-CoV-2 virus occur in related coronaviruses in nature.<ref name="NM-20200317" /> | |||
| ]]] | |||
| Outside the human body, the virus is killed by household ], which bursts its protective bubble.<ref name=":0" /> | |||
| The SARS-CoV-2 virus can infect a wide range of cells and systems of the body. COVID‑19 is most known for affecting the upper respiratory tract (sinuses, nose, and throat) and the lower respiratory tract (windpipe and lungs).<ref name="pmid33132005">{{#invoke:cite journal || vauthors = Harrison AG, Lin T, Wang P | title = Mechanisms of SARS-CoV-2 Transmission and Pathogenesis | journal = Trends in Immunology | volume = 41 | issue = 12 | pages = 1100–1115 | date = December 2020 | pmid = 33132005 | pmc = 7556779 | doi = 10.1016/j.it.2020.10.004 }}</ref> The lungs are the organs most affected by COVID‑19 because the virus accesses host cells via the ] for the enzyme ] (ACE2), which is most abundant on the surface of ]s of the lungs.<ref>{{#invoke:cite journal || vauthors = Verdecchia P, Cavallini C, Spanevello A, Angeli F | title = The pivotal link between ACE2 deficiency and SARS-CoV-2 infection | journal = European Journal of Internal Medicine | volume = 76 | pages = 14–20 | date = June 2020 | pmid = 32336612 | pmc = 7167588 | doi = 10.1016/j.ejim.2020.04.037 }}</ref> The virus uses a special surface glycoprotein called a "]" to connect to the ACE2 receptor and enter the host cell.<ref name="Nature Microbiology">{{#invoke:cite journal || vauthors = Letko M, Marzi A, Munster V | title = Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses | journal = Nature Microbiology | volume = 5 | issue = 4 | pages = 562–569 | date = April 2020 | pmid = 32094589 | pmc = 7095430 | doi = 10.1038/s41564-020-0688-y | doi-access = free | title-link = doi }}</ref> | |||
| === Respiratory tract === | |||
| SARS-CoV-2 is closely related to the original SARS-CoV.<ref name="Zhu24Jan2020">{{cite journal |vauthors=Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W |display-authors=6 |title=A Novel Coronavirus from Patients with Pneumonia in China, 2019 |journal=] |volume=382 |issue=8 |pages=727–733 |date=February 2020 |pmid=31978945 |doi=10.1056/NEJMoa2001017|pmc=7092803 }}</ref> It is thought to have a ] origin. Genetic analysis has revealed that the coronavirus genetically clusters with the genus '']'', in subgenus ] (lineage B) together with two bat-derived strains. It is 96% identical at the whole ] level to other bat coronavirus samples (BatCov RaTG13).<ref name="WHOReport24Feb2020" /> In February 2020, Chinese researchers found that there is only one ] difference in certain parts of the genome sequences between the viruses from ] and those from humans, however, whole-genome comparison to date found at most 92% of genetic material shared between pangolin coronavirus and SARS-CoV-2, which is insufficient to prove pangolins to be the ].<ref name="Cyranoski26Feb2020">{{cite journal |title=Mystery deepens over animal source of coronavirus |journal=Nature |volume=579 |pages=18–19 |date=26 February 2020 |doi=10.1038/d41586-020-00548-w |pmid=32127703 |vauthors=Cyranoski D |issue=7797 |bibcode=2020Natur.579...18C}}</ref> | |||
| Following viral entry, COVID‑19 infects the ciliated epithelium of the nasopharynx and upper airways.<ref>{{#invoke:cite journal || vauthors = Marik PE, Iglesias J, Varon J, Kory P | title = A scoping review of the pathophysiology of COVID-19 | journal = International Journal of Immunopathology and Pharmacology | volume = 35 | pages = 20587384211048026 | date = January 2021 | pmid = 34569339 | pmc = 8477699 | doi = 10.1177/20587384211048026 }}</ref> Autopsies of people who died of COVID‑19 have found ], and lymphocyte-containing inflammatory infiltrates within the lung.<ref name="Cureus">{{#invoke:cite journal || vauthors = Eketunde AO, Mellacheruvu SP, Oreoluwa P | title = A Review of Postmortem Findings in Patients With COVID-19 | journal = Cureus | volume = 12 | issue = 7 | pages = e9438 | date = July 2020 | pmid = 32864262 | pmc = 7451084 | doi = 10.7759/cureus.9438 | publisher = Cureus, Inc. | s2cid = 221352704 | doi-access = free | title-link = doi }}</ref> | |||
| From the ]s of COVID-19 infected lungs, white patches were observed containing fluid known as ] (GGO) or simply ground glass.<ref>Ground glass opacities of the lung before, during and post COVID-19 pandemic - PMC (nih.gov)</ref> This tended to correlate with the clear jelly liquid found in lung autopsies of people who died of COVID-19. One possibility addressed in medical research is that ] (HA) could be the leading factor for this observation of the clear jelly liquid found in the lungs, in what could be hyuralonic storm, in conjunction with ].<ref>{{#invoke:Cite journal ||last1=Ontong |first1=Pawared |last2=Prachayasittikul |first2=Virapong |date=2021-01-15 |title=Unraveled roles of hyaluronan in severe COVID-19 |journal=EXCLI Journal |volume=20 |pages=117–125 |doi=10.17179/excli2020-3215 |issn=1611-2156 |pmc=7868638 |pmid=33564281}}</ref> | |||
| ==Pathophysiology== | |||
| === Nervous system === | |||
| The lungs are the organs most affected by COVID-19 because the virus accesses host cells via the enzyme ], which is most abundant in the ] of the lungs. The virus uses a special surface glycoprotein called a "spike" (]) to connect to ACE2 and enter the host cell.<ref name="Nature Microbiology">{{cite journal | title=Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses | journal=Nature Microbiology | doi=10.1038/s41564-020-0688-y | doi-access=free | pmid=32094589 | date=2020 | vauthors=Letko M, Marzi A, Munster V | volume=5 | issue=4 | pages=562–569 }}</ref> The density of ACE2 in each tissue correlates with the severity of the disease in that tissue and some have suggested that decreasing ACE2 activity might be protective,<ref name="Zhang Penninger Li Zhong p.">{{cite journal | vauthors=Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS | title=Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target | journal=Intensive Care Medicine | date=March 2020 | volume=46 | issue=4 | pages=586–590 |doi=10.1007/s00134-020-05985-9 | doi-access=free | pmid=32125455 | pmc=7079879 }}</ref><ref name="Xu Zhong Deng Peng p.">{{cite journal | vauthors=Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X, Li T, Chen Q | display-authors=6 | title=High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa | journal=International Journal of Oral Science | volume=12 | issue=1 | page=8 | date=February 2020 | doi=10.1038/s41368-020-0074-x |doi-access=free | pmid=32094336 | pmc=7039956 }}</ref> though another view is that increasing ACE2 using ] medications could be protective and that these hypotheses need to be tested.<ref>{{cite journal | vauthors=Gurwitz D | title=Angiotensin receptor blockers as tentative SARS‐CoV‐2 therapeutics | journal=Drug Development Research | doi=10.1002/ddr.21656 | doi-access=free | pmid=32129518 | date=March 2020 }}</ref> As the alveolar disease progresses, respiratory failure might develop and death may follow.<ref name="Xu Zhong Deng Peng p."/> | |||
| One common symptom, loss of smell, results from ], with subsequent damage to the ]s.<ref name="Meunier-2020">{{#invoke:cite journal ||vauthors = Meunier N, Briand L, Jacquin-Piques A, Brondel L, Pénicaud L |title = COVID 19-Induced Smell and Taste Impairments: Putative Impact on Physiology |journal = Frontiers in Physiology |volume = 11 |pages = 625110 |date = June 2020 |pmid = 33574768 |pmc = 7870487 |doi = 10.3389/fphys.2020.625110 |doi-access = free |title-link = doi }}</ref> The involvement of both the central and peripheral nervous system in COVID‑19 has been reported in many medical publications.<ref name="Guerrero2020">{{#invoke:cite journal ||vauthors = Guerrero JI, Barragán LA, Martínez JD, Montoya JP, Peña A, Sobrino FE, Tovar-Spinoza Z, Ghotme KA |title = Central and peripheral nervous system involvement by COVID-19: a systematic review of the pathophysiology, clinical manifestations, neuropathology, neuroimaging, electrophysiology, and cerebrospinal fluid findings |journal = BMC Infectious Diseases |date = June 2021 |volume = 21 |issue = 1 |page = 515 |doi = 10.1186/s12879-021-06185-6| pmid = 34078305 |pmc = 8170436 |doi-access=free |title-link=doi }}</ref> It is clear that many people with ]. The virus is not detected in the ] (CNS) of the majority of people with COVID-19 who also have ]. However, SARS-CoV-2 has been detected at low levels in the brains of those who have died from COVID‑19, but these results need to be confirmed.<ref name="Pezzini2020">{{#invoke:cite journal ||vauthors = Pezzini A, Padovani A |title = Lifting the mask on neurological manifestations of COVID-19 |journal = Nature Reviews. Neurology |volume = 16 |issue = 11 |pages = 636–644 |date = November 2020 |pmid = 32839585 |pmc = 7444680 |doi = 10.1038/s41582-020-0398-3 }}</ref> While virus has been detected in ] of autopsies, the exact mechanism by which it invades the CNS remains unclear and may first involve invasion of peripheral nerves given the low levels of ACE2 in the brain.<ref>{{#invoke:cite journal ||vauthors = Li YC, Bai WZ, Hashikawa T |title = The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients |journal = Journal of Medical Virology |volume = 92 |issue = 6 |pages = 552–555 |date = June 2020 |pmid = 32104915 |pmc = 7228394 |doi = 10.1002/jmv.25728 |title-link = doi |doi-access = free }}</ref><ref>{{#invoke:cite journal ||vauthors = Baig AM, Khaleeq A, Ali U, Syeda H |title = Evidence of the COVID-19 Virus Targeting the CNS: Tissue Distribution, Host-Virus Interaction, and Proposed Neurotropic Mechanisms |journal = ACS Chemical Neuroscience |volume = 11 |issue = 7 |pages = 995–998 |date = April 2020 |pmid = 32167747 |pmc = 7094171 |doi= 10.1021/acschemneuro.0c00122 }}</ref><ref>{{#invoke:cite journal ||vauthors = Yavarpour-Bali H, Ghasemi-Kasman M |title = Update on neurological manifestations of COVID-19 |journal = Life Sciences |volume = 257 |pages = 118063 |date = September 2020 |pmid = 32652139 |pmc = 7346808 |doi = 10.1016/j.lfs.2020.118063 }}</ref> The virus may also enter the bloodstream from the lungs and cross the blood–brain barrier to gain access to the CNS, possibly within an infected white blood cell.<ref name="Pezzini2020" /> | |||
| ] and ] in SARS-CoV-2 infection]] Research conducted when Alpha was the dominant variant has suggested COVID-19 may cause brain damage.<ref>{{#invoke:Cite journal ||last1=Douaud |first1=Gwenaëlle |last2=Lee |first2=Soojin |last3=Alfaro-Almagro |first3=Fidel |last4=Arthofer |first4=Christoph |last5=Wang |first5=Chaoyue |last6=McCarthy |first6=Paul |last7=Lange |first7=Frederik |last8=Andersson |first8=Jesper L. R. |last9=Griffanti |first9=Ludovica |last10=Duff |first10=Eugene |last11=Jbabdi |first11=Saad |last12=Taschler |first12=Bernd |last13=Keating |first13=Peter |last14=Winkler |first14=Anderson M. |last15=Collins |first15=Rory |last16=Matthews |first16= Paul M. |last17=Naomi |first17=Allen |last18=Miller |first18=Karla L. |last19=Nichols |first19=Thomas E. |last20=Smith |first20=Stephen M. |date=March 2022 |title=SARS-CoV-2 is associated with changes in brain structure in UK Biobank |journal=] |volume=604 |issue=7907 |pages=697–707 |doi=10.1038/s41586-022-04569-5 |issn=1476-4687 |lccn=12037118 |oclc=01586310 |pmc=9046077 |pmid=35255491 |bibcode=2022Natur.604..697D |doi-access=free}}</ref> Later research showed that all variants studied (including Omicron) killed brain cells, but the exact cells killed varied by variant.<ref>{{#invoke:Cite journal ||last1=Proust |first1=Alizé | |||
| |last2= Queval|first2= Christophe J. |last3= Harvey|first3= Ruth |last4= Adams|first4= Lorin |last5= Bennett|first5= Michael |last6= Wilkinson |first6= Robert J.|date= 2023 |title= Differential effects of SARS-CoV-2 variants on central nervous system cells and blood–brain barrier functions|journal= Journal of Neuroinflammation |volume=20 |issue=184 |page=184 | |||
| |doi=10.1186/s12974-023-02861-3 | |||
| |pmid=37537664 | |||
| |pmc=10398935 | |||
| |doi-access=free | |||
| }}</ref> It is unknown if such damage is temporary or permanent.<ref>{{#invoke:Cite news ||last1=Geddes |first1=Linda |last2=Sample |first2=Ian |date=7 March 2022 |title=Covid can shrink brain and damage its tissue, finds research |work=] |url=https://www.theguardian.com/world/2022/mar/07/covid-can-shrink-brain-and-damage-its-tissue-finds-research |url-status=live |access-date=4 September 2023 |archive-url=https://web.archive.org/web/20220307161107/https://www.theguardian.com/world/2022/mar/07/covid-can-shrink-brain-and-damage-its-tissue-finds-research |archive-date=7 March 2022}}</ref><ref>{{#invoke:Cite news ||last=Morelle |first=Rebecca |date=7 March 2022 |title=Scans reveal how Covid may change the brain |work=] |publisher=] |url=https://www.bbc.com/news/health-60591487 |access-date=4 September 2023}}</ref> Observed individuals infected with COVID-19 (most with mild cases) experienced an additional 0.2% to 2% of brain tissue lost in regions of the brain connected to the sense of smell compared with uninfected individuals, and the overall effect on the brain was equivalent on average to at least one extra year of normal ageing; infected individuals also scored lower on several cognitive tests. All effects were more pronounced among older ages.<ref>{{#invoke:Cite web||url=https://www.nbcnews.com/health/health-news/long-covid-even-mild-covid-linked-damage-brain-months-infection-rcna18959|title=Even mild Covid is linked to brain damage months after illness, scans show |date=7 March 2022 |publisher=NBC News}}</ref> | |||
| === Gastrointestinal tract === | |||
| The virus also affects gastrointestinal organs as ACE2 is abundantly expressed in the ]ular cells of ], ] and ] ]<ref name=":11">{{Cite journal|last1=Gu|first1=Jinyang|last2=Han|first2=Bing|last3=Wang|first3=Jian|date=27 February 2020|title=COVID-19: Gastrointestinal manifestations and potential fecal-oral transmission|journal=Gastroenterology|volume=|pages=|doi=10.1053/j.gastro.2020.02.054|pmid=32142785|issn=0016-5085}}</ref> as well as ] cells and ]s of the ].<ref>{{Cite journal|last1=Hamming|first1=I.|last2=Timens|first2=W.|last3=Bulthuis|first3=M. L. C.|last4=Lely|first4=A. T.|last5=Navis|first5=G. J.|last6=Goor|first6=H. van|date=2004|title=Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis|journal=The Journal of Pathology|language=en|volume=203|issue=2|pages=631–637|doi=10.1002/path.1570|pmid=15141377|issn=1096-9896}}</ref> | |||
| The virus also affects gastrointestinal organs as ACE2 is abundantly expressed in the ]ular cells of ], ] and ] ]<ref name="Gu-2020">{{#invoke:cite journal || vauthors = Gu J, Han B, Wang J | title = COVID-19: Gastrointestinal Manifestations and Potential Fecal-Oral Transmission | journal = Gastroenterology | volume = 158 | issue = 6 | pages = 1518–1519 | date = May 2020 | pmid = 32142785 | pmc = 7130192 | doi = 10.1053/j.gastro.2020.02.054 }}</ref> as well as ] cells and ]s of the ].<ref name="pmid32343593">{{#invoke:cite journal || vauthors = Mönkemüller K, Fry L, Rickes S | title = COVID-19, coronavirus, SARS-CoV-2 and the small bowel | journal = Revista Espanola de Enfermedades Digestivas | volume = 112 | issue = 5 | pages = 383–388 | date = May 2020 | pmid = 32343593 | doi = 10.17235/reed.2020.7137/2020 | s2cid = 216645754 }}</ref> | |||
| === Cardiovascular system === | |||
| The expanding part of the lungs, ], contain two main types of functioning cells. One cell, ], absorbs from the air, i.e. ]. The other, ], produces ]s, which serve to keep the lungs fluid, clean, infection free, etc. COVID-19 finds a way into a surfactant producing type II cell and smothers it by reproducing COVID-19 virus within it. Each type II cell which perishes to the virus causes an extreme reaction in the lungs. Fluids, pus and dead cell material flood the lung, causing the coronavirus pulmonary disease.<ref name="DrVuong">{{cite web|url=https://www.youtube.com/watch?v=4J0d59dd-qM |author=Doctor Duc C Vuong|title=HOW COVID-19 KILLS--I'm a Surgeon--And Why We Can't Save You |publisher=YouTube |date=23 March 2020 |accessdate=5 April 2020 |archiveurl = |archivedate = |url-status=live}}</ref> | |||
| The virus can cause ] and chronic damage to the ].<ref>{{#invoke:cite journal || vauthors = Almamlouk R, Kashour T, Obeidat S, Bois MC, Maleszewski JJ, Omrani OA, Tleyjeh R, Berbari E, Chakhachiro Z, Zein-Sabatto B, Gerberi D, Tleyjeh IM, Paniz Mondolfi AE, Finn AV, Duarte-Neto AN, Rapkiewicz AV, Frustaci A, Keresztesi AA, Hanley B, Märkl B, Lardi C, Bryce C, Lindner D, Aguiar D, Westermann D, Stroberg E, Duval EJ, Youd E, Bulfamante GP, Salmon I, Auer J, Maleszewski JJ, Hirschbühl K, Absil L, Barton LM, Ferraz da Silva LF, Moore L, Dolhnikoff M, Lammens M, Bois MC, Osborn M, Remmelink M, Nascimento Saldiva PH, Jorens PG, Craver R, Aparecida de Almeida Monteiro R, Scendoni R, Mukhopadhyay S, Suzuki T, Mauad T, Fracasso T, Grimes Z | title = COVID-19-Associated cardiac pathology at the postmortem evaluation: a collaborative systematic review | journal = Clinical Microbiology and Infection | volume = 28 | issue = 8 | pages = 1066–1075 | date = August 2022 | pmid = 35339672 | pmc = 8941843 | doi = 10.1016/j.cmi.2022.03.021 }}</ref><ref name="Zheng-2020">{{#invoke:cite journal || vauthors = Zheng YY, Ma YT, Zhang JY, Xie X | title = COVID-19 and the cardiovascular system | journal = Nature Reviews. Cardiology | volume = 17 | issue = 5 | pages = 259–260 | date = May 2020 | pmid = 32139904 | pmc = 7095524 | doi = 10.1038/s41569-020-0360-5 }}</ref> An acute cardiac injury was found in 12% of infected people admitted to the hospital in Wuhan, China,<ref name="Huang24Jan2020" /> and is more frequent in severe disease.<ref>{{#invoke:Cite web|| title=Coronavirus disease 2019 (COVID-19): Myocardial infarction and other coronary artery disease issues | website=UpToDate | url=https://www.uptodate.com/contents/coronavirus-disease-2019-covid-19-myocardial-infarction-and-other-coronary-artery-disease-issues | access-date=28 September 2020}}</ref> Rates of cardiovascular symptoms are high, owing to the systemic inflammatory response and immune system disorders during disease progression, but acute myocardial injuries may also be related to ACE2 receptors in the heart.<ref name="Zheng-2020" /> ACE2 receptors are highly expressed in the heart and are involved in heart function.<ref name="Zheng-2020" /><ref>{{#invoke:cite journal || vauthors = Turner AJ, Hiscox JA, Hooper NM | title = ACE2: from vasopeptidase to SARS virus receptor | journal = Trends in Pharmacological Sciences | volume = 25 | issue = 6 | pages = 291–4 | date = June 2004 | pmid = 15165741 | pmc = 7119032 | doi = 10.1016/j.tips.2004.04.001 | doi-access = free | title-link = doi }}</ref> | |||
| A high incidence of ] and ] occurs in people transferred to ]s with COVID‑19 infections, and may be related to poor prognosis.<ref>{{#invoke:cite journal || vauthors = Abou-Ismail MY, Diamond A, Kapoor S, Arafah Y, Nayak L | title = The hypercoagulable state in COVID-19: Incidence, pathophysiology, and management | journal = Thrombosis Research | volume = 194 | pages = 101–115 | date = October 2020 | pmid = 32788101 | pmc = 7305763 | doi = 10.1016/j.thromres.2020.06.029 | publisher = Elsevier BV }}</ref> Blood vessel dysfunction and clot formation (as suggested by high ] levels caused by blood clots) may have a significant role in mortality, incidents of clots leading to ]s, and ]s (strokes) within the brain found as complications leading to death in people infected with COVID‑19.<ref name=Science/> Infection may initiate a chain of ]s within the body, including pulmonary vasoconstriction {{ndash}} a possible mechanism in which oxygenation decreases during pneumonia.<ref name="Science">{{#invoke:cite journal || vauthors = Wadman M |doi=10.1126/science.abc3208 |title=How does coronavirus kill? Clinicians trace a ferocious rampage through the body, from brain to toes |journal=Science |date=April 2020 |doi-access = free | title-link = doi }}</ref> Furthermore, damage of ] and ] was found in brain tissue samples of people who died from COVID‑19.<ref>{{#invoke:cite news ||title=NIH study uncovers blood vessel damage and inflammation in COVID-19 patients' brains but no infection |url=https://www.nih.gov/news-events/news-releases/nih-study-uncovers-blood-vessel-damage-inflammation-covid-19-patients-brains-no-infection |access-date=17 January 2021 |work=National Institutes of Health (NIH) |date=30 December 2020 }}</ref><ref>{{#invoke:cite journal || vauthors = Lee MH, Perl DP, Nair G, Li W, Maric D, Murray H, Dodd SJ, Koretsky AP, Watts JA, Cheung V, Masliah E, Horkayne-Szakaly I, Jones R, Stram MN, Moncur J, Hefti M, Folkerth RD, Nath A | title = Microvascular Injury in the Brains of Patients with Covid-19 | journal = The New England Journal of Medicine | volume = 384 | issue = 5 | pages = 481–483 | date = February 2021 | pmid = 33378608 | pmc = 7787217 | doi = 10.1056/nejmc2033369 }}</ref> | |||
| ==Diagnosis== | |||
| COVID{{nbhyph}}19 may also cause substantial structural changes to ]s, sometimes persisting for months after hospital discharge.<ref>{{#invoke:cite journal || vauthors = Kubánková M, Hohberger B, Hoffmanns J, Fürst J, Herrmann M, Guck J, Kräter M | title = Physical phenotype of blood cells is altered in COVID-19 | journal = Biophysical Journal | volume = 120 | issue = 14 | pages = 2838–2847 | date = July 2021 | pmid = 34087216 | pmc = 8169220 | doi = 10.1016/j.bpj.2021.05.025 | bibcode = 2021BpJ...120.2838K }}</ref> ] may result from the virus acting through ACE2-related entry into lymphocytes.<ref>{{#invoke:cite journal|| vauthors = Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, Bikdeli B, Ahluwalia N, Ausiello JC, Wan EY, Freedberg DE, Kirtane AJ, Parikh SA, Maurer MS, Nordvig AS, Accili D, Bathon JM, Mohan S, Bauer KA, Leon MB, Krumholz HM, Uriel N, Mehra MR, Elkind MS, Stone GW, Schwartz A, Ho DD, Bilezikian JP, Landry DW |title=Extrapulmonary manifestations of COVID-19 |journal=Nature Medicine |date=July 2020 |volume=26 |issue=7 |pages=1017–1032 |doi=10.1038/s41591-020-0968-3|pmid=32651579 |s2cid=220462000 | doi-access=free | title-link=doi }}</ref> | |||
| {{main|COVID-19 testing}} | |||
| ] for ]]] | |||
| ] ] test kit for COVID-19<ref>{{cite web|url=https://www.cdc.gov/coronavirus/2019-ncov/about/testing.html|title=CDC Tests for 2019-nCoV|author=|date=5 February 2020|website=Centers for Disease Control and Prevention|url-status=live|archive-url=https://web.archive.org/web/20200214023335/https://www.cdc.gov/coronavirus/2019-ncov/about/testing.html|archive-date=14 February 2020|access-date=12 February 2020| name-list-format = vanc}}</ref>]] | |||
| The WHO has published several testing protocols for the disease.<ref>{{cite web|url=https://www.who.int/publications-detail/laboratory-testing-for-2019-novel-coronavirus-in-suspected-human-cases-20200117|title=Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases|website=] (WHO)|access-date=13 March 2020|archive-url=https://web.archive.org/web/20200317023052/https://www.who.int/publications-detail/laboratory-testing-for-2019-novel-coronavirus-in-suspected-human-cases-20200117|archive-date=17 March 2020|url-status=live}}</ref> The standard method of testing is ] (rRT-PCR).<ref name="20200130cdc">{{cite web |url=https://www.cdc.gov/coronavirus/2019-ncov/summary.html |title=2019 Novel Coronavirus (2019-nCoV) Situation Summary |date=30 January 2020 |website=] |url-status=live |archive-url=https://web.archive.org/web/20200126210549/https://www.cdc.gov/coronavirus/2019-nCoV/summary.html |archive-date=26 January 2020 |access-date=30 January 2020| name-list-format = vanc}}</ref> The test is typically done on respiratory samples obtained by a ], however a nasal swab or ] sample may also be used.<ref name="CDC2020Testing"/><ref name="20200129cdc">{{cite web |url=https://www.cdc.gov/coronavirus/2019-ncov/lab/rt-pcr-detection-instructions.html |title=Real-Time RT-PCR Panel for Detection 2019-nCoV |date=29 January 2020 |website=] |access-date=1 February 2020 |archive-url=https://web.archive.org/web/20200130202031/https://www.cdc.gov/coronavirus/2019-ncov/lab/rt-pcr-detection-instructions.html |archive-date=30 January 2020 |url-status=live | name-list-format = vanc}}</ref> Results are generally available within a few hours to two days.<ref name="globenewswire1977226">{{cite web |url=https://www.globenewswire.com/news-release/2020/01/30/1977226/0/en/Curetis-Group-Company-Ares-Genetics-and-BGI-Group-Collaborate-to-Offer-Next-Generation-Sequencing-and-PCR-based-Coronavirus-2019-nCoV-Testing-in-Europe.html |title=Curetis Group Company Ares Genetics and BGI Group Collaborate to Offer Next-Generation Sequencing and PCR-based Coronavirus (2019-nCoV) Testing in Europe |date=30 January 2020 |website=GlobeNewswire News Room |access-date=1 February 2020 |archive-url=https://web.archive.org/web/20200131201626/https://www.globenewswire.com/news-release/2020/01/30/1977226/0/en/Curetis-Group-Company-Ares-Genetics-and-BGI-Group-Collaborate-to-Offer-Next-Generation-Sequencing-and-PCR-based-Coronavirus-2019-nCoV-Testing-in-Europe.html |archive-date=31 January 2020 |url-status=live | name-list-format = vanc}}</ref><ref name="20200130businessinsider">{{cite web |url=https://www.businessinsider.com/how-to-know-if-you-have-the-coronavirus-pcr-test-2020-1 |title=There's only one way to know if you have the coronavirus, and it involves machines full of spit and mucus |last=Brueck |first=Hilary | name-list-format = vanc |date=30 January 2020 |website=Business Insider |access-date=1 February 2020 |archive-url= https://web.archive.org/web/20200201034232/https://www.businessinsider.com/how-to-know-if-you-have-the-coronavirus-pcr-test-2020-1 |archive-date=1 February 2020 |url-status=live }}</ref> Blood tests can be used, but these require two blood samples taken two weeks apart and the results have little immediate value.<ref>{{cite web |url=https://www.who.int/publications-detail/laboratory-testing-for-2019-novel-coronavirus-in-suspected-human-cases-20200117 |title=Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases |access-date=26 February 2020 |archive-url=https://web.archive.org/web/20200221192745/https://www.who.int/publications-detail/laboratory-testing-for-2019-novel-coronavirus-in-suspected-human-cases-20200117 |archive-date=21 February 2020 |url-status=live }}</ref> Chinese scientists were able to isolate a strain of the coronavirus and publish the ] so laboratories across the world could independently develop ] (PCR) tests to detect infection by the virus.<ref name="Hui14Jan2020" /><ref name="Cohen17Jan20202">{{cite journal | vauthors = Cohen J, Normile D | title = New SARS-like virus in China triggers alarm | journal = Science | volume = 367 | issue = 6475 | pages = 234–35 | date = January 2020 | pmid = 31949058 |doi=10.1126/science.367.6475.234 | bibcode = 2020Sci...367..234C | url = https://mcb.uconn.edu/wp-content/uploads/sites/2341/2020/01/WuhanScience24Jan2020.pdf | access-date = 11 February 2020 | url-status = live | archive-url = https://web.archive.org/web/20200211230310/https://mcb.uconn.edu/wp-content/uploads/sites/2341/2020/01/WuhanScience24Jan2020.pdf | archive-date = 11 February 2020 }}</ref><ref name="ncbiWuhanGenomes">{{cite web |url=https://www.ncbi.nlm.nih.gov/labs/virus/vssi/#/virus?SeqType_s=Nucleotide&VirusLineage_ss=Wuhan%20seafood%20market%20pneumonia%20virus,%20taxid:2697049 |title=Severe acute respiratory syndrome coronavirus 2 data hub |website=NCBI |url-status=live |access-date=4 March 2020 |name-list-format=vanc |archive-url=https://web.archive.org/web/20200321235550/https://www.ncbi.nlm.nih.gov/labs/virus/vssi/#/virus?SeqType_s=Nucleotide&VirusLineage_ss=Wuhan%20seafood%20market%20pneumonia%20virus,%20taxid:2697049 |archive-date=21 March 2020 }}</ref> {{As of|2020|April|4}}, ]s (which may detect active infections and whether a person had been infected in the past) were in development, but not yet widely used.<ref>{{Cite journal|last=Petherick|first=Anna|date=4 April 2020|title=Developing antibody tests for SARS-CoV-2|url=https://doi.org/10.1016/S0140-6736(20)30788-1|journal=The Lancet|volume=395|issue=10230|pages=1101–1102|doi=10.1016/s0140-6736(20)30788-1|issn=0140-6736|via=}}</ref><ref name="Vogel2020">{{cite journal |title=New blood tests for antibodies could show true scale of coronavirus pandemic |last=Vogel| first=Gretchen |journal=Science |issn=0036-8075 |doi=10.1126/science.abb8028|date=2020-03-19}}</ref><ref>{{cite journal | vauthors = Pang J, Wang MX, Ang IY, Tan SH, Lewis RF, Chen JI, Gutierrez RA, Gwee SX, Chua PE, Yang Q, Ng XY, Yap RK, Tan HY, Teo YY, Tan CC, Cook AR, Yap JC, Hsu LY | display-authors = 6 | title = Potential Rapid Diagnostics, Vaccine and Therapeutics for 2019 Novel Coronavirus (2019-nCoV): A Systematic Review | journal = Journal of Clinical Medicine | volume = 9 | issue = 3 | page = 623 | date = February 2020 | pmid = 32110875 | doi = 10.3390/jcm9030623 }}</ref> The FDA approved the first ] on 21 March 2020 for use at the end of that month.<ref>{{cite press release |title= Coronavirus (COVID-19) Update: FDA Issues first Emergency Use Authorization for Point of Care Diagnostic |date= 21 March 2020 |publisher= FDA |url= https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-first-emergency-use-authorization-point-care-diagnostic |access-date= 22 March 2020 |archive-url= https://web.archive.org/web/20200321224700/https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-first-emergency-use-authorization-point-care-diagnostic |archive-date= 21 March 2020 |url-status= live }}</ref> | |||
| === Kidneys === | |||
| Diagnostic guidelines released by Zhongnan Hospital of ] suggested methods for detecting infections based upon clinical features and epidemiological risk. These involved identifying people who had at least two of the following symptoms in addition to a history of travel to ] or contact with other infected people: fever, imaging features of pneumonia, normal or reduced white blood cell count or reduced ] count.<ref name=Jin2020>{{cite journal | vauthors=Jin YH, Cai L, Cheng ZS, Cheng H, Deng T, Fan YP, Fang C, Huang D, Huang LQ, Huang Q, Han Y, Hu B, Hu F, Li BH, Li YR, Liang K, Lin LK, Luo LS, Ma J, Ma LL, Peng ZY, Pan YB, Pan ZY, Ren XQ, Sun HM, Wang Y, Wang YY, Weng H, Wei CJ, Wu DF, Xia J, Xiong Y, Xu HB, Yao XM, Yuan YF, Ye TS, Zhang XC, Zhang YW, Zhang YG, Zhang HM, Zhao Y, Zhao MJ, Zi H, Zeng XT, Wang YY, Wang XH | display-authors=6 | title=A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version) | journal=Military Medical Research | date=February 2020 | volume=7 | issue=1 | page=4 | doi=10.1186/s40779-020-0233-6 | doi-access=free | pmid=32029004 | pmc=7003341 }}</ref> | |||
| Another common cause of death is complications related to the ]s.<ref name="Science" /> Early reports show that up to 30% of people hospitalised with COVID-19 both in China and in New York have experienced some injury to their kidneys, including some persons with no previous kidney problems.<ref>{{#invoke:Cite web|| title=Coronavirus: Kidney Damage Caused by COVID-19 | website=Johns Hopkins Medicine | date=14 May 2020 | url=https://www.hopkinsmedicine.org/health/conditions-and-diseases/coronavirus/coronavirus-kidney-damage-caused-by-covid19 | access-date=25 January 2022}}</ref> | |||
| === Immunopathology === | |||
| A March 2020 review concluded that ] are of little value in early stages, whereas CT scans of the chest are useful even before symptoms occur.<ref name=":0">{{Cite journal|last=Salehi|first=Sana|last2=Abedi|first2=Aidin|last3=Balakrishnan|first3=Sudheer|last4=Gholamrezanezhad|first4=Ali|date=2020-03-14|title=Coronavirus Disease 2019 (COVID-19): A Systematic Review of Imaging Findings in 919 Patients|journal=American Journal of Roentgenology|language=en|pages=1–7|doi=10.2214/AJR.20.23034|issn=0361-803X|pmid=32174129}}</ref> Typical features on CT include bilateral multilobar ] with a peripheral, asymmetric and posterior distribution.<ref name=":0" /> ], ] (lobular septal thickening with variable alveolar filling) and ] develop as the disease evolves.<ref>{{cite journal|last1=Lee|first1=Elaine Y. P.|last2=Ng|first2=Ming-Yen|last3=Khong|first3=Pek-Lan|date=24 February 2020|title=COVID-19 pneumonia: what has CT taught us?|url=https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(20)30134-1/fulltext|journal=The Lancet Infectious Diseases|language=English|volume=0|issue=4|pages=384–385|doi=10.1016/S1473-3099(20)30134-1|issn=1473-3099|pmid=32105641|accessdate=13 March 2020|archive-url=https://web.archive.org/web/20200308143943/https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(20)30134-1/fulltext|archive-date=8 March 2020|url-status=live}}</ref> As of March 2020, the ] recommends that "CT should not be used to screen for or as a first-line test to diagnose COVID-19".<ref>{{cite web|url=https://www.acr.org/Advocacy-and-Economics/ACR-Position-Statements/Recommendations-for-Chest-Radiography-and-CT-for-Suspected-COVID19-Infection|title=ACR Recommendations for the use of Chest Radiography and Computed Tomography (CT) for Suspected COVID-19 Infection|last=|first=|date=2020-03-22|website=American College of Radiology|url-status=live|archive-url=https://web.archive.org/web/20200328055813/https://www.acr.org/Advocacy-and-Economics/ACR-Position-Statements/Recommendations-for-Chest-Radiography-and-CT-for-Suspected-COVID19-Infection|archive-date=28 March 2020|access-date=}}</ref> | |||
| ] to SARS-CoV-2]] | |||
| <gallery mode=packed heights=100px> | |||
| Although SARS-CoV-2 has a tropism for ACE2-expressing epithelial cells of the respiratory tract, people with severe COVID‑19 have symptoms of systemic hyperinflammation. Clinical laboratory findings<!-- Spellings in this paragraph have been meticulously compared link-by-link; if you spot an error, please correct the other article first. --> of elevated ], ], ], as well as the following suggest an underlying immunopathology:<ref name="Huang24Jan2020" /> | |||
| File:COVID19CT2.webp|Typical CT imaging findings | |||
| * ] (GM{{nbh}}CSF) | |||
| File:COVID19CT1.webp|CT imaging of rapid progression stage | |||
| * ] (IP{{nbh}}10) | |||
| </gallery> | |||
| * ] (MCP1) | |||
| * ] (MIP{{nbh}}1{{nbh}}alpha) | |||
| * ] (TNF{{nbh}}α) indicative of ] (CRS) | |||
| ] plays a complex, Janus-faced role in the pathogenesis of COVID-19. Although it promotes the elimination of virus-infected cells, it also upregulates the expression of ACE-2, thereby facilitating the SARS-Cov2 virus to enter cells and to replicate.<ref>{{#invoke:cite journal ||last1=Ziegler |first1=CGK |last2=Allon |first2=SJ |last3=Nyquist |first3=SK |last4=Mbano |first4=IM |last5=Miao |first5=VN |last6=Tzouanas |first6=CN |last7=Cao |first7=Y |last8=Yousif |first8=AS |last9=Bals |first9=J |last10=Hauser |first10=BM |last11=Feldman |first11=J |last12=Muus |first12=C |last13=Wadsworth |first13=MH |last14=Kazer |first14=SW |last15=Hughes |first15=TK |last16=Doran |first16=B |last17=Gatter |first17=GJ |last18=Vukovic |first18=M |last19=Taliaferro |first19=F |last20=Mead |first20=BE |last21=Guo |first21=Z |last22=Wang |first22=JP |last23=Gras |first23=D |last24=Plaisant |first24=M |last25=Ansari |first25=M |last26=Angelidis |first26=I |last27=Adler |first27=H |last28=Sucre |first28=JMS |last29=Taylor |first29=CJ |last30=Lin |first30=B |last31=Waghray |first31=A |last32=Mitsialis |first32=V |last33=Dwyer |first33=DF |last34=Buchheit |first34=KM |last35=Boyce |first35=JA |last36=Barrett |first36=NA |last37=Laidlaw |first37=TM |last38=Carroll |first38=SL |last39=Colonna |first39=L |last40=Tkachev |first40=V |last41=Peterson |first41=CW |last42=Yu |first42=A |last43=Zheng |first43=HB |last44=Gideon |first44=HP |last45=Winchell |first45=CG |last46=Lin |first46=PL |last47=Bingle |first47=CD |last48=Snapper |first48=SB |last49=Kropski |first49=JA |last50=Theis |first50=FJ |last51=Schiller |first51=HB |last52=Zaragosi |first52=LE |last53=Barbry |first53=P |last54=Leslie |first54=A |last55=Kiem |first55=HP |last56=Flynn |first56=JL |last57=Fortune |first57=SM |last58=Berger |first58=B |last59=Finberg |first59=RW |last60=Kean |first60=LS |last61=Garber |first61=M |last62=Schmidt |first62=AG |last63=Lingwood |first63=D |last64=Shalek |first64=AK |last65=Ordovas-Montanes |first65=J |title=SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues. |journal=Cell |series=HCA Lung Biological Network |date=28 May 2020 |volume=181 |issue=5 |pages=1016–1035.e19 |doi=10.1016/j.cell.2020.04.035 |pmid=32413319|pmc=7252096 }}</ref><ref>{{#invoke:cite journal ||last1=Sajuthi |first1=SP |last2=DeFord |first2=P |last3=Li |first3=Y |last4=Jackson |first4=ND |last5=Montgomery |first5=MT |last6=Everman |first6=JL |last7=Rios |first7=CL |last8=Pruesse |first8=E |last9=Nolin |first9=JD |last10=Plender |first10=EG |last11=Wechsler |first11=ME |last12=Mak |first12=ACY |last13=Eng |first13=C |last14=Salazar |first14=S |last15=Medina |first15=V |last16=Wohlford |first16=EM |last17=Huntsman |first17=S |last18=Nickerson |first18=DA |last19=Germer |first19=S |last20=Zody |first20=MC |last21=Abecasis |first21=G |last22=Kang |first22=HM |last23=Rice |first23=KM |last24=Kumar |first24=R |last25=Oh |first25=S |last26=Rodriguez-Santana |first26=J |last27=Burchard |first27=EG |last28=Seibold |first28=MA |title=Type 2 and interferon inflammation regulate SARS-CoV-2 entry factor expression in the airway epithelium. |journal=Nature Communications |date=12 October 2020 |volume=11 |issue=1 |pages=5139 |doi=10.1038/s41467-020-18781-2 |pmid=33046696|pmc=7550582 |bibcode=2020NatCo..11.5139S }}</ref> A competition of negative feedback loops (via protective effects of interferon alpha) and positive feedback loops (via upregulation of ACE-2) is assumed to determine the fate of people with COVID-19.<ref>{{#invoke:cite journal ||last1=Tretter |first1=F |last2=Peters |first2=EMJ |last3=Sturmberg |first3=J |last4=Bennett |first4=J |last5=Voit |first5=E |last6=Dietrich |first6=JW |last7=Smith |first7=G |last8=Weckwerth |first8=W |last9=Grossman |first9=Z |last10=Wolkenhauer |first10=O |last11=Marcum |first11=JA |title=Perspectives of (/memorandum for) systems thinking on COVID-19 pandemic and pathology. |journal=Journal of Evaluation in Clinical Practice |date=28 September 2022 |volume=29 |issue=3 |pages=415–429 |doi=10.1111/jep.13772 |pmid=36168893|pmc=9538129 |s2cid=252566067 }}</ref>{{Update needed|date=December 2024}} | |||
| ===Pathology=== | |||
| Additionally, people with COVID‑19 and ] (ARDS) have classical ] ]s of CRS, including elevated ] (CRP), ] (LDH), ], and ].<ref>{{#invoke:cite journal || vauthors = Zhang C, Wu Z, Li JW, Zhao H, Wang GQ | title = Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality | journal = International Journal of Antimicrobial Agents | volume = 55 | issue = 5 | pages = 105954 | date = May 2020 | pmid = 32234467 | pmc = 7118634 | doi = 10.1016/j.ijantimicag.2020.105954 }}</ref> | |||
| Few data are available about microscopic lesions and the pathophysiology of COVID-19.<ref> {{Webarchive|url=https://web.archive.org/web/20200328202232/https://jcp.bmj.com/content/early/2020/03/20/jclinpath-2020-206522 |date=28 March 2020 }}, Hanley B et al, J Clin Pathol, {{PMID|32198191}}</ref><ref>, Yao XH et al., {{PMID|32172546}}</ref> The main pathological findings at autopsy are: | |||
| * ]: ], ], ] and ] | |||
| * Four types of severity of ] can be observed: | |||
| ** minor ]: minor serous ], minor ] exudation | |||
| ** mild pneumonia: ], ] ], large atypical ]s, interstitial ] with ] ] and ] formation | |||
| ** severe pneumonia: ] (DAD) with diffuse ] ]. DAD is the cause of ] (ARDS) and severe ]. | |||
| ** healing pneumonia: ] of ]s in ] and ] | |||
| ** ] in ]<ref>{{Cite journal|title=Exuberant plasmocytosis in bronchoalveolar lavage of the first patient requiring Extracorporeal Membrane Oxygenation for SARS-CoV-2 in Europe|first1=Marco|last1=Giani|first2=Davide|last2=Seminati|first3=Alberto|last3=Lucchini|first4=Giuseppe|last4=Foti|first5=Fabio|last5=Pagni|date=16 March 2020|journal=Journal of Thoracic Oncology|doi=10.1016/j.jtho.2020.03.008|pmid=32194247|pmc=7118681}}</ref> | |||
| * ]: ] (DIC);<ref>{{Cite journal|title=Disseminated intravascular coagulation in patients with 2019-nCoV pneumonia|first=David|last=Lillicrap|date=1 April 2020|journal=Journal of Thrombosis and Haemostasis: JTH|volume=18|issue=4|pages=786–787|doi=10.1111/jth.14781|pmid=32212240}}</ref> leukoerythroblastic reaction<ref>{{Cite journal|title=Leukoerythroblastic reaction in a patient with COVID-19 infection|first1=Anupam|last1=Mitra|first2=Denis M.|last2=Dwyre|first3=Michael|last3=Schivo|first4=George R.|last4=Thompson|first5=Stuart H.|last5=Cohen|first6=Nam|last6=Ku|first7=John P.|last7=Graff|date=25 March 2020|journal=American Journal of Hematology|doi=10.1002/ajh.25793|pmid=32212392}}</ref> | |||
| * ]: microvesicular ] | |||
| Systemic inflammation results in ], allowing inflammatory lymphocytic and monocytic infiltration of the lung and the heart. In particular, pathogenic GM-CSF-secreting ]s were shown to correlate with the recruitment of inflammatory IL-6-secreting ]s and severe lung pathology in people with COVID‑19.<ref>{{#invoke:cite journal || vauthors = Gómez-Rial J, Rivero-Calle I, Salas A, Martinón-Torres F | title = Role of Monocytes/Macrophages in Covid-19 Pathogenesis: Implications for Therapy | journal = Infection and Drug Resistance | volume = 13 | pages = 2485–2493 | year = 2020 | pmid = 32801787 | pmc = 7383015 | doi = 10.2147/IDR.S258639 | doi-access = free | title-link = doi }}</ref> Lymphocytic infiltrates have also been reported at autopsy.<ref name="Cureus" /> | |||
| ==Prevention== | |||
| === Viral and host factors === | |||
| {{See also|2019–20 coronavirus pandemic#Prevention|flatten the curve|workplace hazard controls for COVID-19}} | |||
| ==== Virus proteins ==== | |||
| ]"—allows healthcare services to better manage the same volume of patients.<ref>{{cite web |last=Wiles |first=Siouxsie |author-link=Siouxsie Wiles |title=The three phases of Covid-19—and how we can make it manageable |url=https://thespinoff.co.nz/society/09-03-2020/the-three-phases-of-covid-19-and-how-we-can-make-it-manageable/ |website=The Spinoff |access-date=9 March 2020 |date=9 March 2020 |name-list-format=vanc |archive-url=https://web.archive.org/web/20200327120015/https://thespinoff.co.nz/society/09-03-2020/the-three-phases-of-covid-19-and-how-we-can-make-it-manageable/ |archive-date=27 March 2020 |url-status=live }}</ref><ref name="Lancet2020Flatten">{{cite journal | vauthors = Anderson RM, Heesterbeek H, Klinkenberg D, Hollingsworth TD | title = How will country-based mitigation measures influence the course of the COVID-19 epidemic? | journal = Lancet | date = March 2020 | volume = 395 | issue = 10228 | pages = 931–934 | pmid = 32164834 | doi = 10.1016/S0140-6736(20)30567-5 | quote = A key issue for epidemiologists is helping policy makers decide the main objectives of mitigation—e.g. minimising morbidity and associated mortality, avoiding an epidemic peak that overwhelms health-care services, keeping the effects on the economy within manageable levels, and flattening the epidemic curve to wait for vaccine development and manufacture on scale and antiviral drug therapies. | doi-access = free }}</ref><ref>{{cite web|url=https://www.vox.com/2020/3/10/21171481/coronavirus-us-cases-quarantine-cancellation|title=How canceled events and self-quarantines save lives, in one chart|first=Eliza|last=Barclay|date=10 March 2020|website=Vox|name-list-format=vanc|access-date=12 March 2020|archive-url=https://web.archive.org/web/20200312161852/https://www.vox.com/2020/3/10/21171481/coronavirus-us-cases-quarantine-cancellation|archive-date=12 March 2020|url-status=live}}</ref>]] | |||
| ] (RAAS)]] | |||
| Multiple viral and host factors affect the pathogenesis of the virus. The ], otherwise known as the spike protein, is the viral component that attaches to the host receptor via the ] receptors. It includes two subunits: S1 and S2. | |||
| * S1 determines the virus-host range and cellular tropism via the receptor-binding domain. | |||
| * S2 mediates the membrane fusion of the virus to its potential cell host via the H1 and HR2, which are ] regions. | |||
| Studies have shown that S1 domain induced ] and ] antibody levels at a much higher capacity. It is the focus spike proteins expression that are involved in many effective COVID‑19 vaccines.<ref name="pmid33340022">{{#invoke:cite journal || vauthors = Dai L, Gao GF | title = Viral targets for vaccines against COVID-19 | journal = Nature Reviews. Immunology | volume = 21 | issue = 2 | pages = 73–82 | date = February 2021 | pmid = 33340022 | pmc = 7747004 | doi = 10.1038/s41577-020-00480-0 |issn=1474-1733 }}</ref> | |||
| Preventive measures to reduce the chances of infection include staying at home, avoiding crowded places, washing hands with soap and water often and for at least 20 seconds, practising good respiratory hygiene and avoiding touching the eyes, nose or mouth with unwashed hands.<ref name="CDC-Prevention & Treatment">{{cite web | url = https://www.cdc.gov/coronavirus/about/prevention.html | author = Centers for Disease Control | title = Coronavirus Disease 2019 (COVID-19): Prevention & Treatment | date = 3 February 2020 | language = en-us | access-date = 10 February 2020 | archive-url = https://web.archive.org/web/20191215193934/https://www.cdc.gov/coronavirus/about/prevention.html | archive-date = 15 December 2019 | url-status = live| author-link = Centers for Disease Control }}</ref><ref name="WHO Advice for Public">{{cite web | url = https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public | title = Advice for Public | vauthors = ((World Health Organization)) | access-date = 10 February 2020 | archive-url = https://web.archive.org/web/20200126025750/https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public | archive-date = 26 January 2020 | url-status = live| author-link = World Health Organization | name-list-format = vanc}}</ref><ref>{{cite web|url=https://www.npr.org/sections/goatsandsoda/2020/03/17/814221111/my-hand-washing-song-readers-offer-lyrics-for-a-20-second-scrub|title=My Hand-Washing Song: Readers Offer Lyrics For A 20-Second Scrub|website=NPR.org|language=en|access-date=20 March 2020|archive-url=https://web.archive.org/web/20200320145553/https://www.npr.org/sections/goatsandsoda/2020/03/17/814221111/my-hand-washing-song-readers-offer-lyrics-for-a-20-second-scrub|archive-date=20 March 2020|url-status=live}}</ref> The CDC recommends covering the mouth and nose with a tissue when coughing or sneezing and recommends using the inside of the elbow if no tissue is available.<ref name = "CDC-Prevention & Treatment"/> They also recommend proper hand hygiene after any cough or sneeze.<ref name = "CDC-Prevention & Treatment"/> ] strategies aim to reduce contact of infected persons with large groups by closing schools and workplaces, restricting travel and cancelling mass gatherings.<ref name="JHUSocialDistancing">{{cite web | first = Lisa Lockerd | last = Maragakis | name-list-format = vanc | title = Coronavirus, Social Distancing and Self Quarantine | url = https://www.hopkinsmedicine.org/health/conditions-and-diseases/coronavirus/coronavirus-social-distancing-and-self-quarantine | website = www.hopkinsmedicine.org | publisher = Johns Hopkins University | access-date = 18 March 2020 | archive-url = https://web.archive.org/web/20200318012357/https://www.hopkinsmedicine.org/health/conditions-and-diseases/coronavirus/coronavirus-social-distancing-and-self-quarantine | archive-date = 18 March 2020 | url-status = live }}</ref> Social distancing also includes that people stay at least {{Convert|6|ft||abbr=}} apart.<ref>{{Cite news|last=Parker-Pope|first=Tara|url=https://www.nytimes.com/2020/03/19/well/live/coronavirus-quarantine-social-distancing.html|title=Deciding How Much Distance You Should Keep|date=19 March 2020|work=The New York Times|access-date=20 March 2020|language=en-US|issn=0362-4331|archive-url=https://web.archive.org/web/20200320003705/https://www.nytimes.com/2020/03/19/well/live/coronavirus-quarantine-social-distancing.html|archive-date=20 March 2020|url-status=live}}</ref> | |||
| The M protein is the viral protein responsible for the transmembrane transport of nutrients. It is the cause of the bud release and the formation of the viral envelope.<ref name="Boopathi-2020">{{#invoke:cite journal || vauthors = Boopathi S, Poma AB, Kolandaivel P | title = Novel 2019 coronavirus structure, mechanism of action, antiviral drug promises and rule out against its treatment | journal = Journal of Biomolecular Structure & Dynamics | pages = 3409–3418 | date = April 2020 | volume = 39 | issue = 9 | pmid = 32306836 | pmc = 7196923 | doi = 10.1080/07391102.2020.1758788 }}</ref> The N and E protein are accessory proteins that interfere with the host's immune response.<ref name="Boopathi-2020" /> | |||
| As a ] is not expected until 2021 at the earliest,<ref>{{cite web | url = https://www.sciencealert.com/who-says-a-coronavirus-vaccine-is-18-months-away | title = Here's Why It's Taking So Long to Develop a Vaccine for the New Coronavirus | website = Science Alert | first1 = Rob | last1 = Grenfell | first2 = Trevor | last2 = Drew | name-list-format = vanc | date = 17 February 2020 | access-date = 26 February 2020 | archive-url = https://web.archive.org/web/20200228010631/https://www.sciencealert.com/who-says-a-coronavirus-vaccine-is-18-months-away | archive-date = 28 February 2020 | url-status = live }}</ref> a key part of managing COVID-19 is trying to decrease the epidemic peak, known as "flattening the ]".<ref name="Lancet2020Flatten" /> This is done by slowing the infection rate to decrease the risk of health services being overwhelmed, allowing for better treatment of current cases and delaying additional cases until effective treatments or a vaccine become available.<ref name="Lancet2020Flatten" /><ref>{{cite web |last=Wiles |first=Siouxsie |title=After 'Flatten the Curve', we must now 'Stop the Spread'. Here's what that means |url=https://thespinoff.co.nz/society/14-03-2020/after-flatten-the-curve-we-must-now-stop-the-spread-heres-what-that-means/ |website=The Spinoff |access-date=13 March 2020 |date=14 March 2020 |archive-url=https://web.archive.org/web/20200326232315/https://thespinoff.co.nz/society/14-03-2020/after-flatten-the-curve-we-must-now-stop-the-spread-heres-what-that-means/ |archive-date=26 March 2020 |url-status=live }}</ref><ref>{{cite journal | vauthors = Anderson RM, Heesterbeek H, Klinkenberg D, Hollingsworth TD | title = How will country-based mitigation measures influence the course of the COVID-19 epidemic? | journal = Lancet | date = March 2020 | volume = 395 | issue = 10228 | pages = 931–934 | pmid = 32164834 | doi = 10.1016/S0140-6736(20)30567-5 }}</ref> | |||
| ==== Host factors ==== | |||
| According to the WHO, the use of masks is recommended only if a person is coughing or sneezing or when one is taking care of someone with a suspected infection.{{update-inline|date=April 2020}}<ref>{{cite web|url=https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public/when-and-how-to-use-masks|title=When and how to use masks|website=] (WHO)|access-date=8 March 2020|name-list-format=vanc|archive-url=https://web.archive.org/web/20200307013848/https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public/when-and-how-to-use-masks|archive-date=7 March 2020|url-status=live}}</ref> Some countries also recommend healthy individuals to wear face masks, including China,<ref name="nhc_masks">{{cite web |url=http://en.nhc.gov.cn/2020-02/07/c_76337.htm |title=For different groups of people: how to choose masks |work=NHC.gov.cn |publisher=National Health Commission of the People's Republic of China |date=7 February 2020 |access-date=22 March 2020 |archive-url= |archive-date= |quote="Disposable medical masks: Recommended for: · People in crowded places · Indoor working environment with a relatively dense population · People going to medical institutions · Children in kindergarten and students at school gathering to study and do other activities" }}{{Dead link|date=April 2020 |bot=InternetArchiveBot |fix-attempted=yes }}</ref> ],<ref>{{cite web |url=https://www.chp.gov.hk/files/pdf/prevention_of_covid_19_en.pdf |title=Prevention of Coronavirus Disease 2019 (COVID-19) |website=] |access-date=22 March 2020 |quote="Wear a surgical mask when taking public transport or staying in crowded places." |archive-url=https://web.archive.org/web/20200321175110/https://www.chp.gov.hk/files/pdf/prevention_of_covid_19_en.pdf |archive-date=21 March 2020 |url-status=live }}</ref> ],<ref>{{cite news |title='Better than nothing': Thailand encourages cloth masks amid surgical mask shortage |url=https://www.reuters.com/article/us-health-coronavirus-thailand-masks/better-than-nothing-thailand-encourages-cloth-masks-amid-surgical-mask-shortage-idUSKBN20Z0UT |first=Jiraporn |last=Kuhakan |work=Reuters |date=12 March 2020 |quote="Thailand's health authorities are encouraging people to make cloth face masks at home to guard against the spread of the coronavirus amid a shortage of surgical masks.{{nbsp}}... The droplet from coughing and sneezing is around five microns and we have tested already that cloth masks can protect against droplets bigger than one micron." |access-date=22 March 2020 |archive-url=https://web.archive.org/web/20200321192522/https://www.reuters.com/article/us-health-coronavirus-thailand-masks/better-than-nothing-thailand-encourages-cloth-masks-amid-surgical-mask-shortage-idUSKBN20Z0UT |archive-date=21 March 2020 |url-status=live }}</ref> Czech Republic,<ref>{{cite web|url=https://www.euronews.com/2020/03/24/coronavirus-czechs-facing-up-to-covid-19-crisis-by-making-masks-mandatory|title=Coronavirus: Czechs facing up to COVID-19 crisis by making masks mandatory|last=|first=|date=2020|website=euronews|url-status=live|archive-url=https://web.archive.org/web/20200330233916/https://www.euronews.com/2020/03/24/coronavirus-czechs-facing-up-to-covid-19-crisis-by-making-masks-mandatory|archive-date=30 March 2020|access-date=}}</ref> and Austria.<ref>{{cite web|url=https://www.cbsnews.com/news/austria-supermarket-face-mask/|title=Austria is making everyone who goes inside a supermarket wear a face mask|website=www.cbsnews.com|language=en-US|access-date=2020-03-31|archive-url=https://web.archive.org/web/20200331192209/https://www.cbsnews.com/news/austria-supermarket-face-mask/|archive-date=31 March 2020|url-status=live}}</ref> In order to meet the need for masks, the WHO estimates that global production will need to increase by 40%. Hoarding and speculation have worsened the problem, with the price of masks increasing sixfold, ] tripled, and gowns doubled.<ref>{{cite press release |title= Shortage of personal protective equipment endangering health workers worldwide |date= 3 March 2020 |publisher= WHO |url= https://www.who.int/news-room/detail/03-03-2020-shortage-of-personal-protective-equipment-endangering-health-workers-worldwide |access-date= 24 March 2020 |archive-url= https://web.archive.org/web/20200305052623/https://www.who.int/news-room/detail/03-03-2020-shortage-of-personal-protective-equipment-endangering-health-workers-worldwide |archive-date= 5 March 2020 |url-status= live }}</ref> Some health experts consider wearing non-medical grade masks and other face coverings like scarves or bandanas a good way to prevent people from touching their mouths and noses, even if non-medical coverings would not protect against a direct sneeze or cough from an infected person.<ref>{{cite web|url=https://www.bostonglobe.com/2020/03/19/opinion/guidance-against-wearing-masks-coronavirus-is-wrong-you-should-cover-your-face/|title=Guidance against wearing masks for the coronavirus is wrong—you should cover your face—The Boston Globe|website=BostonGlobe.com|access-date=22 March 2020|archive-url=https://web.archive.org/web/20200322181032/https://www.bostonglobe.com/2020/03/19/opinion/guidance-against-wearing-masks-coronavirus-is-wrong-you-should-cover-your-face/|archive-date=22 March 2020|url-status=live}}</ref> | |||
| Human ] (hACE2) is the host factor that SARS-CoV-2 virus targets causing COVID‑19. Theoretically, the usage of ]s (ARB) and ]s upregulating ACE2 expression might increase ] with COVID‑19, though animal data suggest some potential protective effect of ARB; however no clinical studies have proven susceptibility or outcomes. Until further data is available, guidelines and recommendations for people with hypertension remain.<ref>{{#invoke:cite journal || vauthors = Kai H, Kai M | title = Interactions of coronaviruses with ACE2, angiotensin II, and RAS inhibitors-lessons from available evidence and insights into COVID-19 | journal = Hypertension Research | volume = 43 | issue = 7 | pages = 648–654 | date = July 2020 | pmid = 32341442 | pmc = 7184165 | doi = 10.1038/s41440-020-0455-8 }}</ref> | |||
| Those diagnosed with COVID-19 or who believe they may be infected are advised by the CDC to stay home except to get medical care, call ahead before visiting a healthcare provider, wear a face mask before entering the healthcare provider's office and when in any room or vehicle with another person, cover coughs and sneezes with a tissue, regularly wash hands with soap and water and avoid sharing personal household items.<ref>{{cite web | url = https://www.cdc.gov/coronavirus/2019-ncov/about/prevention.html | title = Coronavirus Disease 2019 (COVID-19)—Prevention & Treatment | date = 10 March 2020 | work = Centers for Disease Control and Prevention | publisher = U.S. Department of Health & Human Services | access-date = 11 March 2020 | archive-url = https://web.archive.org/web/20200311163637/https://www.cdc.gov/coronavirus/2019-ncov/about/prevention.html | archive-date = 11 March 2020 | url-status = live }}</ref><ref>{{cite web | url = https://www.cdc.gov/coronavirus/2019-ncov/about/steps-when-sick.html | title = What to do if you are sick with 2019 Novel Coronavirus (2019-nCoV) | author = ] | date = 11 February 2020 | language = en-us | url-status = live | archive-url = https://web.archive.org/web/20200214153016/https://www.cdc.gov/coronavirus/2019-ncov/about/steps-when-sick.html | archive-date = 14 February 2020 | access-date = 13 February 2020| name-list-format = vanc}}</ref> The CDC also recommends that individuals wash hands often with soap and water for at least 20 seconds, especially after going to the toilet or when hands are visibly dirty, before eating and after blowing one's nose, coughing or sneezing. It further recommends using an alcohol-based ] with at least 60% alcohol, but only when soap and water are not readily available.<ref name="CDC-Prevention & Treatment" /> | |||
| The effect of the virus on ACE2 cell surfaces leads to leukocytic infiltration, increased blood vessel permeability, alveolar wall permeability, as well as decreased secretion of lung surfactants. These effects cause the majority of the respiratory symptoms. However, the aggravation of local inflammation causes a cytokine storm eventually leading to a ].<ref>{{#invoke:cite journal || vauthors = Chen HX, Chen ZH, Shen HH | title = | journal = Sheng Li Xue Bao | volume = 72 | issue = 5 | pages = 617–630 | date = October 2020 | pmid = 33106832 }}</ref> | |||
| For areas where commercial hand sanitisers are not readily available, the WHO provides two formulations for local production. In these formulations, the antimicrobial activity arises from ] or ]. ] is used to help eliminate ] in the alcohol; it is "not an active substance for hand ]". ] is added as a ].<ref>{{cite book | chapter-url = https://www.ncbi.nlm.nih.gov/books/NBK144054/ | chapter = WHO-recommended handrub formulations | title = WHO Guidelines on Hand Hygiene in Health Care: First Global Patient Safety Challenge Clean Care Is Safer Care. |date=19 March 2009 |publisher=World Health Organization|access-date=19 March 2020 }}</ref> | |||
| <gallery mode=packed heights=200px> | |||
| File:Covid-19-Transmission-graphic-01.gif|Prevention efforts are multiplicative, with effects far beyond that of a single spread. Each avoided case leads to more avoided cases down the line, which in turn can stop the outbreak in its tracks. | |||
| File:COVID19 W ENG.ogv|Handwashing instructions | |||
| </gallery> | |||
| Among healthy adults not exposed to SARS-CoV-2, about 35% have ]s that recognise the SARS-CoV-2 ] (particularly the S2 subunit) and about 50% react to other proteins of the virus, suggesting ] from previous ]s caused by other coronaviruses.<ref>{{#invoke:cite journal ||vauthors=Jeyanathan M, Afkhami S, Smaill F, Miller MS, Lichty BD, Xing Z |date=4 September 2020 |title=Immunological considerations for COVID-19 vaccine strategies |journal=Nature Reviews Immunology |volume=20 |issue=10 |pages=615–632 |doi=10.1038/s41577-020-00434-6 |pmid=32887954 |pmc=7472682 |issn=1474-1741}}</ref> | |||
| ==Management== | |||
| It is unknown whether different persons use similar antibody genes in response to COVID‑19.<ref>{{#invoke:cite journal || vauthors = Zhang Q, Ju B, Ge J, Chan JF, Cheng L, Wang R, Huang W, Fang M, Chen P, Zhou B, Song S, Shan S, Yan B, Zhang S, Ge X, Yu J, Zhao J, Wang H, Liu L, Lv Q, Fu L, Shi X, Yuen KY, Liu L, Wang Y, Chen Z, Zhang L, Wang X, Zhang Z | title = Potent and protective IGHV3-53/3-66 public antibodies and their shared escape mutant on the spike of SARS-CoV-2 | journal = Nature Communications | volume = 12 | issue = 1 | pages = 4210 | date = July 2021 | pmid = 34244522 | pmc = 8270942 | doi = 10.1038/s41467-021-24514-w | bibcode = 2021NatCo..12.4210Z | s2cid = 235786394 }}</ref> | |||
| ] | |||
| === Host cytokine response === | |||
| People are managed with ], which may include fluid therapy, ] and supporting other affected vital organs.<ref name="NatureDale Fisher & David Heymann">{{cite journal | vauthors=Fisher D, Heymann D |title = Q&A: The novel coronavirus outbreak causing COVID-19 |journal=BMC Medicine |volume=18 |issue=1 |page=57 |doi=10.1186/s12916-020-01533-w |doi-access=free |pmid=32106852 |pmc=7047369 | date=February 2020 }}</ref><ref name="KuiFang2020">{{cite journal | vauthors = Kui L, Fang YY, Deng Y, Liu W, Wang MF, Ma JP, Xiao W, Wang YN, Zhong MH, Li CH, Li GC, Liu HG | display-authors = 6 | title = Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province | journal = Chinese Medical Journal | page = 1 | date = February 2020 | pmid = 32044814 |doi=10.1097/CM9.0000000000000744 |doi-access=free }}</ref><ref name="Wang Du Zhu Cao 2020 p.">{{cite journal | vauthors = Wang T, Du Z, Zhu F, Cao Z, An Y, Gao Y, Jiang B | title = Comorbidities and multi-organ injuries in the treatment of COVID-19 | journal = Lancet | date = March 2020 | volume = 395 | issue = 10228 | pages = e52 | pmid = 32171074 | doi = 10.1016/s0140-6736(20)30558-4 | publisher = Elsevier BV }}</ref> The CDC recommends that those who suspect they carry the virus wear a simple face mask.<ref name=CDC2020IfSick/> ] (ECMO) has been used to address the issue of respiratory failure, but its benefits are still under consideration.<ref name="Guan Ni Hu Liang p." /><ref name="Henry 2020 p.">{{cite journal | last=Henry | first=Brandon Michael | name-list-format = vanc | title=COVID-19, ECMO, and lymphopenia: a word of caution | journal=The Lancet Respiratory Medicine | publisher=Elsevier BV | date=2020 | volume=8 | issue=4 | pages=e24 | issn=2213-2600 | doi=10.1016/s2213-2600(20)30119-3 | pmid=32178774 | pmc=7118650 }}</ref> | |||
| ] during ]]] | |||
| The severity of the inflammation can be attributed to the severity of what is known as the ].<ref name="pmid32474885">{{#invoke:cite journal || vauthors = Soy M, Keser G, Atagündüz P, Tabak F, Atagündüz I, Kayhan S | title = Cytokine storm in COVID-19: pathogenesis and overview of anti-inflammatory agents used in treatment | journal = Clinical Rheumatology | volume = 39 | issue = 7 | pages = 2085–2094 | date = July 2020 | pmid = 32474885 | pmc = 7260446 | doi = 10.1007/s10067-020-05190-5 }}</ref> Levels of ], ], interferon-inducible protein 10, and monocyte chemoattractant protein{{spaces}}1 were all associated with COVID‑19 disease severity. Treatment has been proposed to combat the cytokine storm as it remains to be one of the leading causes of ] and mortality in COVID‑19 disease.<ref>{{#invoke:cite journal || vauthors = Quirch M, Lee J, Rehman S | title = Hazards of the Cytokine Storm and Cytokine-Targeted Therapy in Patients With COVID-19: Review | journal = Journal of Medical Internet Research | volume = 22 | issue = 8 | pages = e20193 | date = August 2020 | pmid = 32707537 | pmc = 7428145 | doi = 10.2196/20193 |doi-access=free}}</ref> | |||
| A cytokine storm is due to an acute hyperinflammatory response that is responsible for clinical illness in an array of diseases but in COVID‑19, it is related to worse prognosis and increased fatality. The storm causes acute respiratory distress syndrome, blood clotting events such as strokes, ], ], ], and ]. The production of ], ], ], ], and ], all crucial components of normal immune responses, inadvertently become the causes of a cytokine storm. The cells of the ], the ], ]s, and ]s, are also involved in the release of ]s affecting the nervous system, and effects of cytokine storms toward the ] are not uncommon.<ref>{{#invoke:cite journal || vauthors = Bhaskar S, Sinha A, Banach M, Mittoo S, Weissert R, Kass JS, Rajagopal S, Pai AR, Kutty S | title = Cytokine Storm in COVID-19-Immunopathological Mechanisms, Clinical Considerations, and Therapeutic Approaches: The REPROGRAM Consortium Position Paper | journal = Frontiers in Immunology | volume = 11 | pages = 1648 | year = 2020 | pmid = 32754159 | pmc = 7365905 | doi = 10.3389/fimmu.2020.01648 | doi-access = free | title-link = doi }}</ref> | |||
| The WHO and ] have published recommendations for taking care of people who are hospitalised with COVID-19.<ref name="Cheng2020">{{cite journal | vauthors = Cheng ZJ, Shan J | title = 2019 Novel coronavirus: where we are and what we know | journal = Infection | date = February 2020 | volume = 48 | issue = 2 | pages = 155–163 | pmid = 32072569 |doi=10.1007/s15010-020-01401-y |doi-access=free }}</ref><ref>{{cite web|url=https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected|title=Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected|website=] (WHO)|access-date=13 February 2020|archive-url=https://web.archive.org/web/20200131032122/https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected|archive-date=31 January 2020|url-status=live }}</ref> ] and ] in the US have compiled treatment recommendations from various agencies into a free resource, the ].<ref name="IBCC">{{cite book |last=Farkas |first=Josh | name-list-format = vanc |date=March 2020 |title=COVID-19—The Internet Book of Critical Care |url=https://emcrit.org/ibcc/covid19/ |url-status=live |format=digital |type=Reference manual |language=English |location=USA |publisher=EMCrit |archive-url=https://web.archive.org/web/20200311195758/https://emcrit.org/ibcc/covid19/ |archive-date=11 March 2020 |access-date=13 March 2020}}</ref><ref name="UPenn-IBCC">{{cite web |url=https://guides.library.upenn.edu/covid-19 |title=COVID19—Resources for Health Care Professionals |publisher=] |date=11 March 2020 |access-date=13 March 2020 |archive-url=https://web.archive.org/web/20200314035631/https://guides.library.upenn.edu/covid-19 |archive-date=14 March 2020 |url-status=live }}</ref> | |||
| === |
=== Pregnancy response === | ||
| There are many unknowns for pregnant women during the COVID-19 pandemic. Given that they are prone to have complications and severe disease infection with other types of coronaviruses, they have been identified as a vulnerable group and advised to take supplementary preventive measures.<ref name="Wastnedge_2021">{{#invoke:cite journal||vauthors=Wastnedge EA, Reynolds RM, van Boeckel SR, Stock SJ, Denison FC, Maybin JA, Critchley HO |date=January 2021|title=Pregnancy and COVID-19|journal=Physiological Reviews|volume=101|issue=1|pages=303–318|doi=10.1152/physrev.00024.2020|pmc=7686875|pmid=32969772}}</ref> | |||
| Physiological responses to pregnancy can include: | |||
| Some medical professionals recommend ] (acetaminophen) over ] for first-line use.<ref name="Day 2020 p.">{{cite journal | title = Covid-19: ibuprofen should not be used for managing symptoms, say doctors and scientists | journal = BMJ | volume = 368 | date = 17 March 2020 | pmid = 32184201 | doi = 10.1136/bmj.m1086 | issn=1756-1833 | url = https://www.bmj.com/content/368/bmj.m1086 | access-date = 18 March 2020 | last = Day | first = Michael | pages = m1086 | archive-url = https://web.archive.org/web/20200319181945/https://www.bmj.com/content/368/bmj.m1086 | archive-date = 19 March 2020 | url-status = live }}</ref><ref>{{cite web|url=https://www.nhs.uk/conditions/coronavirus-covid-19/self-isolation-advice/|title=Self-isolation advice—Coronavirus (COVID-19)|date=2020-02-28|website=National Health Service (United Kingdom)|language=en|access-date=2020-03-27|archive-url=https://web.archive.org/web/20200328000128/https://www.nhs.uk/conditions/coronavirus-covid-19/self-isolation-advice/|archive-date=28 March 2020|url-status=live}}</ref> The WHO does not oppose the use of ] (NSAIDs) such as ibuprofen for symptoms,<ref name="AFP 2020b">{{cite web|url=https://www.sciencealert.com/who-recommends-to-avoid-taking-ibuprofen-for-covid-19-symptoms|title=Updated: WHO Now Doesn't Recommend Avoiding Ibuprofen For COVID-19 Symptoms|author=AFP|date=19 March 2020|website=ScienceAlert|access-date=19 March 2020|archive-url=https://web.archive.org/web/20200318222020/https://www.sciencealert.com/who-recommends-to-avoid-taking-ibuprofen-for-covid-19-symptoms|archive-date=18 March 2020|url-status=live}}</ref> and the ] says currently there is no evidence that NSAIDs worsen COVID-19 symptoms.<ref>{{Cite journal|last=Research|first=Center for Drug Evaluation and|date=2020-03-19|title=FDA advises patients on use of non-steroidal anti-inflammatory drugs (NSAIDs) for COVID-19|url=http://www.fda.gov/drugs/drug-safety-and-availability/fda-advises-patients-use-non-steroidal-anti-inflammatory-drugs-nsaids-covid-19|journal=Drug Safety and Availability|language=en|access-date=27 March 2020|archive-url=https://web.archive.org/web/20200327194633/https://www.fda.gov/drugs/drug-safety-and-availability/fda-advises-patients-use-non-steroidal-anti-inflammatory-drugs-nsaids-covid-19|archive-date=27 March 2020|url-status=live}}</ref> | |||
| * Immunological: The immunological response to COVID-19, like other viruses, depends on a working immune system. It adapts during pregnancy to allow the development of the foetus whose genetic load is only partially shared with their mother, leading to a different immunological reaction to infections during the course of pregnancy.<ref name="Wastnedge_2021" /> | |||
| * Respiratory: Many factors can make pregnant women more vulnerable to hard respiratory infections. One of them is the total reduction of the lungs' capacity and inability to clear secretions.<ref name="Wastnedge_2021" /> | |||
| * Coagulation: During pregnancy, there are higher levels of circulating coagulation factors, and the pathogenesis of SARS-CoV-2 infection can be implicated. The thromboembolic events with associated mortality are a risk for pregnant women.<ref name="Wastnedge_2021" /> | |||
| However, from the evidence base, it is difficult to conclude whether pregnant women are at increased risk of grave consequences of this virus.<ref name="Wastnedge_2021" /> | |||
| While theoretical concerns have been raised about ] and ]s, as of 19 March 2020, these are not sufficient to justify stopping these medications.<ref>{{cite web |title=Patients taking ACE-i and ARBs who contract COVID-19 should continue treatment, unless otherwise advised by their physician |url=https://www.hfsa.org/patients-taking-ace-i-and-arbs-who-contract-covid-19-should-continue-treatment-unless-otherwise-advised-by-their-physician/ |access-date=21 March 2020 |archive-url=https://web.archive.org/web/20200321172112/https://www.hfsa.org/patients-taking-ace-i-and-arbs-who-contract-covid-19-should-continue-treatment-unless-otherwise-advised-by-their-physician/ |archive-date=21 March 2020 |url-status=live }}</ref><ref>{{cite press release | title=Patients taking ACE-i and ARBs who contract COVID-19 should continue treatment, unless otherwise advised by their physician | website=American Heart Association | date=17 March 2020 | url=https://newsroom.heart.org/news/patients-taking-ace-i-and-arbs-who-contract-covid-19-should-continue-treatment-unless-otherwise-advised-by-their-physician | access-date=25 March 2020 | archive-url=https://web.archive.org/web/20200324050912/https://newsroom.heart.org/news/patients-taking-ace-i-and-arbs-who-contract-covid-19-should-continue-treatment-unless-otherwise-advised-by-their-physician | archive-date=24 March 2020 | url-status=live }}</ref><ref name="ESCPositionStatement">{{cite web |last=de Simone |first=Giovanni |title=Position Statement of the ESC Council on Hypertension on ACE-Inhibitors and Angiotensin Receptor Blockers |url=https://www.escardio.org/Councils/Council-on-Hypertension-(CHT)/News/position-statement-of-the-esc-council-on-hypertension-on-ace-inhibitors-and-ang |website=Council on Hypertension of the European Society of Cardiology |access-date=24 March 2020 |archive-url=https://web.archive.org/web/20200324073257/https://www.escardio.org/Councils/Council-on-Hypertension-(CHT)/News/position-statement-of-the-esc-council-on-hypertension-on-ace-inhibitors-and-ang |archive-date=24 March 2020 |url-status=live }}</ref> ], such as ], are not recommended unless the disease is complicated by ].<ref name="Vetter Eckerle Kaiser 2020 p.">{{cite journal | vauthors=Vetter P, Eckerle I, Kaiser L | title=Covid-19: a puzzle with many missing pieces | journal=BMJ | volume=368 | pages=m627 | date=February 2020 | pmid=32075791 | doi=10.1136/bmj.m627 |doi-access=free }}</ref><ref>{{cite web |title=Novel Coronavirus—COVID-19: What Emergency Clinicians Need to Know |url=https://www.ebmedicine.net/topics/infectious-disease/COVID-19 |website=www.ebmedicine.net |access-date=9 March 2020 |name-list-format=vanc |archive-url=https://web.archive.org/web/20200314163512/https://www.ebmedicine.net/topics/infectious-disease/COVID-19 |archive-date=14 March 2020 |url-status=live }}</ref> | |||
| In addition to the above, other clinical studies have proved that SARS-CoV-2 can affect the period of pregnancy in different ways. On the one hand, there is little evidence of its impact up to 12 weeks gestation. On the other hand, COVID-19 infection may cause increased rates of unfavourable outcomes in the course of the pregnancy. Some examples of these could be foetal growth restriction, preterm birth, and perinatal mortality, which refers to the foetal death past 22 or 28 completed weeks of pregnancy as well as the death among live-born children up to seven completed days of life.<ref name="Wastnedge_2021" /> For preterm birth, a 2023 review indicates that there appears to be a correlation with COVID-19.<ref>{{#invoke:cite journal ||last1=Digby |first1=Alyson M. |last2=Dahan |first2=Michael H. |title=Obstetrical and gynecologic implications of COVID-19: what have we learned over the first two years of the pandemic |journal=Archives of Gynecology and Obstetrics |date=12 January 2023 |volume=308 |issue=3 |pages=813–819 |doi=10.1007/s00404-022-06847-z|pmid=36633677 |pmc=9838509 }}</ref> | |||
| ===Personal protective equipment=== | |||
| Unvaccinated women in later stages of pregnancy with COVID-19 are more likely than other people to need very intensive care. Babies born to mothers with COVID-19 are more likely to have breathing problems. Pregnant women are strongly encouraged to get ].<ref>{{#invoke:Cite web|| vauthors = Campbell D | title=One in six most critically ill NHS Covid patients are unvaccinated pregnant women | website=The Guardian | date=10 October 2021 | url=https://www.theguardian.com/lifeandstyle/2021/oct/11/one-in-six-most-critically-ill-patients-are-unvaccinated-pregnant-women-with-covid | access-date=25 January 2022 }}</ref> | |||
| Precautions must be taken to minimise the risk of virus transmission, especially in healthcare settings when performing procedures that can generate ]s, such as ] or ].<ref name="Cheung Ho Cheng Cham 2020 p.">{{cite journal | vauthors=Cheung JC, Ho LT, Cheng JV, Cham EY, Lam KN | title=Staff safety during emergency airway management for COVID-19 in Hong Kong | journal=Lancet Respiratory Medicine | date=February 2020 | volume=8 | issue=4 | pages=e19 | doi=10.1016/s2213-2600(20)30084-9 | doi-access=free | pmid=32105633 }}</ref> For healthcare professionals caring for people with COVID-19, the CDC recommends placing the person in an Airborne Infection Isolation Room (AIIR) in addition to using ], contact precautions and airborne precautions.<ref>{{cite web|url=https://www.cdc.gov/coronavirus/2019-ncov/hcp/caring-for-patients-H.pdf|title=What healthcare personnel should know about caring for patients with confirmed or possible coronavirus disease 2|last=|date=12 March 2020|website=CDC|url-status=live|archive-url=|archive-date=|access-date=31 March 2020}}</ref> | |||
| == Diagnosis == | |||
| CDC outlines the specific guidelines for the use of ] (PPE) during the pandemic. The recommended gear includes: | |||
| {{Further|COVID-19 testing}} | |||
| * ] or ]<ref> {{Webarchive|url=https://web.archive.org/web/20190816114738/https://www.cdc.gov/niosh/docs/2018-130/ |date=16 August 2019 }}. The National Institute for Occupational Safety and Health (April 2018). Retrieved 16 March 2020.</ref><ref> {{Webarchive|url=https://web.archive.org/web/20200327073252/https://blogs.cdc.gov/niosh-science-blog/2020/03/16/n95-preparedness/?deliveryName=USCDC_170-DM22692 |date=27 March 2020 }}. NIOSH Science Blog (16 March 2020). Retrieved 16 March 2020.</ref> | |||
| COVID‑19 can provisionally be diagnosed on the basis of symptoms and confirmed using ] (RT-PCR) or other ]ing of infected secretions.<ref name="pmid32621814">{{#invoke:cite journal || vauthors = Li C, Zhao C, Bao J, Tang B, Wang Y, Gu B | title = Laboratory diagnosis of coronavirus disease-2019 (COVID-19) | journal = Clinica Chimica Acta; International Journal of Clinical Chemistry | volume = 510 | pages = 35–46 | date = November 2020 | pmid = 32621814 | pmc = 7329657 | doi = 10.1016/j.cca.2020.06.045 }}</ref><ref name="jTxKm">{{#invoke:cite journal || vauthors = Ai T, Yang Z, Hou H, Zhan C, Chen C, Lv W, Tao Q, Sun Z, Xia L | title = Correlation of Chest CT and RT-PCR Testing for Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases | journal = Radiology | volume = 296 | issue = 2 | pages = E32–E40 | date = August 2020 | pmid = 32101510 | pmc = 7233399 | doi = 10.1148/radiol.2020200642 }}</ref> Along with laboratory testing, chest CT scans may be helpful to diagnose COVID‑19 in individuals with a high clinical suspicion of infection.<ref name="Salehi-2020">{{#invoke:cite journal || vauthors = Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A | title = Coronavirus Disease 2019 (COVID-19): A Systematic Review of Imaging Findings in 919 Patients | journal = AJR. American Journal of Roentgenology | volume = 215 | issue = 1 | pages = 87–93 | date = July 2020 | pmid = 32174129 | doi = 10.2214/AJR.20.23034 | doi-access = free | title-link = doi }}</ref> Detection of a past infection is possible with ]s, which detect ] produced by the body in response to the infection.<ref name="pmid32621814" /> | |||
| * ]<ref>{{cite web |title=Coronavirus Disease 2019 (COVID-19) |url=https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html |website=Centers for Disease Control and Prevention |date=11 February 2020 |accessdate=4 April 2020}}</ref> | |||
| * ]s<ref>{{cite web |title=Coronavirus Disease 2019 (COVID-19) |url=https://www.cdc.gov/coronavirus/2019-ncov/infection-control/control-recommendations.html |website=Centers for Disease Control and Prevention |access-date=11 March 2020 |date=11 February 2020 |name-list-format=vanc |archive-url=https://web.archive.org/web/20200304165907/https://www.cdc.gov/coronavirus/2019-ncov/infection-control/control-recommendations.html |archive-date=4 March 2020 |url-status=live }}</ref><ref>{{cite web |title=Coronavirus Disease 2019 (COVID-19) |url=https://www.cdc.gov/coronavirus/2019-ncov/hcp/caring-for-patients.html |website=Centers for Disease Control and Prevention |access-date=8 March 2020 |date=11 February 2020 |name-list-format=vanc |archive-url=https://web.archive.org/web/20200304165907/https://www.cdc.gov/coronavirus/2019-ncov/hcp/caring-for-patients.html |archive-date=4 March 2020 |url-status=live }}</ref> | |||
| * ]<ref>{{cite web|title=Strategies for Optimizing the Supply of Eye Protection|date=11 February 2020|url=https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/eye-protection.html|publisher=CDC|access-date=23 March 2020|archive-url=https://web.archive.org/web/20200323173916/https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/eye-protection.html|archive-date=23 March 2020|url-status=live}}</ref> | |||
| === Viral testing === | |||
| When available, respirators (instead of facemasks) are preferred.<ref>{{cite web|url=https://www.cdc.gov/coronavirus/2019-ncov/infection-control/control-recommendations.html|title=Interim Infection Prevention and Control Recommendations for Patients with Suspected or Confirmed Coronavirus Disease 2019 (COVID-19) in Healthcare Settings|author=CDC|date=11 February 2020|website=Centers for Disease Control and Prevention|language=en-us|url-status=live|archive-url=https://web.archive.org/web/20200304165907/https://www.cdc.gov/coronavirus/2019-ncov/infection-control/control-recommendations.html|archive-date=4 March 2020|access-date=25 March 2020}}</ref> N95 respirators are approved for industrial settings but the FDA has authorised the masks for use under an ] (EUA). They are designed to protect from airborne particles like dust but effectiveness against a specific biological agent is not guaranteed for off-label uses.<ref>{{cite web|title=Coronavirus Disease 2019 (COVID-19) Frequently Asked Questions|url=https://www.fda.gov/emergency-preparedness-and-response/mcm-issues/coronavirus-disease-2019-covid-19-frehttps://oc.wikipedia.org/Malauti%C3%A1_de_coronavirus_de_2019quently-asked-questions#5e78ba94b86da|website=Food and Drug Administration}}</ref> When masks are not available, the CDC recommends using face shields or, as a last resort, ].<ref>{{cite web|url=https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/face-masks.html|title=Strategies for Optimizing the Supply of Facemasks|date=11 February 2020|publisher=CDC|access-date=23 March 2020|archive-url=https://web.archive.org/web/20200323173927/https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/face-masks.html|archive-date=23 March 2020|url-status=live}}</ref> | |||
| {{Main|COVID-19 testing}} | |||
| ] for ]]] | |||
| The standard methods of testing for presence of SARS-CoV-2 are ]s,<ref name="pmid32621814" /><ref name="20200130cdc">{{#invoke:Cite web||date=30 January 2020|title=2019 Novel Coronavirus (2019-nCoV) Situation Summary|url=https://www.cdc.gov/coronavirus/2019-ncov/summary.html|url-status=live|archive-url=https://web.archive.org/web/20200126210549/https://www.cdc.gov/coronavirus/2019-nCoV/summary.html|archive-date=26 January 2020|access-date=30 January 2020|website=U.S. ] (CDC)}}</ref> which detects the presence of viral RNA fragments.<ref name="WHO_InterimGuidance">{{#invoke:Cite web||title=Coronavirus disease (COVID-19) technical guidance: Laboratory testing for 2019-nCoV in humans |work=] (WHO) |url=https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/laboratory-guidance |access-date=14 March 2020 |archive-url=https://web.archive.org/web/20200315044138/https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/laboratory-guidance |archive-date=15 March 2020 |url-status=live}}</ref> As these tests detect RNA but not infectious virus, its "ability to determine duration of infectivity of patients is limited".<ref name="2k0iS">{{#invoke:cite journal ||vauthors=Bullard J, Dust K, Funk D, Strong JE, Alexander D, Garnett L, Boodman C, Bello A, Hedley A, Schiffman Z, Doan K, Bastien N, Li Y, Van Caeseele PG, Poliquin G |title=Predicting Infectious Severe Acute Respiratory Syndrome Coronavirus 2 From Diagnostic Samples |journal=Clinical Infectious Diseases |volume=71 |issue=10 |pages=2663–2666 |date=December 2020 |pmid=32442256 |pmc=7314198 |doi=10.1093/cid/ciaa638 |doi-access=free |title-link=doi}}</ref> The test is typically done on respiratory samples obtained by a ]; however, a nasal swab or sputum sample may also be used.<ref name="CDC2020Testing">{{#invoke:Cite web||date=11 February 2020|title=Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens from Persons for Coronavirus Disease 2019 (COVID-19) |url=https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html|url-status=live|archive-url=https://web.archive.org/web/20200304165907/https://www.cdc.gov/coronavirus/2019-nCoV/lab/guidelines-clinical-specimens.html|archive-date=4 March 2020|access-date=26 March 2020|website=U.S. ] (CDC)}}</ref><ref name="20200129cdc">{{#invoke:Cite web||date=29 January 2020|title=Real-Time RT-PCR Panel for Detection 2019-nCoV |url=https://www.cdc.gov/coronavirus/2019-ncov/lab/rt-pcr-detection-instructions.html|url-status=live|archive-url=https://web.archive.org/web/20200130202031/https://www.cdc.gov/coronavirus/2019-ncov/lab/rt-pcr-detection-instructions.html|archive-date=30 January 2020|access-date=1 February 2020|website=U.S. ] (CDC)}}</ref> Results are generally available within hours.<ref name="pmid32621814" /> The WHO has published several testing protocols for the disease.<ref>{{#invoke:Cite web||title=Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases|url=https://www.who.int/publications-detail/laboratory-testing-for-2019-novel-coronavirus-in-suspected-human-cases-20200117|url-status=live|archive-url=https://web.archive.org/web/20200317023052/https://www.who.int/publications-detail/laboratory-testing-for-2019-novel-coronavirus-in-suspected-human-cases-20200117|archive-date=17 March 2020|access-date=13 March 2020|website=] (WHO)}}</ref> | |||
| Several laboratories and companies have developed serological tests, which detect antibodies produced by the body in response to infection. Some have been evaluated by ] and approved for use in the UK.<ref name="independent9515466">{{#invoke:cite news||date=14 May 2020|title=NHS staff will be first to get new coronavirus antibody test, medical chief promises|website=The Independent|url=https://www.independent.co.uk/news/uk/home-news/coronavirus-test-antibody-covid-roche-immune-nhs-staff-a9515466.html|access-date=14 May 2020}}</ref> | |||
| ===Mechanical ventilation=== | |||
| The ]'s CEBM has pointed to mounting evidence<ref name="yFMEP">{{#invoke:Cite web|| vauthors = Heneghan C, Jefferson T |date=1 September 2020|title=Virological characterization of COVID-19 patients that test re-positive for SARS-CoV-2 by RT-PCR|url=https://www.cebm.net/study/virological-characterization-of-covid-19-patients-that-test-re-positive-for-sars-cov-2-by-rt-pcr|access-date=19 September 2020|website=CEBM}}</ref><ref name="2exX5">{{#invoke:cite journal || vauthors = Lu J, Peng J, Xiong Q, Liu Z, Lin H, Tan X, Kang M, Yuan R, Zeng L, Zhou P, Liang C, Yi L, du Plessis L, Song T, Ma W, Sun J, Pybus OG, Ke C | title = Clinical, immunological and virological characterization of COVID-19 patients that test re-positive for SARS-CoV-2 by RT-PCR | journal = eBioMedicine | volume = 59 | pages = 102960 | date = September 2020 | pmid = 32853988 | pmc = 7444471 | doi = 10.1016/j.ebiom.2020.102960 }}</ref> that "a good proportion of 'new' mild cases and people re-testing positives after quarantine or discharge from hospital are not infectious, but are simply clearing harmless virus particles which their immune system has efficiently dealt with" and have called for "an international effort to standardize and periodically calibrate testing"<ref name="PQGAv">{{#invoke:Cite web|| vauthors = Spencer E, Jefferson T, Brassey J, Heneghan C |date=11 September 2020|title=When is Covid, Covid?|url=https://www.cebm.net/covid-19/when-is-covid-covid/|access-date=19 September 2020|website=The Centre for Evidence-Based Medicine}}</ref> In September 2020, the UK government issued "guidance for procedures to be implemented in laboratories to provide assurance of positive SARS-CoV-2 RNA results during periods of low prevalence, when there is a reduction in the predictive value of positive test results".<ref name="9Kblp">{{#invoke:Cite web||title=SARS-CoV-2 RNA testing: assurance of positive results during periods of low prevalence |url=https://www.gov.uk/government/publications/sars-cov-2-rna-testing-assurance-of-positive-results-during-periods-of-low-prevalence|access-date=19 September 2020|website=GOV.UK}}</ref> | |||
| Most cases of COVID-19 are not severe enough to require ] (artificial assistance to support breathing), but a percentage of cases do.<ref name="murthy">{{cite journal | vauthors = Murthy S, Gomersall CD, Fowler RA | title = Care for Critically Ill Patients With COVID-19 | journal = JAMA | date = 11 March 2020 | pmid = 32159735 | doi = 10.1001/jama.2020.3633 | url = https://jamanetwork.com/journals/jama/fullarticle/2762996 | access-date = 18 March 2020 | archive-url = https://web.archive.org/web/20200318203852/https://jamanetwork.com/journals/jama/fullarticle/2762996 |archive-date=18 March 2020 |url-status=live}}</ref><ref>{{cite web|author=World Health Organization|date=28 January 2020|title=Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected|url=https://www.who.int/docs/default-source/coronaviruse/clinical-management-of-novel-cov.pdf|journal=|volume=|pages=|via=|access-date=18 March 2020|archive-url=https://web.archive.org/web/20200226041620/https://www.who.int/docs/default-source/coronaviruse/clinical-management-of-novel-cov.pdf|archive-date=26 February 2020|url-status=live}}</ref> Some Canadian doctors recommend the use of invasive ] because this technique limits the spread of ] transmission ].<ref name="murthy"/> Severe cases are most common in older adults (those older than 60 years<ref name="murthy"/> and especially those older than 80 years).<ref>{{Cite document |last1=Ferguson |first1=N. |last2=Laydon |first2=D.|last3=Nedjati Gilani |first3=G. |last4=Imai |first4=N. |last5=Ainslie |first5=K. |last6=Baguelin |first6=M. |last7=Bhatia |first7=S. |last8=Boonyasiri|first8=A.|last9=Cucunuba Perez|first9=Zulma|last10=Cuomo-Dannenburg |first10=G. |last11=Dighe |first11=A. |date=16 March 2020 |title=Report 9: Impact of non-pharmaceutical interventions (NPIs) to reduce COVID19 mortality and healthcare demand|publisher=] |url=http://spiral.imperial.ac.uk/handle/10044/1/77482|doi=10.25561/77482|hdl=20.1000/100|doi-access=free|at=Table 1|journal=|access-date=25 March 2020|archive-url=https://web.archive.org/web/20200321133445/https://spiral.imperial.ac.uk/handle/10044/1/77482|archive-date=21 March 2020|url-status=live}}</ref> Many developed countries do not have enough ], which limits a ]'s capacity to handle a sudden spike in the number of COVID-19 cases severe enough to require hospitalisation.<ref name="VoxCOVID">{{cite news |last=Scott |first=Dylan |title=Coronavirus is exposing all of the weaknesses in the US health system High health care costs and low medical capacity made the US uniquely vulnerable to the coronavirus. |url=https://www.vox.com/policy-and-politics/2020/3/16/21173766/coronavirus-covid-19-us-cases-health-care-system |accessdate=18 March 2020 |publisher=Vox |date=16 March 2020 |archive-url=https://web.archive.org/web/20200318022237/https://www.vox.com/policy-and-politics/2020/3/16/21173766/coronavirus-covid-19-us-cases-health-care-system |archive-date=18 March 2020 |url-status=live}}</ref> This limited capacity is a significant driver of the need to ] (to keep the speed at which new cases occur and thus the number of people sick at one point in time lower).<ref name="VoxCOVID"/> One study in China found 5% were admitted to ]s, 2.3% needed mechanical support of ventilation, and 1.4% died.<ref name="Guan Ni Hu Liang p.">{{cite journal | vauthors = Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DS, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS | display-authors = 6 | title = Clinical Characteristics of Coronavirus Disease 2019 in China | journal = The New England Journal of Medicine | date = February 2020 | pmid = 32109013 | doi = 10.1056/nejmoa2002032 | publisher = Massachusetts Medical Society | doi-access = free }}</ref> Around 20–30% of the people in hospital with pneumonia from COVID-19 needed ICU care for respiratory support.<ref>{{cite web|url=https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html|title=Coronavirus Disease 2019 (COVID-19)|last=|date=2020-02-11|website=Centers for Disease Control and Prevention|language=en-us|access-date=2020-03-29|archive-url=https://web.archive.org/web/20200302201644/https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html|archive-date=2 March 2020|url-status=live}}</ref> | |||
| === Imaging === | |||
| ===Acute respiratory distress syndrome=== | |||
| <noinclude>] scan of a person with COVID-19 shows lesions (bright regions) in the lungs]]</noinclude> | |||
| ] | |||
| ] | |||
| Chest CT scans may be helpful to diagnose COVID‑19 in individuals with a high clinical suspicion of infection but are not recommended for routine screening.<ref name="Salehi-2020" /><ref name="acr.org">{{#invoke:Cite web||date=22 March 2020|title=ACR Recommendations for the use of Chest Radiography and Computed Tomography (CT) for Suspected COVID-19 Infection|url=https://www.acr.org/Advocacy-and-Economics/ACR-Position-Statements/Recommendations-for-Chest-Radiography-and-CT-for-Suspected-COVID19-Infection|url-status=live|archive-url=https://web.archive.org/web/20200328055813/https://www.acr.org/Advocacy-and-Economics/ACR-Position-Statements/Recommendations-for-Chest-Radiography-and-CT-for-Suspected-COVID19-Infection|archive-date=28 March 2020|website=American College of Radiology}}</ref> Bilateral multilobar ] with a peripheral, asymmetric, and posterior distribution are common in early infection.<ref name="Salehi-2020" /><ref>{{#invoke:cite journal || vauthors = Pormohammad A, Ghorbani S, Khatami A, Razizadeh MH, Alborzi E, Zarei M, Idrovo JP, Turner RJ | title = Comparison of influenza type A and B with COVID-19: A global systematic review and meta-analysis on clinical, laboratory and radiographic findings | journal = Reviews in Medical Virology | pages = e2179 | date = October 2020 | pmid = 33035373 | pmc = 7646051 | doi = 10.1002/rmv.2179 | s2cid = 222255245 | title-link = doi | volume = 31 | issue = 3 | doi-access = free }}</ref> Subpleural dominance, ] (lobular septal thickening with variable alveolar filling), and ] may appear as the disease progresses.<ref name="Salehi-2020" /><ref>{{#invoke:cite journal || vauthors = Lee EY, Ng MY, Khong PL | title = COVID-19 pneumonia: what has CT taught us? | journal = The Lancet. Infectious Diseases | volume = 20 | issue = 4 | pages = 384–385 | date = April 2020 | pmid = 32105641 | pmc = 7128449 | doi = 10.1016/S1473-3099(20)30134-1 | title-link = doi | doi-access = free }}</ref> Characteristic imaging features on chest ]s and ] (CT) of people who are symptomatic include asymmetric peripheral ground-glass opacities without ]s.<ref name="AJR">{{#invoke:cite journal || vauthors = Li Y, Xia L | title = Coronavirus Disease 2019 (COVID-19): Role of Chest CT in Diagnosis and Management | journal = AJR. American Journal of Roentgenology | volume = 214 | issue = 6 | pages = 1280–1286 | date = June 2020 | pmid = 32130038 | doi = 10.2214/AJR.20.22954 | s2cid = 212416282 }}</ref> | |||
| Many groups have created ] that include imagery such as the ] which has compiled an international online database of imaging findings for confirmed cases.<ref name="Cx70C">{{#invoke:Cite web||title=COVID-19 Database |url=https://www.sirm.org/category/senza-categoria/covid-19/ |website=Società Italiana di Radiologia Medica e Interventistica |access-date=11 March 2020 |language=it}}</ref> Due to overlap with other infections such as ], imaging without confirmation by rRT-PCR is of limited ] in identifying COVID‑19.<ref name="AJR" /> A large study in China compared chest CT results to PCR and demonstrated that though imaging is less specific for the infection, it is faster and more ].<ref name="jTxKm" /> | |||
| ] becomes more complex as ] develops in COVID-19 and oxygenation becomes increasingly difficult.<ref name="LancetRespMar2020" /> Ventilators capable of ] and high ]<ref>{{cite journal |last1=Briel |first1=Matthias |last2=Meade |first2=Maureen |last3=Mercat |first3=Alain |last4=Brower |first4=Roy G. |last5=Talmor |first5=Daniel |last6=Walter |first6=Stephen D. |last7=Slutsky |first7=Arthur S. |last8=Pullenayegum |first8=Eleanor |last9=Zhou |first9=Qi |last10=Cook |first10=Deborah |last11=Brochard |first11=Laurent |last12=Richard |first12=Jean-Christophe M. |last13=Lamontagne |first13=François |last14=Bhatnagar |first14=Neera |last15=Stewart |first15=Thomas E. |last16=Guyatt |first16=Gordon |title=Higher vs Lower Positive End-Expiratory Pressure in Patients With Acute Lung Injury and Acute Respiratory Distress Syndrome |journal=JAMA |date=3 March 2010 |volume=303 |issue=9 |pages=865–73 |doi=10.1001/jama.2010.218|pmid=20197533 }}</ref> are needed to maximise oxygen delivery while minimising the risk of ] and ].<ref name="barotrauma">{{cite book |last1=Diaz |first1=Raiko |last2=Heller |first2=Daniel |title=Barotrauma And Mechanical Ventilation |url=https://www.ncbi.nlm.nih.gov/books/NBK545226/ |website=StatPearls |publisher=StatPearls Publishing |date=2020|pmid=31424810 }}</ref> High PEEP may not be available on older ventilators. | |||
| === Coding === | |||
| {| class="wikitable" style="text-align:center; margin-right:1em;" | |||
| In late 2019, the WHO assigned emergency ] disease codes U07.1 for deaths from lab-confirmed SARS-CoV-2 infection and U07.2 for deaths from clinically or epidemiologically diagnosed COVID‑19 without lab-confirmed SARS-CoV-2 infection.<ref name="ICD10_2019_U07p2">{{#invoke:Cite web||year=2019|title=ICD-10 Version:2019|website=] (WHO) |url=https://icd.who.int/browse10/2019/en#/U07.1|access-date=31 March 2020 |url-status=live|archive-url=https://archive.today/20200331004754/https://icd.who.int/browse10/2019/en%23/U07.1|archive-date=31 March 2020|quote=U07.2{{snd}}COVID-19, virus not identified{{snd}}COVID-19 NOS{{snd}}Use this code when COVID-19 is diagnosed clinically or epidemiologically but laboratory testing is inconclusive or not available. Use additional code, if desired, to identify pneumonia or other manifestations}}</ref> | |||
| |+ style="background:#E5AFAA;" | Options for ARDS<ref name=LancetRespMar2020>{{cite journal |last1=Matthay |first1=Michael A. |last2=Aldrich |first2=J. Matthew |last3=Gotts |first3=Jeffrey E. |title=Treatment for severe acute respiratory distress syndrome from COVID-19 |journal=The Lancet Respiratory Medicine |date=March 2020 |doi=10.1016/S2213-2600(20)30127-2|pmid=32203709 |pmc=7118607 }}</ref> | |||
| |- style="background: #E5AFAA;text-align:center;" | |||
| ! Therapy | |||
| ! Recommendations | |||
| |- | |||
| | ] | |||
| | For ] <93%. May prevent the need for intubation and ventilation | |||
| |- | |||
| | ] | |||
| | 6mL per kg and can be reduced to 4mL/kg | |||
| |- | |||
| | ] | |||
| | Keep below 30 ] if possible (high ] (35 per minute) may be required) | |||
| |- | |||
| | ] | |||
| | Moderate to high levels | |||
| |- | |||
| | ]ing | |||
| | For worsening oxygenation | |||
| |- | |||
| | ] | |||
| | Goal is a negative balance of 0.5–1] per day | |||
| |- | |||
| | ] | |||
| | For secondary bacterial infections | |||
| |- | |||
| | ] | |||
| | Not recommended | |||
| |} | |||
| === |
=== Pathology === | ||
| The main pathological findings at autopsy are: | |||
| * ]: ], ] and ]<ref name="Cureus" /> | |||
| * Lung findings: | |||
| ** Minor serous ], minor ] exudation<ref name="Cureus" /> | |||
| ** Pulmonary oedema, ] ], large atypical ]s, interstitial ] with ] ] and ] formation<ref name="Cureus" /> | |||
| ** ] (DAD) with diffuse ] ]. DAD is the cause of ] (ARDS) and severe ].<ref name="Cureus" /> | |||
| ** ] of ]s in ] and ]<ref name="Cureus" /> | |||
| ** ] in ] (BAL)<ref>{{#invoke:cite journal || vauthors = Giani M, Seminati D, Lucchini A, Foti G, Pagni F | title = Exuberant Plasmocytosis in Bronchoalveolar Lavage Specimen of the First Patient Requiring Extracorporeal Membrane Oxygenation for SARS-CoV-2 in Europe | journal = Journal of Thoracic Oncology | volume = 15 | issue = 5 | pages = e65–e66 | date = May 2020 | pmid = 32194247 | pmc = 7118681 | doi = 10.1016/j.jtho.2020.03.008 }}</ref> | |||
| * Blood and vessels: ] (DIC);<ref>{{#invoke:cite journal || vauthors = Lillicrap D | title = Disseminated intravascular coagulation in patients with 2019-nCoV pneumonia | journal = Journal of Thrombosis and Haemostasis | volume = 18 | issue = 4 | pages = 786–787 | date = April 2020 | pmid = 32212240 | pmc = 7166410 | doi = 10.1111/jth.14781 }}</ref> ] reaction,<ref>{{#invoke:cite journal || vauthors = Mitra A, Dwyre DM, Schivo M, Thompson GR, Cohen SH, Ku N, Graff JP | title = Leukoerythroblastic reaction in a patient with COVID-19 infection | journal = American Journal of Hematology | volume = 95 | issue = 8 | pages = 999–1000 | date = August 2020 | pmid = 32212392 | pmc = 7228283 | doi = 10.1002/ajh.25793 | title-link = doi | doi-access = free }}</ref> ],<ref name="SatturwarFowkes2021">{{#invoke:cite journal || vauthors = Satturwar S, Fowkes M, Farver C, Wilson AM, Eccher A, Girolami I, Pujadas E, Bryce C, Salem F, El Jamal SM, Paniz-Mondolfi A, Petersen B, Gordon RE, Reidy J, Fraggetta F, Marshall DA, Pantanowitz L | title = Postmortem Findings Associated With SARS-CoV-2: Systematic Review and Meta-analysis | journal = The American Journal of Surgical Pathology | volume = 45 | issue = 5 | pages = 587–603 | date = May 2021 | pmid = 33481385 | doi = 10.1097/PAS.0000000000001650 | pmc = 8132567 | s2cid = 231679276 }}</ref> ]<ref name="SatturwarFowkes2021" /> | |||
| * Heart: ] necrosis<ref name="SatturwarFowkes2021" /> | |||
| * Liver: microvesicular ]<ref name="Cureus" /> | |||
| * Nose: ]<ref name="Meunier-2020" /> | |||
| * Brain: ]<ref name="SatturwarFowkes2021" /> | |||
| * Kidneys: acute tubular damage.<ref name="SatturwarFowkes2021" /> | |||
| * Spleen: ] depletion.<ref name="SatturwarFowkes2021" /> | |||
| == Prevention == | |||
| {{see also|Coronavirus disease 2019#Research|label 1=Research}} | |||
| <noinclude> | |||
| No medications are approved to treat the disease by the WHO although some are recommended by individual national medical authorities.<ref name="LiDeClerq">{{cite journal | vauthors = Li G, De Clercq E | title = Therapeutic options for the 2019 novel coronavirus (2019-nCoV) | journal = Nature Reviews. Drug Discovery | volume = 19 | issue = 3 | pages = 149–150 | date = March 2020 | pmid = 32127666 | doi = 10.1038/d41573-020-00016-0 | doi-access = free }}</ref> Research into potential treatments started in January 2020,<ref>{{Cite news|url=https://www.reuters.com/article/us-china-health-hospital-idUSKBN20B1M6|title=Chinese doctors using plasma therapy on coronavirus, WHO says 'very valid' approach|newspaper=Reuters|date=17 February 2020|via=www.reuters.com|access-date=19 March 2020|archive-url=https://web.archive.org/web/20200304173709/https://www.reuters.com/article/us-china-health-hospital-idUSKBN20B1M6|archive-date=4 March 2020|url-status=live}}</ref> and several antiviral drugs are in clinical trials.<ref name="Reut_NIH_Moderna_3months" /><ref name="clinicaltrialsarena">{{cite web|url=https://www.clinicaltrialsarena.com/analysis/coronavirus-mers-cov-drugs/|title=Coronavirus outbreak: Vaccines/drugs in the pipeline for Covid-19|last=Duddu|first=Praveen|date=19 February 2020|work=clinicaltrialsarena.com|archive-url=https://web.archive.org/web/20200219184512/https://www.clinicaltrialsarena.com/analysis/coronavirus-mers-cov-drugs/|archive-date=19 February 2020|name-list-format=vanc}}</ref> Although new medications may take until 2021 to develop,<ref>{{cite journal|vauthors=Lu H|date=28 January 2020|title=Drug treatment options for the 2019-new coronavirus (2019-nCoV).|journal=Biosci Trends|volume=14|issue=1|pages=69–71|doi=10.5582/bst.2020.01020|pmid=31996494}}</ref> several of the medications being tested are already approved for other uses or are already in advanced testing.<ref name="LiDeClerq" /> Antiviral medication may be tried in people with severe disease.<ref name="NatureDale Fisher & David Heymann" /> The WHO recommended volunteers take part in trials of the effectiveness and safety of potential treatments.<ref name="ThomReut_notreatment_20200205">{{cite news|last1=Nebehay|first1=Stephanie|url=https://www.reuters.com/article/us-china-health-treatments-who-idUSKBN1ZZ1M6|title=WHO: 'no known effective' treatments for new coronavirus|date=5 February 2020|access-date=5 February 2020|url-status=live |archive-url=https://web.archive.org/web/20200205155653/https://www.reuters.com/article/us-china-health-treatments-who-idUSKBN1ZZ1M6|archive-date=5 February 2020|agency=]|last2=Kelland|first2=Kate|last3=Liu|first3=Roxanne|name-list-format=vanc}}</ref> | |||
| {{Further|COVID-19 vaccine|Workplace hazard controls for COVID-19|Pandemic prevention|Non-pharmaceutical intervention (epidemiology){{!}}Non-pharmaceutical intervention|Pandemic predictions and preparations prior to the COVID-19 pandemic{{!}}Preparations prior to COVID-19|COVID-19 surveillance|COVID-19 apps}} | |||
| <!-- THE FOLLOWING TWO PARAGRAPHS ARE TRANSCLUDED INTO THE COVID-19 PANDEMIC ARTICLE --> | |||
| </noinclude> | |||
| ] | |||
| Preventive measures to reduce the chances of infection include getting vaccinated, staying at home, wearing a mask in public, avoiding crowded places, keeping distance from others, ventilating indoor spaces, managing potential exposure durations,<ref>{{#invoke:Cite web||url=https://reallycorrect.com/ReallyCorrectWp1/covid-19-safety-information-ideas/#Viral-Load-Exposure-Factors|title=Viral Load Exposure Factors|website=ReallyCorrect.com}}</ref> washing hands with soap and water often and for at least twenty seconds, practising good respiratory hygiene, and avoiding touching the eyes, nose, or mouth with unwashed hands.<ref name="CDC042020">{{#invoke:Cite web||date=28 June 2020|title=Recommendation Regarding the Use of Cloth Face Coverings, Especially in Areas of Significant Community-Based Transmission|work=U.S. ] (CDC) |url=https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/cloth-face-cover.html}}</ref><ref>{{#invoke:Cite web||title=Scientific Brief: SARS-CoV-2 and Potential Airborne Transmission |url=https://www.cdc.gov/coronavirus/2019-ncov/more/scientific-brief-sars-cov-2.html |website=COVID-19 Published Science and Research |date=11 February 2020 |publisher=U.S. ] (CDC) |access-date=30 October 2020}}</ref> | |||
| Those diagnosed with COVID‑19 or who believe they may be infected are advised by the CDC to stay home except to get medical care, call ahead before visiting a healthcare provider, ] before entering the healthcare provider's office and when in any room or vehicle with another person, cover coughs and sneezes with a tissue, regularly wash hands with soap and water and avoid sharing personal household items.<ref name="CDC2020IfSick">{{#invoke:Cite web||date=5 April 2020|title=What to Do if You Are Sick|url=https://www.cdc.gov/coronavirus/2019-ncov/if-you-are-sick/steps-when-sick.html|url-status=live|archive-url=https://web.archive.org/web/20200214153016/https://www.cdc.gov/coronavirus/2019-ncov/about/steps-when-sick.html|archive-date=14 February 2020|access-date=24 April 2020|website=U.S. ] (CDC)|vauthors=((Centers for Disease Control and Prevention))}}</ref><ref>{{#invoke:Cite web||date=10 March 2020|title=Coronavirus Disease 2019 (COVID-19) – Prevention & Treatment |url=https://www.cdc.gov/coronavirus/2019-ncov/about/prevention.html|url-status=live|archive-url=https://web.archive.org/web/20200311163637/https://www.cdc.gov/coronavirus/2019-ncov/about/prevention.html|archive-date=11 March 2020|access-date=11 March 2020|work=U.S. ] (CDC)}}</ref> | |||
| ===Information technology=== | |||
| The first ] was granted regulatory approval on 2{{spaces}}December 2020 by the UK medicines regulator ].<ref name="ukgov12-2">{{#invoke:Cite web||title=UK medicines regulator gives approval for first UK COVID-19 vaccine |url=https://www.gov.uk/government/news/uk-medicines-regulator-gives-approval-for-first-uk-covid-19-vaccine |publisher=Medicines and Healthcare Products Regulatory Agency, Government of the UK |access-date=2 December 2020 |date=2 December 2020}}</ref> It was evaluated for ] (EUA) status by the US ], and in several other countries.<ref name="mueller">{{#invoke:cite news || vauthors = Mueller B |title=U.K. Approves Pfizer Coronavirus Vaccine, a First in the West |url=https://www.nytimes.com/2020/12/02/world/europe/pfizer-coronavirus-vaccine-approved-uk.html |archive-url=https://web.archive.org/web/20201202071559/https://www.nytimes.com/2020/12/02/world/europe/pfizer-coronavirus-vaccine-approved-uk.html |archive-date=2 December 2020 |url-access=subscription |url-status=live |access-date=2 December 2020 |work=The New York Times |date=2 December 2020}}</ref> Initially, the US ] guidelines do not recommend any medication for prevention of COVID‑19, before or after exposure to the SARS-CoV-2 virus, outside the setting of a clinical trial.<ref name="NIHGuidelines2020">{{#invoke:Cite web||title=COVID-19 Treatment Guidelines |url=https://covid19treatmentguidelines.nih.gov/introduction/ |website=nih.gov |publisher=National Institutes of Health |access-date=21 April 2020}}</ref><ref name="Sanders202022" /> Without a vaccine, other prophylactic measures, or effective treatments, a key part of managing COVID‑19 is trying to decrease and delay the epidemic peak, known as "flattening the ]".<ref name="Lancet2020Flatten">{{#invoke:cite journal || vauthors = Anderson RM, Heesterbeek H, Klinkenberg D, Hollingsworth TD | title = How will country-based mitigation measures influence the course of the COVID-19 epidemic? | journal = Lancet | volume = 395 | issue = 10228 | pages = 931–934 | date = March 2020 | pmid = 32164834 | pmc = 7158572 | doi = 10.1016/S0140-6736(20)30567-5 | quote = A key issue for epidemiologists is helping policy makers decide the main objectives of mitigation{{snd}}e.g. minimising morbidity and associated mortality, avoiding an epidemic peak that overwhelms health-care services, keeping the effects on the economy within manageable levels, and flattening the epidemic curve to wait for vaccine development and manufacture on scale and antiviral drug therapies. | doi-access = free | title-link = doi }}</ref> This is done by slowing the infection rate to decrease the risk of health services being overwhelmed, allowing for better treatment of active cases, and delaying additional cases until effective treatments or a vaccine become available.<ref name="Lancet2020Flatten" /><ref name="Wiles">{{#invoke:Cite web|| vauthors = Wiles S |title=After 'Flatten the Curve', we must now 'Stop the Spread'. Here's what that means |url=https://thespinoff.co.nz/society/14-03-2020/after-flatten-the-curve-we-must-now-stop-the-spread-heres-what-that-means/ |website=The Spinoff |access-date=13 March 2020 |date=14 March 2020 |archive-url=https://web.archive.org/web/20200326232315/https://thespinoff.co.nz/society/14-03-2020/after-flatten-the-curve-we-must-now-stop-the-spread-heres-what-that-means/ |archive-date=26 March 2020 |url-status=live}}</ref> | |||
| {{See also|Government by algorithm}} | |||
| === Vaccine === | |||
| In February 2020, China launched a ] to deal with the disease outbreak.<ref>{{cite news |title=China launches coronavirus 'close contact' app |url=https://www.bbc.com/news/technology-51439401 |access-date=7 March 2020 |work=BBC News |date=11 February 2020 |name-list-format=vanc |archive-url=https://web.archive.org/web/20200228003957/https://www.bbc.com/news/technology-51439401 |archive-date=28 February 2020 |url-status=live }}</ref> Users are asked to enter their name and ID number. The app is able to detect 'close contact' using surveillance data and therefore a potential risk of infection. Every user can also check the status of three other users. If a potential risk is detected, the app not only recommends self-quarantine, it also alerts local health officials.<ref>{{cite web |last1=Chen |first1=Angela | name-list-format = vanc |title=China's coronavirus app could have unintended consequences |url=https://www.technologyreview.com/s/615199/coronavirus-china-app-close-contact-surveillance-covid-19-technology/ |website=MIT Technology Review |access-date=7 March 2020 }}</ref> | |||
| {{Main|COVID-19 vaccine}} | |||
| ] | |||
| ] | |||
| <!-- TO EDIT THIS SECTION, GO TO ]. --> | |||
| {{Excerpt|COVID-19 vaccine|hat=no|paragraphs=4-7}} | |||
| === Face masks and respiratory hygiene === | |||
| ] analytics on cellphone data, ] technology, ] and ] are used to track infected people and people whom they contacted in South Korea, Taiwan and Singapore.<ref>{{cite news |title=Gov in the Time of Corona |url=https://govinsider.asia/innovation/gov-in-the-time-of-corona/ |access-date=20 March 2020 |work=GovInsider |date=19 March 2020 |archive-url=https://web.archive.org/web/20200320125215/https://govinsider.asia/innovation/gov-in-the-time-of-corona/ |archive-date=20 March 2020 |url-status=live }}</ref><ref>{{cite news |last=Manancourt |first=Vincent |title=Coronavirus tests Europe's resolve on privacy |url=https://www.politico.eu/article/coronavirus-tests-europe-resolve-on-privacy-tracking-apps-germany-italy/ |access-date=20 March 2020 |work=POLITICO |date=10 March 2020 |archive-url=https://web.archive.org/web/20200320105744/https://www.politico.eu/article/coronavirus-tests-europe-resolve-on-privacy-tracking-apps-germany-italy/ |archive-date=20 March 2020 |url-status=live }}</ref> In March 2020, the Israeli government enabled security agencies to track mobile phone data of people supposed to have coronavirus. The measure was taken to enforce quarantine and protect those who may come into contact with infected citizens.<ref>{{Cite news|last=Tidy|first=Joe|url=https://www.bbc.com/news/technology-51930681|title=Coronavirus: Israel enables emergency spy powers|date=17 March 2020|work=BBC News|access-date=18 March 2020|language=en-GB|archive-url=https://web.archive.org/web/20200318113608/https://www.bbc.com/news/technology-51930681|archive-date=18 March 2020|url-status=live}}</ref> Also in March 2020, ] shared aggregated phone location data with the German federal government agency, ], in order to research and prevent the spread of the virus.<ref name="heise-handydaten">{{cite news |last=Bünte |first=Oliver |title=Corona-Krise: Deutsche Telekom liefert anonymisierte Handydaten an RKI |url=https://www.heise.de/newsticker/meldung/Corona-Krise-Deutsche-Telekom-liefert-anonymisierte-Handydaten-an-RKI-4685191.html |accessdate=25 March 2020 |work=Heise Online |date=18 March 2020 |language=German |trans-title=Corona crisis: Deutsche Telekom delivers anonymized cell phone data to RKI |archive-url=https://web.archive.org/web/20200324115410/https://www.heise.de/newsticker/meldung/Corona-Krise-Deutsche-Telekom-liefert-anonymisierte-Handydaten-an-RKI-4685191.html |archive-date=24 March 2020 |url-status=live }}</ref> Russia deployed facial recognition technology to detect quarantine breakers.<ref>{{cite news |title=Moscow deploys facial recognition technology for coronavirus quarantine |url=https://www.reuters.com/article/us-china-health-moscow-technology-idUSKBN20F1RZ |access-date=20 March 2020 |work=Reuters |date=21 February 2020 |language=en |archive-url=https://web.archive.org/web/20200222215731/https://www.reuters.com/article/us-china-health-moscow-technology-idUSKBN20F1RZ |archive-date=22 February 2020 |url-status=live }}</ref> Italian regional health commissioner ] said he has been informed by mobile phone operators that "40% of people are continuing to move around anyway".<ref>{{cite news |title=Italians scolded for flouting lockdown as death toll nears 3,000 |url=https://www.post-gazette.com/news/world/2020/03/18/Italy-coronavirus-475-deaths-one-day-death-toll-2978-COVID-19-doctors/stories/202003180182 |access-date=20 March 2020 |work=Pittsburgh Post-Gazette |language=en |archive-url=https://web.archive.org/web/20200320110555/https://www.post-gazette.com/news/world/2020/03/18/Italy-coronavirus-475-deaths-one-day-death-toll-2978-COVID-19-doctors/stories/202003180182 |archive-date=20 March 2020 |url-status=live }}</ref> German government conducted a 48 hours weekend ] with more than 42.000 participants.<ref>{{cite web |title=Kreative Lösungen gesucht |url=https://www.bundesregierung.de/breg-de/themen/coronavirus/wir-vs-virus-1731968 |website=Startseite |language=de |access-date=23 March 2020 |archive-url=https://web.archive.org/web/20200324085627/https://www.bundesregierung.de/breg-de/themen/coronavirus/wir-vs-virus-1731968 |archive-date=24 March 2020 |url-status=live }}</ref><ref>{{cite news |last1=Dannewitz |first1=Juliane |title=Hackathon Germany: #WirvsVirus |url=https://www.datenschutzbeauftragter-info.de/hackathon-germany-wirvsvirus/ |work=Datenschutzbeauftragter |date=23 March 2020 |language=de-DE}}</ref> Also the president of Estonia, ], made a global call for creative solutions against the spread of coronavirus.<ref>{{cite news |first=Andrew |last=Whyte |title=President makes global call to combat coronavirus via hackathon |url=https://news.err.ee/1067171/president-makes-global-call-to-combat-coronavirus-via-hackathon |work=ERR |date=21 March 2020 |language=en |access-date=23 March 2020 |archive-url=https://web.archive.org/web/20200324050421/https://news.err.ee/1067171/president-makes-global-call-to-combat-coronavirus-via-hackathon |archive-date=24 March 2020 |url-status=live }}</ref> | |||
| {{Main|Face masks during the COVID-19 pandemic}} | |||
| ] | |||
| {{Excerpt|Face masks during the COVID-19 pandemic|hat=no|paragraphs=2-4}} | |||
| === Indoor ventilation and avoiding crowded indoor spaces === | |||
| ===Psychological support=== | |||
| The CDC states that avoiding crowded indoor spaces reduces the risk of COVID-19 infection.<ref name="CDC-2020b">{{#invoke:Cite web||last=CDC|date=11 February 2020|title=Scientific Brief: SARS-CoV-2 Transmission|url=https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/sars-cov-2-transmission.html|access-date=10 May 2021|website=U.S. ] (CDC) }}</ref> When indoors, increasing the rate of air change, decreasing recirculation of air and increasing the use of outdoor air can reduce transmission.<ref name="CDC-2020b" /><ref name="ecdc_transmission2">{{#invoke:Cite web||date=7 September 2020|title=Transmission of COVID-19 |url=https://www.ecdc.europa.eu/en/covid-19/latest-evidence/transmission|access-date=14 October 2020|publisher=]}}</ref> The WHO recommends ] and ] in public spaces to help clear out infectious aerosols.<ref name="CDCasof07092020" /><ref name="KoFZO">{{#invoke:cite AV media ||date=30 October 2020 |url=https://www.youtube.com/watch?v=XJC1f7F4qtc |title=WHO's Science in 5 on COVID-19 – Ventilation – 30 October 2020 |publisher=] |via=] |access-date=8 December 2022 |archive-url=https://web.archive.org/web/20221025043909/http://www.youtube.com/watch?v=XJC1f7F4qtc |archive-date=25 October 2022 |url-status=live}}</ref><ref name="Lancetdroplet05272020">{{#invoke:cite journal||vauthors=Somsen GA, van Rijn C, Kooij S, Bem RA, Bonn D|date=July 2020|title=Small droplet aerosols in poorly ventilated spaces and SARS-CoV-2 transmission|journal=The Lancet. Respiratory Medicine|publisher=Elsesier|volume=8|issue=7|pages=658–659 |doi=10.1016/S2213-2600(20)30245-9|pmc=7255254|pmid=32473123}}</ref> | |||
| Exhaled respiratory particles can build-up within enclosed spaces with inadequate ]. The risk of COVID‑19 infection increases especially in spaces where people engage in physical exertion or raise their voice (e.g., exercising, shouting, singing) as this increases exhalation of respiratory droplets. Prolonged exposure to these conditions, typically more than 15 minutes, leads to higher risk of infection.<ref name="CDC-2020b" /> | |||
| {{see also|Mental health during the 2019–20 coronavirus pandemic}} | |||
| Individuals may experience distress from quarantine, travel restrictions, side effects of treatment or fear of the infection itself. To address these concerns, the ] published a national guideline for psychological crisis intervention on 27 January 2020.<ref name="Xiang4Feb2020">{{cite journal | vauthors = Xiang YT, Yang Y, Li W, Zhang L, Zhang Q, Cheung T, Ng CH | display-authors = 6 | title = Timely mental health care for the 2019 novel coronavirus outbreak is urgently needed | journal = The Lancet. Psychiatry | volume = 7 | issue = 3 | pages = 228–29 | date = March 2020 | pmid = 32032543 |doi=10.1016/S2215-0366(20)30046-8 |doi-access=free }}</ref><ref name="Kang5Feb2020">{{cite journal | vauthors = Kang L, Li Y, Hu S, Chen M, Yang C, Yang BX, Wang Y, Hu J, Lai J, Ma X, Chen J, Guan L, Wang G, Ma H, Liu Z | display-authors = 6 | title = The mental health of medical workers in Wuhan, China dealing with the 2019 novel coronavirus | journal = The Lancet. Psychiatry | volume = 7 | issue = 3 | pages = e14 | date = March 2020 | pmid = 32035030 |doi=10.1016/S2215-0366(20)30047-X |doi-access=free }}</ref> | |||
| ] with large natural inlets can move stale air directly to the exhaust in ] while significantly reducing the concentration of droplets and particles. ] reduces energy consumption and maintenance costs but may lack ] and ]. Displacement ventilation can also be achieved mechanically with higher energy and maintenance costs. The use of large ducts and openings helps to prevent mixing in closed environments. Recirculation and mixing should be avoided because recirculation prevents dilution of harmful particles and redistributes possibly contaminated air, and mixing increases the concentration and range of infectious particles and keeps larger particles in the air.<ref>{{#invoke:cite journal ||vauthors=Lipinski T, Ahmad D, Serey N, Jouhara H |date=1 November 2020 |title=Review of ventilation strategies to reduce the risk of disease transmission in high occupancy buildings |url=https://www.sciencedirect.com/science/article/pii/S266620272030032X |journal=International Journal of Thermofluids |volume=7–8 |pages=100045 |doi=10.1016/j.ijft.2020.100045 |bibcode=2020IJTf....700045L |s2cid=221642242 |issn=2666-2027}}</ref> | |||
| ==Prognosis== | |||
| === Hand-washing and hygiene === | |||
| {{Primary sources|date=March 2020}} | |||
| {{Main|Hand washing}} | |||
| {{multiple image | |||
| ] hand washing and wearing face masks during the ].]] | |||
| | align = right | |||
| Thorough hand hygiene after any cough or sneeze is required.<ref name="nhs.uk-2020">{{#invoke:Cite web||date=2 June 2020|title=Social distancing: what you need to do – Coronavirus (COVID-19) |url=https://www.nhs.uk/conditions/coronavirus-covid-19/social-distancing/what-you-need-to-do/|access-date=18 August 2020|website=nhs.uk}}</ref> The WHO also recommends that individuals wash hands often with soap and water for at least twenty seconds, especially after going to the toilet or when hands are visibly dirty, before eating and after blowing one's nose.<ref name="WHO-2020b">{{#invoke:Cite web||title=Advice for the public on COVID-19 – World Health Organization|url=https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public|access-date=18 August 2020|website=] (WHO)}}</ref> When soap and water are not available, the CDC recommends using an alcohol-based ] with at least 60% alcohol.<ref>{{#invoke:Cite web||date=11 February 2020|title=COVID-19 and Your Health|url=https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/hand-sanitizer.html|access-date=23 March 2021|website=U.S. ] (CDC)|quote=To prevent the spread of germs, including COVID-19, CDC recommends washing hands with soap and water whenever possible because it reduces the amount of many types of germs and chemicals on hands. But if soap and water are not readily available, using a hand sanitizer with at least 60% alcohol can help you avoid getting sick and spreading germs to others.}}</ref> For areas where commercial hand sanitisers are not readily available, the WHO provides two formulations for local production. In these formulations, the antimicrobial activity arises from ] or ]. ] is used to help eliminate ]s in the alcohol; it is "not an active substance for hand ]". ] is added as a ].<ref>{{#invoke:cite book||title=WHO Guidelines on Hand Hygiene in Health Care: First Global Patient Safety Challenge Clean Care Is Safer Care|date=19 March 2009|publisher=] (WHO)|chapter=WHO-recommended handrub formulations|access-date=19 March 2020|chapter-url=https://www.ncbi.nlm.nih.gov/books/NBK144054/}}</ref> | |||
| | direction = vertical | |||
| | total_width = 400 | |||
| | caption_align = center | |||
| <!--image 1--> | |||
| | image1 = Severity-of-coronavirus-cases-in-China-1.png | |||
| | alt1 = The severity of diagnosed cases in China | |||
| | caption1 = The severity of diagnosed COVID-19 cases in China<ref>{{cite journal |last1=Roser |first1=Max |last2=Ritchie |first2=Hannah |last3=Ortiz-Ospina |first3=Esteban |name-list-format=vanc |title=Coronavirus Disease (COVID-19) |url=https://ourworldindata.org/coronavirus |journal=Our World in Data |access-date=12 March 2020 |date=4 March 2020 |archive-url=https://web.archive.org/web/20200319171947/https://ourworldindata.org/coronavirus |archive-date=19 March 2020 |url-status=live }}</ref> | |||
| <!--image 2--> | |||
| | image2 = Illustration of SARS-COV-2 Case Fatality Rate 200228 01-1.png | |||
| | alt2 = 3D Medical Animation Still Shot graph showing Case Fatality rates by age group from SARS-COV-2 in China. | |||
| | caption2 = Case fatality rates by age group in China. Data through 11 February 2020.<ref name="Epidemiology2020Feb17" /> | |||
| <!--image 3--> | |||
| | image3 = Comorbidity and severity in covid-19 data from China CDC Weekly 2020, 2(8), pp. 113-122 (cropped).png | |||
| | alt3 = Case fatality rate depending on other health problems | |||
| | caption3 = Case fatality rate in China depending on other health problems. Data through 11 February 2020.<ref name="Epidemiology2020Feb17" /> | |||
| <!--image 4--> | |||
| | image4 = Covid-19-total-confirmed-cases-vs-total-confirmed-deaths.png | |||
| | alt4 = Case fatality rate by country and number of cases | |||
| | caption4 = The number of deaths vs total cases by country and approximate case fatality rate<ref>{{cite journal |last1=Roser |first1=Max |last2=Ritchie |first2=Hannah |last3=Ortiz-Ospina |first3=Esteban |name-list-format=vanc |title=Coronavirus Disease (COVID-19) |url=https://ourworldindata.org/coronavirus |journal=Our World in Data |access-date=6 April 2020 |date=6 April 2020}}</ref> | |||
| }} | |||
| === Social distancing === | |||
| The severity of COVID-19 varies. The disease may take a mild course with few or no symptoms, resembling other common upper respiratory diseases such as the ]. Mild cases typically recover within two weeks, while those with severe or critical diseases may take three to six weeks to recover. Among those who have died, the time from symptom onset to death has ranged from two to eight weeks.<ref name="WHOReport24Feb2020" /> | |||
| {{Main|Social distancing measures related to the COVID-19 pandemic}} | |||
| Social distancing (also known as physical distancing) includes ] actions intended to slow the spread of the disease by minimising close contact between individuals. Methods include quarantines; travel restrictions; and the closing of schools, workplaces, stadiums, theatres, or shopping centres. Individuals may apply social distancing methods by staying at home, limiting travel, avoiding crowded areas, using no-contact greetings, and physically distancing themselves from others.<ref>{{#invoke:cite journal || vauthors = Nussbaumer-Streit B, Mayr V, Dobrescu AI, Chapman A, Persad E, Klerings I, Wagner G, Siebert U, Ledinger D, Zachariah C, Gartlehner G |date=September 2020 |title=Quarantine alone or in combination with other public health measures to control COVID-19: a rapid review |journal=The Cochrane Database of Systematic Reviews |volume=2020 |issue=9 |pages=CD013574 |doi=10.1002/14651858.CD013574.pub2 |issn=1469-493X |pmc=8133397 |pmid=33959956 }}</ref> | |||
| In 2020, outbreaks occurred in prisons due to crowding and an inability to enforce adequate social distancing.<ref name="Hawks2020" /><ref>{{#invoke:Cite web|| vauthors = Waldstein D |title=To Fight Virus in Prisons, C.D.C. Suggests More Screenings |website=The New York Times |date=6 May 2020 |url=https://www.nytimes.com/2020/05/06/health/coronavirus-prisons-cdc.html |archive-url=https://web.archive.org/web/20200507161241/https://www.nytimes.com/2020/05/06/health/coronavirus-prisons-cdc.html |archive-date=7 May 2020 |url-access=subscription |url-status=live |access-date=14 May 2020}}</ref> In the United States, the prisoner population is ageing and many of them are at high risk for poor outcomes from COVID‑19 due to high rates of coexisting heart and lung disease, and poor access to high-quality healthcare.<ref name="Hawks2020">{{#invoke:cite journal || vauthors = Hawks L, Woolhandler S, McCormick D | title = COVID-19 in Prisons and Jails in the United States | journal = JAMA Internal Medicine | volume = 180 | issue = 8 | pages = 1041–1042 | date = August 2020 | pmid = 32343355 | doi = 10.1001/jamainternmed.2020.1856 | doi-access = free | title-link = doi }}</ref> | |||
| Children are susceptible to the disease, but are likely to have milder symptoms and a lower chance of severe disease than adults; in those younger than 50 years, the risk of death is less than 0.5%, while in those older than 70 it is more than 8%.<ref name="Lu Zhang Du Zhang p.">{{cite journal|display-authors=6|vauthors=Lu X, Zhang L, Du H, Zhang J, Li YY, Qu J, Zhang W, Wang Y, Bao S, Li Y, Wu C, Liu H, Liu D, Shao J, Peng X, Yang Y, Liu Z, Xiang Y, Zhang F, Silva RM, Pinkerton KE, Shen K, Xiao H, Xu S, Wong GW|date=18 March 2020|title=SARS-CoV-2 Infection in Children|journal=New England Journal of Medicine|publisher=Massachusetts Medical Society|page=|doi=10.1056/nejmc2005073|issn=0028-4793|pmid=32187458}}</ref><ref name="pediatrics_tong">{{cite journal | vauthors = Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z, Tong S | journal = Pediatrics | title = Epidemiological Characteristics of 2143 Pediatric Patients With 2019 Coronavirus Disease in China | date = 2020 | pages = e20200702 | url = https://pediatrics.aappublications.org/content/pediatrics/early/2020/03/16/peds.2020-0702.full.pdf | doi = 10.1542/peds.2020-0702 | pmid = 32179660 | access-date = 16 March 2020 | archive-url = https://web.archive.org/web/20200317223427/https://pediatrics.aappublications.org/content/pediatrics/early/2020/03/16/peds.2020-0702.full.pdf | archive-date = 17 March 2020 | url-status = live }}</ref> ] may be at higher risk for severe infection with COVID-19 based on data from other similar viruses, like ] and ], but data for COVID-19 is lacking.<ref>{{cite journal | vauthors = Fang L, Karakiulakis G, Roth M | title = Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? | journal = ] | volume = 395 | issue = 10224 | pages = e40 | date = March 2020 | pmid = 32171062 | doi = 10.1016/S0140-6736(20)30311-1 | pmc = 7118626 }}</ref><ref name="CDC 2020children">{{cite web|url=https://www.cdc.gov/coronavirus/2019-ncov/specific-groups/children-faq.html|title=Coronavirus Disease 2019 (COVID-19)|author=|date=11 February 2020|website=Centers for Disease Control and Prevention|access-date=2 March 2020|name-list-format=vanc|archive-url=https://web.archive.org/web/20200302064104/https://www.cdc.gov/coronavirus/2019-ncov/specific-groups/children-faq.html|archive-date=2 March 2020|url-status=live}}</ref> | |||
| === Surface cleaning === | |||
| In some people, COVID-19 may affect the lungs causing ]. In those most severely affected, COVID-19 may rapidly progress to ] (ARDS) causing respiratory failure, septic shock or multi-organ failure.<ref name="Heymann Shindo 2020 pp. 542–545">{{cite journal | vauthors = Heymann DL, Shindo N | collaboration = WHO Scientific and Technical Advisory Group for Infectious Hazards | title = COVID-19: what is next for public health? | journal = Lancet | volume = 395 | issue = 10224 | pages = 542–545 | date = February 2020 | pmid = 32061313 | doi = 10.1016/s0140-6736(20)30374-3 | publisher = Elsevier BV }}</ref><ref>{{cite book | vauthors = Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R | chapter = Features, Evaluation and Treatment Coronavirus (COVID-19) | date = 2020 | pmid = 32150360 | chapter-url = http://www.ncbi.nlm.nih.gov/books/NBK554776/ | access-date = 18 March 2020 | publisher = StatPearls Publishing | location = Treasure Island (FL) | title = StatPearls }}</ref> Complications associated with COVID-19 include ], ] and damage to the heart, kidneys and liver. Clotting abnormalities, specifically an increase in ], have been described in 6% of those admitted to hospital with COVID-19, while abnormal kidney function is seen in 4% of this group.<ref name="Zhou Yu Du Fan 2020 p.">{{cite journal|display-authors=6|vauthors=Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L|date=2020|title=Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study|journal=The Lancet|volume=395|issue=10229|pages=1054–1062|publisher=Elsevier BV|doi=10.1016/s0140-6736(20)30566-3|issn=0140-6736|pmid=32171076}}</ref> Liver injury as shown by blood markers of liver damage is frequently seen in severe cases.<ref>{{cite journal | vauthors = Xu L, Liu J, Lu M, Yang D, Zheng X | title = Liver injury during highly pathogenic human coronavirus infections | journal = Liver International | date = March 2020 | pmid = 32170806 | doi = 10.1111/liv.14435 }}</ref> | |||
| After being expelled from the body, coronaviruses can survive on surfaces for hours to days. If a person touches the dirty surface, they may deposit the virus at the eyes, nose, or mouth where it can enter the body and cause infection.<ref name="CDCTrans">{{#invoke:Cite web||url=https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/how-covid-spreads.html |title=How COVID-19 Spreads |date=18 September 2020 |website=U.S. ] (CDC) |url-status=live |archive-url=https://web.archive.org/web/20200919224920/https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/how-covid-spreads.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fprepare%2Ftransmission.html |archive-date=19 September 2020 |access-date=20 September 2020}}</ref> Evidence indicates that contact with infected surfaces is not the main driver of COVID‑19,<ref>{{#invoke:cite journal || vauthors = Goldman E | title = Exaggerated risk of transmission of COVID-19 by fomites | journal = The Lancet. Infectious Diseases | volume = 20 | issue = 8 | pages = 892–893 | date = August 2020 | pmid = 32628907 | pmc = 7333993 | doi = 10.1016/S1473-3099(20)30561-2 }}</ref><ref>{{#invoke:Cite web|| vauthors = Weixel N |date=5 April 2021|title=CDC says risk of COVID-19 transmission on surfaces 1 in 10,000|url=https://thehill.com/policy/healthcare/546541-cdc-risk-of-covid-transmission-on-surfaces-is-low|access-date=19 December 2021|website=The Hill}}</ref><ref name="cdc.gov">{{#invoke:Cite web||date=5 April 2021|title=Science Brief: SARS-CoV-2 and Surface (Fomite) Transmission for Indoor Community Environments|url=https://www.cdc.gov/coronavirus/2019-ncov/more/science-and-research/surface-transmission.html|url-status=live |website=U.S. ] (CDC)|archive-url=https://web.archive.org/web/20210405151126/https://www.cdc.gov/coronavirus/2019-ncov/more/science-and-research/surface-transmission.html |archive-date=5 April 2021 }}</ref> leading to recommendations for optimised disinfection procedures to avoid issues such as the increase of ] through the use of inappropriate cleaning products and processes.<ref name="disinfection-foodindus" /><ref>{{#invoke:cite journal || vauthors = Rezasoltani S, Yadegar A, Hatami B, Asadzadeh Aghdaei H, Zali MR | title = Antimicrobial Resistance as a Hidden Menace Lurking Behind the COVID-19 Outbreak: The Global Impacts of Too Much Hygiene on AMR | journal = Frontiers in Microbiology | volume = 11 | pages = 590683 | year = 2020 | pmid = 33384670 | pmc = 7769770 | doi = 10.3389/fmicb.2020.590683 | doi-access = free | title-link = doi }}</ref> ] and other surface sanitation has been criticised as ], giving a false sense of security against something primarily spread through the air.<ref>{{#invoke:Cite web|| vauthors = Thompson D |date=8 February 2021|title=Hygiene Theater Is Still a Huge Waste of Time|url=https://www.theatlantic.com/ideas/archive/2021/02/hygiene-theater-still-waste/617939/|access-date=27 February 2021|website=The Atlantic }}</ref><ref>{{#invoke:Cite web|| vauthors = Thompson D |date=27 July 2020|title=Hygiene Theater Is a Huge Waste of Time |url=https://www.theatlantic.com/ideas/archive/2020/07/scourge-hygiene-theater/614599/|access-date=27 February 2021|website=The Atlantic }}</ref> | |||
| The amount of time that the virus can survive depends significantly on the type of surface, the temperature, and the humidity.<ref name="Bueckert-2020">{{#invoke:cite journal || vauthors = Bueckert M, Gupta R, Gupta A, Garg M, Mazumder A | title = Infectivity of SARS-CoV-2 and Other Coronaviruses on Dry Surfaces: Potential for Indirect Transmission | journal = Materials | volume = 13 | issue = 22 | page = 5211 | date = November 2020 | pmid = 33218120 | pmc = 7698891 | doi = 10.3390/ma13225211 | bibcode = 2020Mate...13.5211B | doi-access = free | title-link = doi }}</ref> Coronaviruses die very quickly when exposed to the ] in ].<ref name="Bueckert-2020" /> Like other enveloped viruses, SARS-CoV-2 survives longest when the temperature is at ] or lower, and when the ] is low (<50%).<ref name="Bueckert-2020" /> | |||
| Some studies have found that the ] (NLR) may be helpful in early screening for severe illness.<ref>{{cite journal|title=Dysregulation of immune response in patients with COVID-19 in Wuhan, China|journal=Clinical Infectious Diseases|doi=10.1093/cid/ciaa248|date=12 March 2020|last1=Tian|first1=Dai-Shi|last2=Wang|first2=Wei|last3=Shang|first3=Ke|last4=Ma|first4=Ke|last5=Xie|first5=Cuihong|last6=Tao|first6=Yu|last7=Yang|first7=Sheng|last8=Zhang|first8=Shuoqi|last9=Hu|first9=Ziwei|last10=Zhou|first10=Luoqi|last11=Qin|first11=Chuan|pmid=32161940|pmc=7108125}}</ref> | |||
| On many surfaces, including glass, some types of plastic, stainless steel, and skin, the virus can remain infective for several days indoors at room temperature, or even about a week under ideal conditions.<ref name="Bueckert-2020" /><ref>{{#invoke:cite journal || vauthors = Bhardwaj R, Agrawal A | title = How coronavirus survives for days on surfaces | journal = Physics of Fluids | volume = 32 | issue = 11 | pages = 111706 | date = November 2020 | pmid = 33281435 | pmc = 7713872 | doi = 10.1063/5.0033306 | bibcode = 2020PhFl...32k1706B }}</ref> On some surfaces, including cotton fabric and copper, the virus usually dies after a few hours.<ref name="Bueckert-2020" /> The virus dies faster on porous surfaces than on non-porous surfaces due to capillary action within pores and faster aerosol droplet evaporation.<ref>{{#invoke:cite journal || vauthors = Chatterjee S, Murallidharan JS, Agrawal A, Bhardwaj R | title = Why coronavirus survives longer on impermeable than porous surfaces | journal = Physics of Fluids | volume = 33 | issue = 2 | pages = 021701 | date = February 2021 | pmid = 33746485 | pmc = 7978145 | doi = 10.1063/5.0037924 | bibcode = 2021PhFl...33b1701C }}</ref><ref name="cdc.gov" /><ref name="Bueckert-2020" /> However, of the many surfaces tested, two with the longest survival times are N95 respirator masks and surgical masks, both of which are considered porous surfaces.<ref name="Bueckert-2020" /> | |||
| Many of those who die of COVID-19 have ], including ], ] and ].<ref name=":8">{{cite web |url=https://www.who.int/dg/speeches/detail/who-director-general-s-statement-on-the-advice-of-the-ihr-emergency-committee-on-novel-coronavirus |title=WHO Director-General's statement on the advice of the IHR Emergency Committee on Novel Coronavirus |website=] (WHO) }}</ref> The ] reported that out of 8.8% of deaths where ] were available for review, 97.2% of sampled patients had at least one ] with the average patient having 2.7 diseases.<ref name="ISSCharacteristics">{{Cite report|vauthors=Palmieri L, Andrianou X, Barbariol P, Bella A, Bellino S, Benelli E, Bertinato L, Boros S, Brambilla G, Calcagnini G, Canevelli M, Castrucci MR, Censi F, Ciervo A, Colaizzo E, D'Ancona F, Del Manso M, Donfrancesco C, Fabiani M, Filia A, Floridia M, Giuliano M, Grisetti T, Langer M, Lega I, Lo Noce C, Maiozzi P, Malchiodi Albedi F, Manno V, Martini M, Mateo Urdiales A, Mattei E, Meduri C, Meli P, Minelli G, Nebuloni M, Nisticò L, Nonis M, Onder G, Palmisano L, Petrosillo N, Pezzotti P, Pricci F, Punzo O, Puro V, Raparelli V, Rezza G, Riccardo F, Rota MC, Salerno P, Serra D, Siddu A, Stefanelli P, Tamburo De Bella M, Tiple D, Unim B, Vaianella L, Vanacore N, Vichi M, Villani ER, Brusaferro S|display-authors= 6|title=Characteristics of COVID-19 patients dying in Italy Report based on available data on April 2th, 2020|url=https://www.epicentro.iss.it/en/coronavirus/bollettino/Report-COVID-2019_2_april_2020.pdf|date=3 April 2020|publisher=]|access-date=3 April 2020|url-status=live}}</ref> According to the same report, the median time between onset of symptoms and death was ten days, with five being spent hospitalised. However, patients transferred to an ICU had a median time of seven days between hospitalisation and death.<ref name="ISSCharacteristics" /> In a study of early cases, the median time from exhibiting initial symptoms to death was 14 days, with a full range of six to 41 days.<ref>{{cite journal | vauthors = Wang W, Tang J, Wei F | title = Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China | journal = Journal of Medical Virology | volume = 92 | issue = 4 | pages = 441–47 | date = April 2020 | pmid = 31994742 |doi=10.1002/jmv.25689 |doi-access=free }}</ref> In a study by the ] (NHC) of China, men had a death rate of 2.8% while women had a death rate of 1.7%.<ref name="WM2020Feb26">{{cite web |title=Coronavirus Age, Sex, Demographics (COVID-19) |url=https://www.worldometers.info/coronavirus/coronavirus-age-sex-demographics/ |website=www.worldometers.info |access-date=26 February 2020 |archive-url=https://web.archive.org/web/20200227112932/https://www.worldometers.info/coronavirus/coronavirus-age-sex-demographics/ |archive-date=27 February 2020 |url-status=live | name-list-format = vanc}}</ref> ] examinations of post-mortem lung samples show ] with cellular fibromyxoid ]s in both lungs. Viral ] changes were observed in the ]. The lung picture resembled ] (ARDS).<ref name="WHOReport24Feb2020" /> In 11.8% of the deaths reported by the National Health Commission of China, heart damage was noted by elevated levels of ] or cardiac arrest.<ref name="Zheng Ma Zhang Xie p." /> | |||
| The CDC says that in most situations, cleaning surfaces with soap or detergent, not disinfecting, is enough to reduce risk of transmission.<ref name="cdc.gov" /><ref>{{#invoke:cite news|| vauthors = Anthes E |date=8 April 2021|title=Has the Era of Overzealous Cleaning Finally Come to an End?|work=The New York Times|url=https://www.nytimes.com/2021/04/08/health/coronavirus-hygiene-cleaning-surfaces.html |archive-url=https://ghostarchive.org/archive/20211228/https://www.nytimes.com/2021/04/08/health/coronavirus-hygiene-cleaning-surfaces.html |archive-date=28 December 2021 |url-access=limited|access-date=12 April 2021| url-status=live }}</ref> The CDC recommends that if a COVID‑19 case is suspected or confirmed at a facility such as an office or day care, all areas such as offices, bathrooms, common areas, shared electronic equipment like tablets, touch screens, keyboards, remote controls, and ATMs used by the ill persons should be disinfected.<ref name="sxygw">{{#invoke:Cite web||date=11 February 2020|title=Interim Recommendations for US Community Facilities with Suspected/Confirmed Coronavirus Disease 2019|url=https://www.cdc.gov/coronavirus/2019-ncov/community/organizations/cleaning-disinfection.html|access-date=4 April 2020|publisher=U.S. ] (CDC)}}</ref> Surfaces may be decontaminated with the following: | |||
| Availability of medical resources and the ] of a region may also affect mortality.<ref name="Ji Ma Peppelenbosch Pan 2020 p.">{{cite journal | vauthors=Ji Y, Ma Z, Peppelenbosch MP, Pan Q | title=Potential association between COVID-19 mortality and health-care resource availability | journal=Lancet Global Health | volume= 8| date=February 2020 | issue=4 | pages=e480 | pmid=32109372 | doi=10.1016/S2214-109X(20)30068-1 | doi-access=free }}</ref> Estimates of the mortality from the condition vary because of those regional differences,<ref name="pmid32159317">{{cite journal | vauthors = Li XQ, Cai WF, Huang LF, Chen C, Liu YF, Zhang ZB, Yuan J, Li TG, Wang M | display-authors = 6 | title = | language = Chinese | journal = Zhonghua Liu Xing Bing Xue Za Zhi = Zhonghua Liuxingbingxue Zazhi | volume = 41 | issue = 5 | pages = 634–637 | date = March 2020 | pmid = 32159317 | doi = 10.3760/cma.j.cn112338-20200228-00209 }}</ref> but also because of ] difficulties. The under-counting of mild cases can cause the mortality rate to be overestimated.<ref>{{cite journal | vauthors = Jung SM, Akhmetzhanov AR, Hayashi K, Linton NM, Yang Y, Yuan B, Kobayashi T, Kinoshita R, Nishiura H | display-authors = 6 | title = Real-Time Estimation of the Risk of Death from Novel Coronavirus (COVID-19) Infection: Inference Using Exported Cases | journal = Journal of Clinical Medicine | volume = 9 | issue = 2 | page = 523 | date = February 2020 | pmid = 32075152 | doi = 10.3390/jcm9020523 | pmc = 7074479 }}</ref> However, the fact that deaths are the result of cases contracted in the past can mean the current mortality rate is underestimated.<ref>{{cite journal | vauthors = Chughtai A, Malik A | title = Is Coronavirus disease (COVID-19) case fatality ratio underestimated? | journal = Global Biosecurity | date = March 2020 | volume = 1 | issue = 3 | doi = 10.31646/gbio.56 | doi-broken-date = 19 March 2020 }}</ref><ref>{{cite journal | vauthors = Baud D, Qi X, Nielsen-Saines K, Musso D, Pomar L, Favre G | title = Real estimates of mortality following COVID-19 infection. | journal = The Lancet Infectious Diseases | date = March 2020 | doi = 10.1016/S1473-3099(20)30195-X | pmid = 32171390 | pmc = 7118515 }}</ref> | |||
| * 62–71% ] | |||
| * 50–100% ] | |||
| * 0.1% ] | |||
| * 0.5% ] | |||
| * 0.2–7.5% ] | |||
| * 50–200 ppm ] | |||
| Other solutions, such as ] and ], are less effective. ] may also be used,<ref name="CDCasof07092020">{{#invoke:Cite web||date=9 July 2020|title=COVID-19 Employer Information for Office Buildings|url=https://www.cdc.gov/coronavirus/2019-ncov/community/office-buildings.html|access-date=9 July 2020|website=U.S. ] (CDC)|vauthors=((National Center for Immunization and Respiratory Diseases (NCIRD)))}}</ref> although popular devices require {{val|5|-|10|u=min}} exposure and may deteriorate some materials over time.<ref>{{#invoke:cite news ||title=Yes, UV phone sanitizers work. That doesn't mean you need one. |url=https://www.washingtonpost.com/lifestyle/2021/02/16/uv-sanitizer-phone-covid-germs/ |access-date=29 April 2022 |newspaper=The Washington Post |date=16 February 2021}}</ref> A datasheet listing the authorised substances to disinfection in the food industry (including suspension or surface tested, kind of surface, use dilution, disinfectant and inoculum volumes) can be seen in the supplementary material of a 2021 ] article.<ref name="disinfection-foodindus">{{#invoke:cite journal || vauthors = Pedreira A, Taşkın Y, García MR | title = A Critical Review of Disinfection Processes to Control SARS-CoV-2 Transmission in the Food Industry | journal = Foods | volume = 10 | issue = 2 | page = 283 | date = January 2021 | pmid = 33572531 | pmc = 7911259 | doi = 10.3390/foods10020283 | s2cid = 231900820 | doi-access = free | title-link = doi }}</ref> | |||
| Concerns have been raised about long-term ]e of the disease. The ] found a drop of 20% to 30% in lung capacity in some people who recovered from the disease, and lung scans suggested organ damage.<ref>{{cite web |last1=Cheung |first1=Elizabeth |name-list-format=vanc |title=Some recovered Covid-19 patients may have lung damage, doctors say |url=https://www.scmp.com/news/hong-kong/health-environment/article/3074988/coronavirus-some-recovered-patients-may-have |website=] |language=en |date=13 March 2020 |access-date=15 March 2020 |archive-url=https://web.archive.org/web/20200315172445/https://www.scmp.com/news/hong-kong/health-environment/article/3074988/coronavirus-some-recovered-patients-may-have |archive-date=15 March 2020 |url-status=live }}</ref> | |||
| === Self-isolation === | |||
| <!-- PLEASE DO NOT ADD INSTRUCTIONS HERE, SEE WIKIPEDIA:NOTHOWTO. --> | |||
| ] at home has been recommended for those diagnosed with COVID‑19 and those who suspect they have been infected. Health agencies have issued detailed instructions for proper self-isolation.<ref name="pmid33012884">{{#invoke:cite journal||vauthors=Patiño-Lugo DF, Vélez M, Velásquez Salazar P, Vera-Giraldo CY, Vélez V, Marín IC, Ramírez PA, Quintero SP, Castrillón Martínez E, Pineda Higuita DA, Henandez G|date=June 2020|title=Non-pharmaceutical interventions for containment, mitigation and suppression of COVID-19 infection|journal=Colombia Medica|volume=51|issue=2|pages=e4266|doi=10.25100/cm.v51i2.4266|pmc=7518730 |pmid=33012884}}</ref> Many governments have mandated or recommended self-quarantine for entire populations. The strongest self-quarantine instructions have been issued to those in high-risk groups.<ref name="lZR3i">{{#invoke:Cite web||title=COVID-19 Informational Resources for High-Risk Groups {{!}} Keeping Education ACTIVE {{!}} Partnership to Fight Chronic Disease|url=https://www.fightchronicdisease.org/resources/covid-19-informational-resources-high-risk-groups|access-date=31 May 2020|website=fightchronicdisease.org}}</ref> Those who may have been exposed to someone with COVID‑19 and those who have recently travelled to a country or region with the widespread transmission have been advised to self-quarantine for 14 days from the time of last possible exposure.<ref>{{#invoke:Cite web||url=https://www.cdc.gov/coronavirus/2019-ncov/if-you-are-sick/quarantine.html |title=Quarantine and Isolation |date=29 July 2021 |publisher=U.S. ] (CDC) |access-date=12 August 2021 }}</ref> | |||
| === International travel-related control measures === | |||
| A 2021 Cochrane rapid review found that based upon low-certainty evidence, international travel-related control measures such as restricting cross-border travel may help to contain the spread of COVID‑19.<ref name="Burns-2021">{{#invoke:cite journal || vauthors = Burns J, Movsisyan A, Stratil JM, Biallas RL, Coenen M, Emmert-Fees KM, Geffert K, Hoffmann S, Horstick O, Laxy M, Klinger C, Kratzer S, Litwin T, Norris S, Pfadenhauer LM, von Philipsborn P, Sell K, Stadelmaier J, Verboom B, Voss S, Wabnitz K, Rehfuess E | title = International travel-related control measures to contain the COVID-19 pandemic: a rapid review | journal = The Cochrane Database of Systematic Reviews | volume = 2021 | pages = CD013717 | date = March 2021 | issue = 3 | pmid = 33763851 | doi = 10.1002/14651858.CD013717.pub2 | pmc = 8406796 | s2cid = 232356197 | collaboration = Cochrane Public Health Group }}</ref> Additionally, symptom/exposure-based screening measures at borders may miss many positive cases.<ref name="Burns-2021" /> While test-based border screening measures may be more effective, it could also miss many positive cases if only conducted upon arrival without follow-up. The review concluded that a minimum 10-day quarantine may be beneficial in preventing the spread of COVID‑19 and may be more effective if combined with an additional control measure like border screening.<ref name="Burns-2021" /> | |||
| == Treatment == | |||
| {{Main|Treatment and management of COVID-19}} | |||
| <!-- CONTENT TRANSCLUDED. CAN BE EDITED WITHIN ] --> | |||
| {{Excerpt|Treatment and management of COVID-19|paragraphs=1-4|hat=no}} | |||
| == Prognosis and risk factors == | |||
| <noinclude>{{See also|COVID-19 pandemic death rates by country}}</noinclude> | |||
| <!-- THE FOLLOWING TWO PARAGRAPHS ARE TRANSCLUDED INTO THE COVID-19 PANDEMIC ARTICLE --> | |||
| The severity of COVID‑19 varies. The disease may take a mild course with few or no symptoms, resembling other common upper respiratory diseases such as the ]. In 3–4% of cases (7.4% for those over age 65) symptoms are severe enough to cause hospitalisation.<ref name="pmid33087398">{{#invoke:cite journal || vauthors = Doshi P | title = Will covid-19 vaccines save lives? Current trials aren't designed to tell us | journal = BMJ | volume = 371 | pages = m4037 | date = October 2020 | pmid = 33087398 | doi = 10.1136/bmj.m4037 | s2cid = 224817161 }}</ref> Mild cases typically recover within two weeks, while those with severe or critical diseases may take three to six weeks to recover. Among those who have died, the time from symptom onset to death has ranged from two to eight weeks.<ref name="WHOReport24Feb2020" /> The Italian ] reported that the median time between the onset of symptoms and death was twelve days, with seven being hospitalised. However, people transferred to an ICU had a median time of ten days between hospitalisation and death.<ref name="ISSCharacteristics" /> Abnormal sodium levels during hospitalisation with COVID-19 are associated with poor prognoses: high sodium with a greater risk of death, and low sodium with an increased chance of needing ventilator support.<ref>{{#invoke:cite journal || vauthors = Tzoulis P, Waung JA, Bagkeris E, Hussein Z, Biddanda A, Cousins J, Dewsnip A, Falayi K, McCaughran W, Mullins C, Naeem A, Nwokolo M, Quah H, Bitat S, Deyab E, Ponnampalam S, Bouloux PM, Montgomery H, Baldeweg SE | title = Dysnatremia is a Predictor for Morbidity and Mortality in Hospitalized Patients with COVID-19 | journal = The Journal of Clinical Endocrinology and Metabolism | volume = 106 | issue = 6 | pages = 1637–1648 | date = May 2021 | pmid = 33624101 | pmc = 7928894 | doi = 10.1210/clinem/dgab107 }}</ref><ref>{{#invoke:cite journal || vauthors = Tzoulis P, Grossman AB, Baldeweg SE, Bouloux P, Kaltsas G | title = MANAGEMENT OF ENDOCRINE DISEASE: Dysnatraemia in COVID-19: prevalence, prognostic impact, pathophysiology, and management | journal = European Journal of Endocrinology | volume = 185 | issue = 4 | pages = R103–R111 | date = September 2021 | pmid = 34370712 | pmc = 8428074 | doi = 10.1530/EJE-21-0281 }}</ref> Prolonged ] time and elevated ] levels on admission to the hospital are associated with severe course of COVID‑19 and with a transfer to ICU.<ref>{{#invoke:cite journal || vauthors = Baranovskii DS, Klabukov ID, Krasilnikova OA, Nikogosov DA, Polekhina NV, Baranovskaia DR, Laberko LA, Maneksha S, Harry TV, Durbin RP | title = Prolonged prothrombin time as an early prognostic indicator of severe acute respiratory distress syndrome in patients with COVID-19 related pneumonia | journal = Current Medical Research and Opinion | volume = 229 | issue = 6 | pages = 21–25 | date = December 1975 | pmid = 33210948<!-- Despite the error message it generates, the PMID is correct and valid as of 29 November 2020. --> | pmc = 7738209 | doi = 10.1080/03007995.2020.1853510 | s2cid = 227065216 }}</ref><ref>{{#invoke:cite journal || vauthors = Christensen B, Favaloro EJ, Lippi G, Van Cott EM | title = Hematology Laboratory Abnormalities in Patients with Coronavirus Disease 2019 (COVID-19) | journal = Seminars in Thrombosis and Hemostasis | volume = 46 | issue = 7 | pages = 845–849 | date = October 2020 | pmid = 32877961 | pmc = 7645834 | doi = 10.1055/s-0040-1715458 }}</ref> | |||
| Some early studies suggest 10% to 20% of people with COVID‑19 will experience ].<ref name="NIHRreportSep20">{{#invoke:cite journal||date=15 October 2020 |title=Living with Covid19 |url=https://evidence.nihr.ac.uk/themedreview/living-with-covid19/ |periodical=NIHR Themed Reviews |publisher=] |doi=10.3310/themedreview_41169 |doi-access=free |title-link=doi}}</ref><ref name="HvIJa">{{#invoke:Cite web||date=6 June 2020|title=How long does COVID-19 last?|url=https://covid.joinzoe.com/post/covid-long-term|access-date=15 October 2020|publisher=UK COVID Symptom Study}}</ref> A majority of those who were admitted to hospital with severe disease report long-term problems including fatigue and shortness of breath.<ref name="UniWashingtonSep20">{{#invoke:Cite web||url=https://www.doh.wa.gov/Portals/1/Documents/1600/coronavirus/SummaryCOVIDLong%20termHealthEffects9-1-2020.pdf |title=Summary of COVID-19 Long Term Health Effects: Emerging evidence and Ongoing Investigation |publisher=] |date=1 September 2020 |access-date=15 October 2020 |archive-date=18 December 2020 |archive-url=https://web.archive.org/web/20201218080009/https://www.doh.wa.gov/Portals/1/Documents/1600/coronavirus/SummaryCOVIDLong%20termHealthEffects9-1-2020.pdf |url-status=dead}}</ref> On 30 October 2020, WHO chief ] warned that "to a significant number of people, the COVID virus poses a range of serious long-term effects". He has described the vast spectrum of COVID‑19 symptoms that fluctuate over time as "really concerning". They range from fatigue, a cough and shortness of breath, to inflammation and injury of major organs{{snd}}including the lungs and heart, and also neurological and psychologic effects. Symptoms often overlap and can affect any system in the body. Infected people have reported cyclical bouts of fatigue, headaches, months of complete exhaustion, mood swings, and other symptoms. Tedros therefore concluded that a strategy of achieving ] by infection, rather than vaccination, is "morally unconscionable and unfeasible".<ref name="OaTsI">{{#invoke:Cite web|| title=Long-term symptoms of COVID-19 'really concerning', says WHO chief | website=UN News | date=30 October 2020 | url=https://news.un.org/en/story/2020/10/1076562 | access-date=7 March 2021}}</ref> | |||
| In terms of hospital readmissions about 9% of 106,000 individuals had to return for hospital treatment within two months of discharge. The average to readmit was eight days since first hospital visit. There are several risk factors that have been identified as being a cause of multiple admissions to a hospital facility. Among these are advanced age (above 65 years of age) and presence of a chronic condition such as diabetes, COPD, heart failure or chronic kidney disease.<ref name="rArHO">{{#invoke:Cite web||title=Coronavirus disease 2019 (COVID-19) – Prognosis |url=https://bestpractice.bmj.com/topics/en-us/3000168/prognosis |website=BMJ |access-date=15 November 2020}}</ref><ref name="CtbMg">{{#invoke:cite journal || vauthors = Lavery AM, Preston LE, Ko JY, Chevinsky JR, DeSisto CL, Pennington AF, Kompaniyets L, Datta SD, Click ES, Golden T, Goodman AB, Mac Kenzie WR, Boehmer TK, Gundlapalli AV | title = Characteristics of Hospitalized COVID-19 Patients Discharged and Experiencing Same-Hospital Readmission – United States, March–August 2020 | journal = MMWR. Morbidity and Mortality Weekly Report | volume = 69 | issue = 45 | pages = 1695–1699 | date = November 2020 | pmid = 33180754 | pmc = 7660660 | doi = 10.15585/mmwr.mm6945e2 }}</ref> | |||
| According to ]s smokers are more likely to require intensive care or die compared to non-smokers.<ref>{{#invoke:cite journal || vauthors = Vardavas CI, Nikitara K | title = COVID-19 and smoking: A systematic review of the evidence | journal = Tobacco Induced Diseases | volume = 18 | pages = 20 | date = March 2020 | pmid = 32206052 | pmc = 7083240 | doi = 10.18332/tid/119324 }}</ref><ref name="engin-review">{{#invoke:cite journal || vauthors = Engin AB, Engin ED, Engin A | title = Two important controversial risk factors in SARS-CoV-2 infection: Obesity and smoking | journal = Environmental Toxicology and Pharmacology | volume = 78 | pages = 103411 | date = August 2020 | pmid = 32422280 | pmc = 7227557 | doi = 10.1016/j.etap.2020.103411 | bibcode = 2020EnvTP..7803411E }}</ref> Acting on the same ACE2 pulmonary receptors affected by smoking, air pollution has been correlated with the disease.<ref name="engin-review" /> Short-term<ref>{{#invoke:cite journal || vauthors = Setti L, Passarini F, De Gennaro G, Barbieri P, Licen S, Perrone MG, Piazzalunga A, Borelli M, Palmisani J, Di Gilio A, Rizzo E, Colao A, Piscitelli P, Miani A | title = Potential role of particulate matter in the spreading of COVID-19 in Northern Italy: first observational study based on initial epidemic diffusion | journal = BMJ Open | volume = 10 | issue = 9 | pages = e039338 | date = September 2020 | pmid = 32973066 | doi = 10.1136/bmjopen-2020-039338 | pmc = 7517216 }}</ref> and chronic<ref>{{#invoke:cite journal || vauthors = Wu X, Nethery RC, Sabath MB, Braun D, Dominici F | title = Air pollution and COVID-19 mortality in the United States: Strengths and limitations of an ecological regression analysis | journal = Science Advances | volume = 6 | issue = 45 | pages = eabd4049 | date = November 2020 | pmid = 33148655 | doi = 10.1126/sciadv.abd4049 | pmc = 7673673 | bibcode = 2020SciA....6.4049W }}</ref> exposure to air pollution seems to enhance morbidity and mortality from COVID‑19.<ref>{{#invoke:cite journal|| vauthors = Pansini R, Fornacca D |date=June 2021|title=Early Spread of COVID-19 in the Air-Polluted Regions of Eight Severely Affected Countries|journal=Atmosphere|volume=12|issue=6|pages=795|doi=10.3390/atmos12060795|bibcode=2021Atmos..12..795P|doi-access = free | title-link = doi }}</ref><ref>{{#invoke:cite journal || vauthors = Comunian S, Dongo D, Milani C, Palestini P | title = Air Pollution and Covid-19: The Role of Particulate Matter in the Spread and Increase of Covid-19's Morbidity and Mortality | journal = International Journal of Environmental Research and Public Health | volume = 17 | issue = 12 | pages = 4487 | date = June 2020 | pmid = 32580440 | doi = 10.3390/ijerph17124487 | pmc = 7345938 | doi-access = free | title-link = doi }}</ref><ref>{{#invoke:cite journal || vauthors = Domingo JL, Marquès M, Rovira J | title = Influence of airborne transmission of SARS-CoV-2 on COVID-19 pandemic. A review | journal = Environmental Research | volume = 188 | pages = 109861 | date = September 2020 | pmid = 32718835 | pmc = 7309850 | doi = 10.1016/j.envres.2020.109861 | bibcode = 2020ER....18809861D }}</ref> Pre-existing heart and lung diseases<ref>{{#invoke:Cite web||url=https://www.mayoclinic.org/diseases-conditions/coronavirus/in-depth/coronavirus-who-is-at-risk/art-20483301|title=COVID-19: Who's at higher risk of serious symptoms?|website=Mayo Clinic}}</ref> and also ], especially in conjunction with ], contributes to an increased health risk of COVID‑19.<ref name="engin-review" /><ref>{{#invoke:cite journal || vauthors = Tamara A, Tahapary DL | title = Obesity as a predictor for a poor prognosis of COVID-19: A systematic review | journal = Diabetes & Metabolic Syndrome | volume = 14 | issue = 4 | pages = 655–659 | date = July 2020 | pmid = 32438328 | pmc = 7217103 | doi = 10.1016/j.dsx.2020.05.020 | doi-access = free | title-link = doi }}</ref><ref>{{#invoke:cite journal || vauthors = Petrakis D, Margină D, Tsarouhas K, Tekos F, Stan M, Nikitovic D, Kouretas D, Spandidos DA, Tsatsakis A | title = Obesity – A risk factor for increased COVID-19, severity and lethality (Review) | journal = Molecular Medicine Reports | volume = 22 | issue = 1 | pages = 9–19 | date = July 2020 | pmid = 32377709 | pmc = 7248467 | doi = 10.3892/mmr.2020.11127 | doi-access = free | title-link = doi }}</ref><ref>{{#invoke:cite journal ||vauthors=Roca-Fernández A, Dennis A, Nicholls R, McGonigle J, Kelly M, Banerjee R, Banerjee A, Sanyal AJ |date=29 March 2021 |title=Hepatic Steatosis, Rather Than Underlying Obesity, Increases the Risk of Infection and Hospitalization for COVID-19 |journal=Frontiers in Medicine |volume=8 |page=636637 |doi=10.3389/fmed.2021.636637 |pmid=33855033 |pmc=8039134 |issn=2296-858X|doi-access = free | title-link = doi }}</ref> | |||
| It is also assumed that those that are immunocompromised are at higher risk of getting severely sick from SARS-CoV-2.<ref>{{#invoke:Cite web||url=https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/immunocompromised.html|title=Coronavirus Disease 2019 (COVID-19)|date=11 February 2020|website=U.S. ] (CDC) }}</ref> One research study that looked into the COVID‑19 infections in hospitalised kidney transplant recipients found a mortality rate of 11%.<ref>{{#invoke:cite journal || vauthors = Devresse A, Belkhir L, Vo B, Ghaye B, Scohy A, Kabamba B, Goffin E, De Greef J, Mourad M, De Meyer M, Yombi JC, Kanaan N | title = COVID-19 Infection in Kidney Transplant Recipients: A Single-Center Case Series of 22 Cases From Belgium | journal = Kidney Medicine | volume = 2 | issue = 4 | pages = 459–466 | date = November 2020 | pmid = 32775986 | pmc = 7295531 | doi = 10.1016/j.xkme.2020.06.001 }}</ref> | |||
| Men with untreated ] were 2.4 times more likely than men with eugonadism to be hospitalised if they contracted COVID-19; Hypogonad men treated with ] were less likely to be hospitalised for COVID-19 than men who were not treated for hypogonadism.<ref name="jama">{{#invoke:cite journal || vauthors = Dhindsa S, Champion C, Deol E, Lui M, Campbell R, Newman J, Yeggalam A, Nadella S, Ahir V, Shrestha E, Kannampallil T, Diwan A | title = Association of Male Hypogonadism With Risk of Hospitalization for COVID-19 | journal = JAMA Network Open | volume = 5 | issue = 9 | pages = e2229747 | date = September 2022 | pmid = 36053534 | doi = 10.1001/jamanetworkopen.2022.29747 | pmc = 9440397 }}</ref> | |||
| === Genetic risk factors === | |||
| ] plays an important role in the ability to fight off Covid.<ref name="pmid33888907">{{#invoke:cite journal || vauthors = Shelton JF, Shastri AJ, Ye C, Weldon CH, Filshtein-Sonmez T, Coker D, Symons A, Esparza-Gordillo J, Aslibekyan S, Auton A | title = Trans-ancestry analysis reveals genetic and nongenetic associations with COVID-19 susceptibility and severity | journal = Nature Genetics | volume = 53 | issue = 6 | pages = 801–808 | date = June 2021 | pmid = 33888907 | doi = 10.1038/s41588-021-00854-7 | s2cid = 233372385 }}</ref> For instance, those that do not produce detectable ]s or produce ] against these may get much sicker from COVID‑19.<ref>{{#invoke:Cite web||title=One in Seven Dire COVID Cases May Result from a Faulty Immune Response|url=https://www.scientificamerican.com/article/one-in-seven-dire-covid-cases-may-result-from-a-faulty-immune-response/|website=Scientific American|vauthors=Wallis C}}</ref><ref>{{#invoke:cite journal || vauthors = Bastard P, Rosen LB, Zhang Q, Michailidis E, Hoffmann HH, Zhang Y, Dorgham K, Philippot Q, Rosain J, Béziat V, Manry J, Shaw E, Haljasmägi L, Peterson P, Lorenzo L, Bizien L, Trouillet-Assant S, Dobbs K, de Jesus AA, Belot A, Kallaste A, Catherinot E, Tandjaoui-Lambiotte Y, Le Pen J, Kerner G, Bigio B, Seeleuthner Y, Yang R, Bolze A, Spaan AN, Delmonte OM, Abers MS, Aiuti A, Casari G, Lampasona V, Piemonti L, Ciceri F, Bilguvar K, Lifton RP, Vasse M, Smadja DM, Migaud M, Hadjadj J, Terrier B, Duffy D, Quintana-Murci L, van de Beek D, Roussel L, Vinh DC, Tangye SG, Haerynck F, Dalmau D, Martinez-Picado J, Brodin P, Nussenzweig MC, Boisson-Dupuis S, Rodríguez-Gallego C, Vogt G, Mogensen TH, Oler AJ, Gu J, Burbelo PD, Cohen JI, Biondi A, Bettini LR, D'Angio M, Bonfanti P, Rossignol P, Mayaux J, Rieux-Laucat F, Husebye ES, Fusco F, Ursini MV, Imberti L, Sottini A, Paghera S, Quiros-Roldan E, Rossi C, Castagnoli R, Montagna D, Licari A, Marseglia GL, Duval X, Ghosn J, Tsang JS, Goldbach-Mansky R, Kisand K, Lionakis MS, Puel A, Zhang SY, Holland SM, Gorochov G, Jouanguy E, Rice CM, Cobat A, Notarangelo LD, Abel L, Su HC, Casanova JL | title = Autoantibodies against type I IFNs in patients with life-threatening COVID-19 | journal = Science | volume = 370 | issue = 6515 | pages = eabd4585 | date = October 2020 | pmid = 32972996 | pmc = 7857397 | doi = 10.1126/science.abd4585 | s2cid = 221914095 | title-link = doi | doi-access = free }}</ref> ] is able to detect ] effector genes.<ref>{{#invoke:cite journal || vauthors = Fusco DN, Brisac C, John SP, Huang YW, Chin CR, Xie T, Zhao H, Jilg N, Zhang L, Chevaliez S, Wambua D, Lin W, Peng L, Chung RT, Brass AL | title = A genetic screen identifies interferon-α effector genes required to suppress hepatitis C virus replication | journal = Gastroenterology | volume = 144 | issue = 7 | pages = 1438–49, 1449.e1-9 | date = June 2013 | pmid = 23462180 | pmc = 3665646 | doi = 10.1053/j.gastro.2013.02.026 }}</ref> Some genetic variants are risk factors in specific populations. For instance, an ] of the ] gene (dedicator of cytokinesis 2 gene) is a common risk factor in Asian populations but much less common in Europe. The mutation leads to lower expression of DOCK2 especially in younger people with severe COVID-19 infections.<ref>{{#invoke:cite journal || vauthors = Namkoong H, Edahiro R, Takano T, Nishihara H, Shirai Y, Sonehara K, Tanaka H, Azekawa S, Mikami Y, Lee H, Hasegawa T, Okudela K, Okuzaki D, Motooka D, Kanai M, Naito T, Yamamoto K, Wang QS, Saiki R, Ishihara R, Matsubara Y, Hamamoto J, Hayashi H, Yoshimura Y, Tachikawa N, Yanagita E, Hyugaji T, Shimizu E, Katayama K, Kato Y, Morita T, Takahashi K, Harada N, Naito T, Hiki M, Matsushita Y, Takagi H, Aoki R, Nakamura A, Harada S, Sasano H, Kabata H, Masaki K, Kamata H, Ikemura S, Chubachi S, Okamori S, Terai H, Morita A, Asakura T, Sasaki J, Morisaki H, Uwamino Y, Nanki K, Uchida S, Uno S, Nishimura T, Ishiguro T, Isono T, Shibata S, Matsui Y, Hosoda C, Takano K, Nishida T, Kobayashi Y, Takaku Y, Takayanagi N, Ueda S, Tada A, Miyawaki M, Yamamoto M, Yoshida E, Hayashi R, Nagasaka T, Arai S, Kaneko Y, Sasaki K, Tagaya E, Kawana M, Arimura K, Takahashi K, Anzai T, Ito S, Endo A, Uchimura Y, Miyazaki Y, Honda T, Tateishi T, Tohda S, Ichimura N, Sonobe K, Sassa CT, Nakajima J, Nakano Y, Nakajima Y, Anan R, Arai R, Kurihara Y, Harada Y, Nishio K, Ueda T, Azuma M, Saito R, Sado T, Miyazaki Y, Sato R, Haruta Y, Nagasaki T, Yasui Y, Hasegawa Y, Mutoh Y, Kimura T, Sato T, Takei R, Hagimoto S, Noguchi Y, Yamano Y, Sasano H, Ota S, Nakamori Y, Yoshiya K, Saito F, Yoshihara T, Wada D, Iwamura H, Kanayama S, Maruyama S, Yoshiyama T, Ohta K, Kokuto H, Ogata H, Tanaka Y, Arakawa K, Shimoda M, Osawa T, Tateno H, Hase I, Yoshida S, Suzuki S, Kawada M, Horinouchi H, Saito F, Mitamura K, Hagihara M, Ochi J, Uchida T, Baba R, Arai D, Ogura T, Takahashi H, Hagiwara S, Nagao G, Konishi S, Nakachi I, Murakami K, Yamada M, Sugiura H, Sano H, Matsumoto S, Kimura N, Ono Y, Baba H, Suzuki Y, Nakayama S, Masuzawa K, Namba S, Suzuki K, Naito Y, Liu YC, Takuwa A, Sugihara F, Wing JB, Sakakibara S, Hizawa N, Shiroyama T, Miyawaki S, Kawamura Y, Nakayama A, Matsuo H, Maeda Y, Nii T, Noda Y, Niitsu T, Adachi Y, Enomoto T, Amiya S, Hara R, Yamaguchi Y, Murakami T, Kuge T, Matsumoto K, Yamamoto Y, Yamamoto M, Yoneda M, Kishikawa T, Yamada S, Kawabata S, Kijima N, Takagaki M, Sasa N, Ueno Y, Suzuki M, Takemoto N, Eguchi H, Fukusumi T, Imai T, Fukushima M, Kishima H, Inohara H, Tomono K, Kato K, Takahashi M, Matsuda F, Hirata H, Takeda Y, Koh H, Manabe T, Funatsu Y, Ito F, Fukui T, Shinozuka K, Kohashi S, Miyazaki M, Shoko T, Kojima M, Adachi T, Ishikawa M, Takahashi K, Inoue T, Hirano T, Kobayashi K, Takaoka H, Watanabe K, Miyazawa N, Kimura Y, Sado R, Sugimoto H, Kamiya A, Kuwahara N, Fujiwara A, Matsunaga T, Sato Y, Okada T, Hirai Y, Kawashima H, Narita A, Niwa K, Sekikawa Y, Nishi K, Nishitsuji M, Tani M, Suzuki J, Nakatsumi H, Ogura T, Kitamura H, Hagiwara E, Murohashi K, Okabayashi H, Mochimaru T, Nukaga S, Satomi R, Oyamada Y, Mori N, Baba T, Fukui Y, Odate M, Mashimo S, Makino Y, Yagi K, Hashiguchi M, Kagyo J, Shiomi T, Fuke S, Saito H, Tsuchida T, Fujitani S, Takita M, Morikawa D, Yoshida T, Izumo T, Inomata M, Kuse N, Awano N, Tone M, Ito A, Nakamura Y, Hoshino K, Maruyama J, Ishikura H, Takata T, Odani T, Amishima M, Hattori T, Shichinohe Y, Kagaya T, Kita T, Ohta K, Sakagami S, Koshida K, Hayashi K, Shimizu T, Kozu Y, Hiranuma H, Gon Y, Izumi N, Nagata K, Ueda K, Taki R, Hanada S, Kawamura K, Ichikado K, Nishiyama K, Muranaka H, Nakamura K, Hashimoto N, Wakahara K, Sakamoto K, Omote N, Ando A, Kodama N, Kaneyama Y, Maeda S, Kuraki T, Matsumoto T, Yokote K, Nakada TA, Abe R, Oshima T, Shimada T, Harada M, Takahashi T, Ono H, Sakurai T, Shibusawa T, Kimizuka Y, Kawana A, Sano T, Watanabe C, Suematsu R, Sageshima H, Yoshifuji A, Ito K, Takahashi S, Ishioka K, Nakamura M, Masuda M, Wakabayashi A, Watanabe H, Ueda S, Nishikawa M, Chihara Y, Takeuchi M, Onoi K, Shinozuka J, Sueyoshi A, Nagasaki Y, Okamoto M, Ishihara S, Shimo M, Tokunaga Y, Kusaka Y, Ohba T, Isogai S, Ogawa A, Inoue T, Fukuyama S, Eriguchi Y, Yonekawa A, Kan-O K, Matsumoto K, Kanaoka K, Ihara S, Komuta K, Inoue Y, Chiba S, Yamagata K, Hiramatsu Y, Kai H, Asano K, Oguma T, Ito Y, Hashimoto S, Yamasaki M, Kasamatsu Y, Komase Y, Hida N, Tsuburai T, Oyama B, Takada M, Kanda H, Kitagawa Y, Fukuta T, Miyake T, Yoshida S, Ogura S, Abe S, Kono Y, Togashi Y, Takoi H, Kikuchi R, Ogawa S, Ogata T, Ishihara S, Kanehiro A, Ozaki S, Fuchimoto Y, Wada S, Fujimoto N, Nishiyama K, Terashima M, Beppu S, Yoshida K, Narumoto O, Nagai H, Ooshima N, Motegi M, Umeda A, Miyagawa K, Shimada H, Endo M, Ohira Y, Watanabe M, Inoue S, Igarashi A, Sato M, Sagara H, Tanaka A, Ohta S, Kimura T, Shibata Y, Tanino Y, Nikaido T, Minemura H, Sato Y, Yamada Y, Hashino T, Shinoki M, Iwagoe H, Takahashi H, Fujii K, Kishi H, Kanai M, Imamura T, Yamashita T, Yatomi M, Maeno T, Hayashi S, Takahashi M, Kuramochi M, Kamimaki I, Tominaga Y, Ishii T, Utsugi M, Ono A, Tanaka T, Kashiwada T, Fujita K, Saito Y, Seike M, Watanabe H, Matsuse H, Kodaka N, Nakano C, Oshio T, Hirouchi T, Makino S, Egi M, Omae Y, Nannya Y, Ueno T, Katayama K, Ai M, Fukui Y, Kumanogoh A, Sato T, Hasegawa N, Tokunaga K, Ishii M, Koike R, Kitagawa Y, Kimura A, Imoto S, Miyano S, Ogawa S, Kanai T, Fukunaga K, Okada Y | title = DOCK2 is involved in the host genetics and biology of severe COVID-19 | journal = Nature | volume = 609 | issue = 7928 | pages = 754–760 | date = September 2022 | pmid = 35940203 | pmc = 9492544 | doi = 10.1038/s41586-022-05163-5 | bibcode = 2022Natur.609..754N }}</ref> In fact, many other genes and genetic variants have been found that determine the outcome of SARS-CoV-2 infections.<ref>{{#invoke:cite journal || vauthors = Kousathanas A, Pairo-Castineira E, Rawlik K, Stuckey A, Odhams CA, Walker S, Russell CD, Malinauskas T, Wu Y, Millar J, Shen X, Elliott KS, Griffiths F, Oosthuyzen W, Morrice K, Keating S, Wang B, Rhodes D, Klaric L, Zechner M, Parkinson N, Siddiq A, Goddard P, Donovan S, Maslove D, Nichol A, Semple MG, Zainy T, Maleady-Crowe F, Todd L, Salehi S, Knight J, Elgar G, Chan G, Arumugam P, Patch C, Rendon A, Bentley D, Kingsley C, Kosmicki JA, Horowitz JE, Baras A, Abecasis GR, Ferreira MA, Justice A, Mirshahi T, Oetjens M, Rader DJ, Ritchie MD, Verma A, Fowler TA, Shankar-Hari M, Summers C, Hinds C, Horby P, Ling L, McAuley D, Montgomery H, Openshaw PJ, Elliott P, Walsh T, Tenesa A, Fawkes A, Murphy L, Rowan K, Ponting CP, Vitart V, Wilson JF, Yang J, Bretherick AD, Scott RH, Hendry SC, Moutsianas L, Law A, Caulfield MJ, Baillie JK | title = Whole-genome sequencing reveals host factors underlying critical COVID-19 | journal = Nature | volume = 607 | issue = 7917 | pages = 97–103 | date = July 2022 | pmid = 35255492 | pmc = 9259496 | doi = 10.1038/s41586-022-04576-6 | bibcode = 2022Natur.607...97K }}</ref> | |||
| === Children === | |||
| {{See also|Impact of the COVID-19 pandemic on children}} | |||
| While very young children have experienced lower rates of infection, older children have a rate of infection that is similar to the population as a whole.<ref>{{#invoke:Cite web||title=COVID-19 in children and the role of school settings in transmission – first update |url=https://www.ecdc.europa.eu/en/publications-data/children-and-school-settings-covid-19-transmission |website=European Centre for Disease Prevention and Control |access-date=6 April 2021 |date=23 December 2020}}</ref><ref>{{#invoke:Cite web||title=Estimated Disease Burden of COVID-19 |url=https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/burden.html |website=U.S. ] (CDC) |access-date=6 April 2021 |date=11 February 2020}}</ref> Children are likely to have milder symptoms and are at lower risk of severe disease than adults.<ref name="Reardon">{{#invoke:cite journal || vauthors = Reardon S |title=Why don't kids tend to get as sick from Covid-19? |journal=] |date=2 September 2021 |doi=10.1146/knowable-090121-1 |s2cid=239653475 |url=https://knowablemagazine.org/article/health-disease/2021/why-dont-kids-tend-get-sick-covid19 |access-date=7 September 2021}}</ref> The CDC reports that in the US roughly a third of hospitalised children were admitted to the ICU,<ref>{{#invoke:Cite web||title=Information for Pediatric Healthcare Providers |url=https://www.cdc.gov/coronavirus/2019-ncov/hcp/pediatric-hcp.html |website=U.S. ] (CDC) |access-date=6 April 2021 |date=11 February 2020}}</ref> while a European multinational study of hospitalised children from June 2020, found that about 8% of children admitted to a hospital needed intensive care.<ref>{{#invoke:cite journal || vauthors = Götzinger F, Santiago-García B, Noguera-Julián A, Lanaspa M, Lancella L, Calò Carducci FI, Gabrovska N, Velizarova S, Prunk P, Osterman V, Krivec U, Lo Vecchio A, Shingadia D, Soriano-Arandes A, Melendo S, Lanari M, Pierantoni L, Wagner N, L'Huillier AG, Heininger U, Ritz N, Bandi S, Krajcar N, Roglić S, Santos M, Christiaens C, Creuven M, Buonsenso D, Welch SB, Bogyi M, Brinkmann F, Tebruegge M | title = COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study | journal = The Lancet. Child & Adolescent Health | volume = 4 | issue = 9 | pages = 653–661 | date = September 2020 | pmid = 32593339 | pmc = 7316447 | doi = 10.1016/S2352-4642(20)30177-2 }}</ref> Four of the 582 children (0.7%) in the European study died, but the actual mortality rate may be "substantially lower" since milder cases that did not seek medical help were not included in the study.<ref>{{#invoke:cite journal || vauthors = Fang L, Karakiulakis G, Roth M | title = Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? | journal = The Lancet. Respiratory Medicine | volume = 8 | issue = 4 | pages = e21 | date = April 2020 | pmid = 32171062 | pmc = 7118626 | doi = 10.1016/S0140-6736(20)30311-1 }}</ref><ref name="CDC 2020children">{{#invoke:Cite web||url=https://www.cdc.gov/coronavirus/2019-ncov/specific-groups/children-faq.html|title=Coronavirus Disease 2019 (COVID-19)|date=11 February 2020|website=U.S. ] (CDC) |access-date=2 March 2020|archive-url=https://web.archive.org/web/20200302064104/https://www.cdc.gov/coronavirus/2019-ncov/specific-groups/children-faq.html|archive-date=2 March 2020|url-status=live}}</ref> | |||
| === Long-term effects === | |||
| {{Further|Long COVID}} | |||
| Around 10% to 30% of non-hospitalised people with COVID-19 go on to develop ]. For those that do need hospitalisation, the incidence of long-term effects is over 50%.<ref name="davis">{{#invoke:cite journal ||vauthors=Davis HE, McCorkell L, Vogel JM, Topol EJ |date=March 2023 |title=Long COVID: major findings, mechanisms and recommendations |journal=Nature Reviews. Microbiology |volume=21 |issue=3 |pages=133–146 |doi=10.1038/s41579-022-00846-2 |pmc=9839201 |pmid=36639608}}</ref> Long COVID is an often severe multisystem disease with a large set of symptoms. There are likely various, possibly coinciding, causes.<ref name="davis" /> Organ damage from the acute infection can explain a part of the symptoms, but long COVID is also observed in people where organ damage seems to be absent.<ref name="pmid35594336">{{#invoke:cite journal ||vauthors=Castanares-Zapatero D, Chalon P, Kohn L, Dauvrin M, Detollenaere J, Maertens de Noordhout C, Primus-de Jong C, Cleemput I, Van den Heede K |date=December 2022 |title=Pathophysiology and mechanism of long COVID: a comprehensive review |url= |journal=Annals of Medicine |volume=54 |issue=1 |pages=1473–1487 |doi=10.1080/07853890.2022.2076901 |pmc=9132392 |pmid=35594336}}</ref> | |||
| By a variety of mechanisms, the lungs are the organs most affected in COVID{{nbhyph}}19.<ref name="Torres">{{#invoke:cite journal || vauthors = Torres-Castro R, Vasconcello-Castillo L, Alsina-Restoy X, Solis-Navarro L, Burgos F, Puppo H, Vilaró J | title = Respiratory function in patients post-infection by COVID-19: a systematic review and meta-analysis | journal = Pulmonology | date = November 2020 | volume = 27 | issue = 4 | pages = 328–337 | pmid = 33262076 | pmc = 7687368 | doi = 10.1016/j.pulmoe.2020.10.013 | publisher = Elsevier BV | s2cid = 227162748 }}</ref> In people requiring hospital admission, up to 98% of CT scans performed show lung abnormalities after 28 days of illness even if they had clinically improved.<ref>{{#invoke:cite journal || vauthors = Shaw B, Daskareh M, Gholamrezanezhad A | title = The lingering manifestations of COVID-19 during and after convalescence: update on long-term pulmonary consequences of coronavirus disease 2019 (COVID-19) | journal = La Radiologia Medica | volume = 126 | issue = 1 | pages = 40–46 | date = January 2021 | pmid = 33006087 | pmc = 7529085 | doi = 10.1007/s11547-020-01295-8 }}</ref> People with advanced age, severe disease, prolonged ICU stays, or who smoke are more likely to have long-lasting effects, including pulmonary fibrosis.<ref name="Rai">{{#invoke:cite journal || vauthors = Zhao YM, Shang YM, Song WB, Li QQ, Xie H, Xu QF, Jia JL, Li LM, Mao HL, Zhou XM, Luo H, Gao YF, Xu AG | title = Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery | journal = eClinicalMedicine | volume = 25 | pages = 100463 | date = August 2020 | pmc = 7654356 | doi = 10.1016/j.ijtb.2020.11.003 | pmid = 32838236 }}</ref> Overall, approximately one-third of those investigated after four weeks will have findings of ] or reduced lung function as measured by ], even in asymptomatic people, but with the suggestion of continuing improvement with the passing of more time.<ref name="Torres" /> After severe disease, lung function can take anywhere from three months to a year or more to return to previous levels.<ref>{{#invoke:Cite news||title=COVID-19 Lung Damage |url=https://www.hopkinsmedicine.org/health/conditions-and-diseases/coronavirus/what-coronavirus-does-to-the-lungs |publisher=Johns Hopkins Medicine |access-date=21 May 2022 |date=28 February 2022}}</ref> | |||
| The risks of ], ], psychotic disorders, and ] or seizures persists at an increased level two years after infection.<ref>{{#invoke:cite journal ||vauthors=Taquet M, Sillett R, Zhu L, Mendel J, Camplisson I, Dercon Q, Harrison PJ |date=August 2022 |title=Neurological and psychiatric risk trajectories after SARS-CoV-2 infection: an analysis of 2-year retrospective cohort studies including 1 284 437 patients |journal=The Lancet Psychiatry |doi=10.1016/S2215-0366(22)00260-7 |pmid=35987197 |pmc=9385200 |s2cid=251626731 |issn=2215-0366 |volume=9 |issue=10 |pages=815–827}}</ref> | |||
| === Immunity === | |||
| {{See also|COVID-19 vaccine}} | |||
| ] to SARS-CoV-2 infection]] | |||
| The ] by humans to SARS-CoV-2 virus occurs as a combination of the ] and antibody production,<ref>{{#invoke:Cite web||url=https://www.ecdc.europa.eu/en/covid-19/latest-evidence/immune-responses |title=Immune responses and correlates of protective immunity against SARS-CoV-2 |date=18 May 2021 |publisher=European Centre for Disease Prevention and Control |access-date=3 June 2021}}</ref> just as with most other infections.<ref>{{#invoke:cite journal || vauthors = Vabret N, Britton GJ, Gruber C, Hegde S, Kim J, Kuksin M, Levantovsky R, Malle L, Moreira A, Park MD, Pia L, Risson E, Saffern M, Salomé B, Esai Selvan M, Spindler MP, Tan J, van der Heide V, Gregory JK, Alexandropoulos K, Bhardwaj N, Brown BD, Greenbaum B, Gümüş ZH, Homann D, Horowitz A, Kamphorst AO, Curotto de Lafaille MA, Mehandru S, Merad M, Samstein RM | title = Immunology of COVID-19: Current State of the Science | journal = Immunity | volume = 52 | issue = 6 | pages = 910–941 | date = June 2020 | pmid = 32505227 | pmc = 7200337 | doi = 10.1016/j.immuni.2020.05.002 | doi-access = free | title-link = doi }}</ref> B cells interact with T cells and begin dividing before selection into the plasma cell, partly on the basis of their affinity for antigen.<ref>{{#invoke:cite journal || vauthors = Wang Z, Muecksch F, Schaefer-Babajew D, Finkin S, Viant C, Gaebler C, Hoffmann HH, Barnes CO, Cipolla M, Ramos V, Oliveira TY, Cho A, Schmidt F, Da Silva J, Bednarski E, Aguado L, Yee J, Daga M, Turroja M, Millard KG, Jankovic M, Gazumyan A, Zhao Z, Rice CM, Bieniasz PD, Caskey M, Hatziioannou T, Nussenzweig MC | title = Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection | journal = Nature | volume = 595 | issue = 7867 | pages = 426–431 | date = July 2021 | pmid = 34126625 | pmc = 8277577 | doi = 10.1038/s41586-021-03696-9 | bibcode = 2021Natur.595..426W }}</ref> Since SARS-CoV-2 has been in the human population only since December 2019, it remains unknown if the ] is long-lasting in people who recover from the disease.<ref name="CohenJI2020Dec">{{#invoke:cite journal || vauthors = Cohen JI, Burbelo PD | title = Reinfection with SARS-CoV-2: Implications for Vaccines | journal = Clinical Infectious Diseases | date = December 2020 | pmid = 33338197 | pmc = 7799323 | doi = 10.1093/cid/ciaa1866 | s2cid = 229323810 | title-link = doi | volume = 73 | issue = 11 | pages = e4223–e4228 | doi-access = free }}</ref> The presence of neutralising antibodies in blood strongly correlates with protection from infection, but the level of neutralising antibody declines with time. Those with asymptomatic or mild disease had undetectable levels of neutralising antibody two months after infection. In another study, the level of neutralising antibodies fell four-fold one to four months after the onset of symptoms. However, the lack of antibodies in the blood does not mean antibodies will not be rapidly produced upon reexposure to SARS-CoV-2. Memory B cells specific for the spike and nucleocapsid proteins of SARS-CoV-2 last for at least six months after the appearance of symptoms.<ref name="CohenJI2020Dec" /> | |||
| As of August 2021, reinfection with COVID‑19 was possible but uncommon. The first case of reinfection was documented in August 2020.<ref name="Wang-2021">{{#invoke:cite journal || vauthors = Wang J, Kaperak C, Sato T, Sakuraba A | title = COVID-19 reinfection: a rapid systematic review of case reports and case series | journal = Journal of Investigative Medicine | volume = 69 | issue = 6 | pages = 1253–1255 | date = August 2021 | pmid = 34006572 | doi = 10.1136/jim-2021-001853 |issn=1081-5589 | s2cid = 234773697 }}</ref> A systematic review found 17 cases of confirmed reinfection in medical literature as of May 2021.<ref name="Wang-2021" /> With the Omicron variant, as of 2022, reinfections have become common, albeit it is unclear how common.<ref name="abc-reinfections"/> ]s are thought to likely be less severe than primary infections, especially if one was previously infected by the same variant.<ref name="abc-reinfections">{{#invoke:cite news ||title=How soon after catching COVID-19 can you get it again? |url=https://www.abc.net.au/news/health/2022-05-03/covid-19-reinfection-what-are-the-odds-of-catching-it-twice/101024180 |access-date=24 June 2022 |work=ABC News |date=2 May 2022 }}</ref>{{additional citation needed|date=July 2022}} | |||
| == Mortality == | |||
| {{Main|COVID-19 pandemic|COVID-19 pandemic death rates by country}} | |||
| Several measures are commonly used to quantify mortality.<ref>{{#invoke:cite book||vauthors=((Centers for Disease Control and Prevention)) |chapter-url=https://www.cdc.gov/csels/dsepd/ss1978/lesson3/section3.html |title=Principles of Epidemiology in Public Health Practice |edition=Third |chapter=Lesson 3: Measures of Risk Section 3: Mortality Frequency Measures |date=May 2012|publisher=U.S. ] (CDC)|access-date=28 March 2020|archive-date=28 February 2020|archive-url= https://web.archive.org/web/20200228150607/https://www.cdc.gov/csels/dsepd/ss1978/lesson3/section3.html |url-status=live |id=No. SS1978}}</ref> These numbers vary by region and over time and are influenced by the volume of testing, healthcare system quality, treatment options, time since the initial outbreak, and population characteristics such as age, sex, and overall health.<ref name=":0">{{#invoke:cite journal ||url=https://ourworldindata.org/covid-mortality-risk |title=What do we know about the risk of dying from COVID-19? |vauthors=Ritchie H, Roser M |date=25 March 2020 |veditors=Chivers T |journal=] |url-status=live |access-date=28 March 2020 |archive-date=28 March 2020 |archive-url=https://web.archive.org/web/20200328192730/https://ourworldindata.org/covid-mortality-risk}}</ref> | |||
| The ] reflects the number of deaths within a specific demographic group divided by the population of that demographic group. Consequently, the mortality rate reflects the prevalence as well as the severity of the disease within a given population. Mortality rates are highly correlated to age, with relatively low rates for young people and relatively high rates among the elderly.<ref name="JAMAPedsCOVID19">{{#invoke:cite journal || vauthors = Castagnoli R, Votto M, Licari A, Brambilla I, Bruno R, Perlini S, Rovida F, Baldanti F, Marseglia GL | title = Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection in Children and Adolescents: A Systematic Review | journal = JAMA Pediatrics | volume = 174 | issue = 9 | pages = 882–889 | date = September 2020 | pmid = 32320004 | doi = 10.1001/jamapediatrics.2020.1467 | doi-access = free | title-link = doi }}</ref><ref name="Lu Zhang Du Zhang p.">{{#invoke:cite journal || vauthors = Lu X, Zhang L, Du H, Zhang J, Li YY, Qu J, Zhang W, Wang Y, Bao S, Li Y, Wu C, Liu H, Liu D, Shao J, Peng X, Yang Y, Liu Z, Xiang Y, Zhang F, Silva RM, Pinkerton KE, Shen K, Xiao H, Xu S, Wong GW | title = SARS-CoV-2 Infection in Children | journal = The New England Journal of Medicine | volume = 382 | issue = 17 | pages = 1663–1665 | date = April 2020 | pmid = 32187458 | pmc = 7121177 | doi = 10.1056/nejmc2005073 | publisher = Massachusetts Medical Society }}</ref><ref name="pediatrics_tong">{{#invoke:cite journal || vauthors = Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z, Tong S | title = Epidemiology of COVID-19 Among Children in China | journal = Pediatrics | volume = 145 | issue = 6 | pages = e20200702 | date = June 2020 | pmid = 32179660 | doi = 10.1542/peds.2020-0702 | s2cid = 219118986 | doi-access = free | title-link = doi }}</ref> In fact, one relevant factor of mortality rates is the age structure of the countries' populations. For example, the case fatality rate for COVID‑19 is lower in India than in the US since India's younger population represents a larger percentage than in the US.<ref name="Dehingia-2021">{{#invoke:cite journal||title=Sex differences in COVID-19 case fatality: do we know enough?|journal=The Lancet. Global Health|vauthors=Dehingia N|year=2021|volume=9|issue=1|pages=e14–e15|doi=10.1016/S2214-109X(20)30464-2|pmid=33160453|pmc=7834645}}</ref> | |||
| === Case fatality rate === | |||
| The ] (CFR) reflects the number of deaths divided by the number of diagnosed cases within a given time interval. Based on Johns Hopkins University statistics, the global death-to-case ratio is {{Cases in the COVID-19 pandemic|ratio|editlink=|ref=no}} ({{Cases in the COVID-19 pandemic|deaths|editlink=|ref=no}}/{{Cases in the COVID-19 pandemic|confirmed|editlink=|ref=no}}) as of {{Cases in the COVID-19 pandemic|date|editlink=|ref=no}}.{{Cases in the COVID-19 pandemic|ref=yes}} The number varies by region.<ref>{{#invoke:cite journal || vauthors = Lazzerini M, Putoto G | title = COVID-19 in Italy: momentous decisions and many uncertainties | journal = The Lancet. Global Health | volume = 8 | issue = 5 | pages = e641–e642 | date = May 2020 | pmid = 32199072 | pmc = 7104294 | doi = 10.1016/S2214-109X(20)30110-8 }}</ref><ref name=":0" /> | |||
| <gallery mode="packed" heights=140> | |||
| Cumulative confirmed COVID-19 cases.svg|Total confirmed cases over time | |||
| World map of total confirmed COVID-19 cases per million people.svg|Total confirmed cases of COVID‑19 per million people<ref>{{#invoke:Cite web||title=Total confirmed cases of COVID-19 per million people |url=https://ourworldindata.org/grapher/total-confirmed-cases-of-covid-19-per-million-people |website=Our World in Data |access-date=21 June 2022 |archive-url=https://web.archive.org/web/20200319163452/https://ourworldindata.org/grapher/total-confirmed-cases-of-covid-19-per-million-people |archive-date=19 March 2020 |url-status=live}}{{update inline|reason=referenced page and the image updated without coordination, archived version obsolete|month=August 2020|date=August 2020}}</ref> | |||
| Daily and total confirmed COVID-19 deaths, World.svg|Total confirmed deaths over time | |||
| World map of total confirmed COVID-19 deaths per million people by country.svg|Total confirmed deaths due to COVID‑19 per million people<ref>{{#invoke:Cite web||title=Cumulative confirmed COVID-19 deaths per million people |url=https://ourworldindata.org/grapher/total-covid-deaths-per-million |website=] }}</ref> | |||
| </gallery> | |||
| === Infection fatality rate === | |||
| A key metric in gauging the severity of COVID‑19 is the ] (IFR), also referred to as the ''infection fatality ratio'' or ''infection fatality risk''.<ref>{{#invoke:cite journal || vauthors = Mallapaty S | title = How deadly is the coronavirus? Scientists are close to an answer | journal = Nature | volume = 582 | issue = 7813 | pages = 467–468 | date = June 2020 | pmid = 32546810 | doi = 10.1038/d41586-020-01738-2 | s2cid = 219726496 | doi-access = free | title-link = doi | bibcode = 2020Natur.582..467M }}</ref><ref>{{#invoke:cite journal || vauthors = Alwan NA, Burgess RA, Ashworth S, Beale R, Bhadelia N, Bogaert D, Dowd J, Eckerle I, Goldman LR, Greenhalgh T, Gurdasani D, Hamdy A, Hanage WP, Hodcroft EB, Hyde Z, Kellam P, Kelly-Irving M, Krammer F, Lipsitch M, McNally A, McKee M, Nouri A, Pimenta D, Priesemann V, Rutter H, Silver J, Sridhar D, Swanton C, Walensky RP, Yamey G, Ziauddeen H | title = Scientific consensus on the COVID-19 pandemic: we need to act now | journal = Lancet | volume = 396 | issue = 10260 | pages = e71–e72 | date = October 2020 | pmid = 33069277 | pmc = 7557300 | doi = 10.1016/S0140-6736(20)32153-X }}</ref><ref>{{#invoke:cite journal || vauthors = Meyerowitz-Katz G, Merone L | title = A systematic review and meta-analysis of published research data on COVID-19 infection fatality rates | journal = International Journal of Infectious Diseases | volume = 101 | pages = 138–148 | date = December 2020 | pmid = 33007452 | pmc = 7524446 | doi = 10.1016/j.ijid.2020.09.1464 }}</ref> This metric is calculated by dividing the total number of deaths from the disease by the total number of infected individuals; hence, in contrast to the ], the IFR incorporates asymptomatic and undiagnosed infections as well as reported cases.<ref name="urlGeneralized trapezoidal ogive curves for fatality rate modeling">{{#invoke:cite journal || vauthors = Zhang D, Hu M, Ji Q | title = Financial markets under the global pandemic of COVID-19 | journal = Finance Research Letters | volume = 36 | pages = 101528 | date = October 2020 | pmc = 7402242 | doi = 10.1016/j.csfx.2020.100043 | pmid = 32837360 | bibcode = 2020CSFX....500043D }}</ref> | |||
| ==== Estimates ==== | |||
| ] | |||
| ] | |||
| A December 2020 systematic review and meta-analysis estimated that population IFR during the first wave of the pandemic was about 0.5% to 1% in many locations (including France, Netherlands, New Zealand, and Portugal), 1% to 2% in other locations (Australia, England, Lithuania, and Spain), and exceeded 2% in Italy.<ref name="EJE_levinetal">{{#invoke:cite journal || vauthors = Levin AT, Hanage WP, Owusu-Boaitey N, Cochran KB, Walsh SP, Meyerowitz-Katz G | title = Assessing the age specificity of infection fatality rates for COVID-19: systematic review, meta-analysis, and public policy implications | journal = European Journal of Epidemiology | volume = 35 | issue = 12 | pages = 1123–1138 | date = December 2020 | pmid = 33289900 | pmc = 7721859 | doi = 10.1007/s10654-020-00698-1 | doi-access = free | title-link = doi }} ] Text was copied from this source, which is available under a {{Webarchive|url=https://web.archive.org/web/20171016050101/https://creativecommons.org/licenses/by/4.0/ |date=16 October 2017 }}.</ref> That study also found that most of these differences in IFR reflected corresponding differences in the age composition of the population and age-specific infection rates; in particular, the metaregression estimate of IFR is very low for children and younger adults (e.g., 0.002% at age 10 and 0.01% at age 25) but increases progressively to 0.4% at age 55, 1.4% at age 65, 4.6% at age 75, and 15% at age 85.<ref name="EJE_levinetal" /> These results were also highlighted in a December 2020 report issued by the WHO.<ref>{{#invoke:cite journal||title=Background paper on Covid-19 disease and vaccines: prepared by the Strategic Advisory Group of Experts (SAGE) on immunization working group on COVID-19 vaccines|date=22 December 2020|url=https://apps.who.int/iris/handle/10665/338095|website=] (WHO) |hdl=10665/338095 | author=World Health Organization }}</ref> | |||
| {| class="wikitable" | {| class="wikitable" | ||
| |+ class="nowrap"| IFR estimate per age group<br />(to December 2020)<ref name="EJE_levinetal" /> | |||
| |+Case fatality rates (%) by age and country | |||
| ! Age group !! IFR | |||
| |- | |- | ||
| | 0–34 || 0.004% | |||
| !Age | |||
| !0–9 | |||
| !10–19 | |||
| !20–29 | |||
| !30–39 | |||
| !40–49 | |||
| !50–59 | |||
| !60–69 | |||
| !70–79 | |||
| !80-89 | |||
| !90+ | |||
| |- | |- | ||
| | 35–44 || 0.068% | |||
| |China as of 11 February<ref name="Epidemiology2020Feb17" /> | |||
| |style="text-align:center;"|0.0 | |||
| |style="text-align:center;"|0.2 | |||
| |style="text-align:center;"|0.2 | |||
| |style="text-align:center;"|0.2 | |||
| |style="text-align:center;"|0.4 | |||
| |style="text-align:center;"|1.3 | |||
| |style="text-align:center;"|3.6 | |||
| |style="text-align:center;"|8.0 | |||
| |colspan="2" style="text-align:center;"|14.8 | |||
| |- | |- | ||
| | 45–54 || 0.23% | |||
| |Denmark as of 6 April<ref name="SSIReport">{{cite report|url=https://www.ssi.dk/-/media/ssi-files/covid19-overvaagningsrapport-06042020-hu4v.pdf|title=COVID-19 i Danmark: Epidemiologisk overvågningsrapport den 6. april 2020|date=6 April 2020|publisher=]|language=Danish|access-date=6 April 2020|url-status=live}}</ref> | |||
| |colspan="6" style="text-align:center;"|0.2 | |||
| |style="text-align:center;"|3.2 | |||
| |style="text-align:center;"|11.0 | |||
| |style="text-align:center;"|20.3 | |||
| |style="text-align:center;"|32.6 | |||
| |- | |- | ||
| | 55–64 || 0.75% | |||
| |Italy as of 6 April<ref name="ISSReport">{{cite report|url=https://www.epicentro.iss.it/coronavirus/bollettino/Bollettino-sorveglianza-integrata-COVID-19_6-aprile-2020.pdf|title=Epidemia COVID-19. Aggiornamento nazionale 6 aprile 2020|last=|first=|date=6 April 2020|publisher=]|issue=|doi=|location=Rome|volume=|language=Italian|pmid=|access-date=7 April 2020|url-status=live}}</ref> | |||
| |style="text-align:center;"|0.1 | |||
| |style="text-align:center;"|0.0 | |||
| |style="text-align:center;"|0.1 | |||
| |style="text-align:center;"|0.4 | |||
| |style="text-align:center;"|0.8 | |||
| |style="text-align:center;"|2.3 | |||
| |style="text-align:center;"|8.4 | |||
| |style="text-align:center;"|22.7 | |||
| |style="text-align:center;"|30.6 | |||
| |style="text-align:center;"|26.8 | |||
| |- | |- | ||
| | 65–74 || 2.5% | |||
| |Netherlands as of 3 April<ref name="RIVMReport">{{cite report|url=https://www.rivm.nl/sites/default/files/2020-04/COVID-19_WebSite_rapport_20200406_1005_1.pdf|title=Epidemiologische situatie COVID-19 in Nederland 06 april 2020|date=6 April 2020|publisher=]|location=Bilthoven|language=Dutch|access-date=6 April 2020|url-status=live}}</ref> | |||
| |style="text-align:center;"|0.0 | |||
| |style="text-align:center;"|0.0 | |||
| |style="text-align:center;"|0.1 | |||
| |style="text-align:center;"|0.1 | |||
| |style="text-align:center;"|0.4 | |||
| |style="text-align:center;"|1.2 | |||
| |style="text-align:center;"|6.2 | |||
| |style="text-align:center;"|16.0 | |||
| |style="text-align:center;"|25.1 | |||
| |style="text-align:center;"|22.0 | |||
| |- | |- | ||
| | 75–84 || 8.5% | |||
| |South Korea as of 5 April<ref name="KCDCReport">{{cite report|url=https://www.cdc.go.kr/board/board.es?mid=a30402000000&bid=0030|title=The updates on COVID-19 in Korea as of 5 April|date=5 April 2020|publisher=]|access-date=6 April 2020|url-status=live}}</ref> | |||
| |style="text-align:center;"|0.0 | |||
| |style="text-align:center;"|0.0 | |||
| |style="text-align:center;"|0.0 | |||
| |style="text-align:center;"|0.1 | |||
| |style="text-align:center;"|0.2 | |||
| |style="text-align:center;"|0.7 | |||
| |style="text-align:center;"|1.9 | |||
| |style="text-align:center;"|7.5 | |||
| |colspan="2" style="text-align:center;"|19.7 | |||
| |- | |- | ||
| | 85 + || 28.3% | |||
| |Spain as of 5 April<ref name="MSCBSReport">{{cite report|url=https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov-China/documentos/Actualizacion_67_COVID-19.pdf|title=Actualización nº 67. Enfermedad por el coronavirus (COVID-19).|date=6 April 2020|publisher=]|language=Spanish|access-date=6 April 2020|url-status=live}}</ref> | |||
| |style="text-align:center;"|0.4 | |||
| |style="text-align:center;"|0.2 | |||
| |style="text-align:center;"|0.1 | |||
| |style="text-align:center;"|0.2 | |||
| |style="text-align:center;"|0.4 | |||
| |style="text-align:center;"|0.9 | |||
| |style="text-align:center;"|2.8 | |||
| |style="text-align:center;"|9.4 | |||
| |style="text-align:center;"|18.9 | |||
| |style="text-align:center;"|22.9 | |||
| |} | |} | ||
| An analysis of those IFR rates indicates that COVID{{nbhyph}}19 is hazardous not only for the elderly but also for middle-aged adults, for whom the infection fatality rate of COVID-19 is two orders of magnitude greater than the annualised risk of a fatal automobile accident and far more dangerous than seasonal ].<ref name="EJE_levinetal" /> | |||
| {| class="wikitable" | |||
| |+Case fatality rates (%) by age in the United States | |||
| ==== Earlier estimates of IFR ==== | |||
| At an early stage of the pandemic, the World Health Organization reported estimates of IFR between 0.3% and 1%.<ref>{{#invoke:Cite web||title=Coronavirus disease 2019 (COVID-19) Situation Report – 30 |url=https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200219-sitrep-30-covid-19.pdf |access-date=3 June 2020 |date=19 February 2020}}</ref><ref>{{#invoke:Cite web||title=Coronavirus disease 2019 (COVID-19) Situation Report – 31 |url=https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200220-sitrep-31-covid-19.pdf |access-date=23 April 2020 |date=20 February 2020}}</ref> On 2{{spaces}}July, The WHO's chief scientist reported that the average IFR estimate presented at a two-day WHO expert forum was about 0.6%.<ref name="NYT-20200704dm">{{#invoke:cite news || vauthors = McNeil Jr DG |title=The Pandemic's Big Mystery: How Deadly Is the Coronavirus? – Even with more than 500,000 dead worldwide, scientists are struggling to learn how often the virus kills. Here's why |url=https://www.nytimes.com/2020/07/04/health/coronavirus-death-rate.html |archive-url=https://web.archive.org/web/20200704152005/https://www.nytimes.com/2020/07/04/health/coronavirus-death-rate.html |archive-date=4 July 2020 |url-access=subscription |url-status=live |date=4 July 2020 |work=The New York Times |access-date=6 July 2020}}</ref><ref>{{#invoke:cite news ||title=Global Research and Innovation Forum on COVID-19: Virtual Press Conference |url=https://www.who.int/docs/default-source/coronaviruse/virtual-press-conference---2-july---update-on-covid-19-r-d.pdf |publisher=] (WHO) |date=2 July 2020}}</ref> In August, the WHO found that studies incorporating data from broad serology testing in Europe showed IFR estimates converging at approximately 0.5–1%.<ref>{{#invoke:Cite web||title=Estimating mortality from COVID-19|url=https://www.who.int/news-room/commentaries/detail/estimating-mortality-from-covid-19|access-date=21 September 2020|website=] (WHO)}}</ref> Firm lower limits of IFRs have been established in a number of locations such as New York City and Bergamo in Italy since the IFR cannot be less than the population fatality rate. (After sufficient time however, people can get reinfected).<ref>{{#invoke:cite journal|| vauthors = Shaffer C |date=23 October 2021|title=Covid-19 still rife in Iran |journal=New Scientist|volume=252|issue=3357|pages=10–11|doi=10.1016/S0262-4079(21)01865-0|pmid=34720322|issn=0262-4079|pmc=8536311|bibcode=2021NewSc.252...10S}}</ref> As of 10 July, in New York City, with a population of 8.4 million, 23,377 individuals (18,758 confirmed and 4,619 probable) have died with COVID‑19 (0.3% of the population).<ref>{{#invoke:Cite web||title=COVID-19: Data |url=https://www1.nyc.gov/site/doh/covid/covid-19-data.page |publisher=City of New York}}</ref> Antibody testing in New York City suggested an IFR of ≈0.9%,<ref>{{#invoke:cite SSRN||title=SARS-CoV-2, COVID-19, Infection Fatality Rate (IFR) Implied by the Serology, Antibody, Testing in New York City| vauthors = Wilson L |date=May 2020|ssrn=3590771}}</ref> and ≈1.4%.<ref>{{#invoke:cite journal || vauthors = Yang W, Kandula S, Huynh M, Greene SK, Van Wye G, Li W, Chan HT, McGibbon E, Yeung A, Olson D, Fine A, Shaman J | title = Estimating the infection-fatality risk of SARS-CoV-2 in New York City during the spring 2020 pandemic wave: a model-based analysis | journal = The Lancet. Infectious Diseases | volume = 21 | issue = 2 | pages = 203–212 | date = February 2021 | pmid = 33091374 | pmc = 7572090 | doi = 10.1016/s1473-3099(20)30769-6 }}</ref> In ], 0.6% of the population has died.<ref>{{#invoke:Cite web||url=https://medium.com/bccp-uc-berkeley/how-deadly-is-covid-19-data-science-offers-answers-from-italy-mortality-data-58abedf824cf|title=How deadly is COVID-19? Data Science offers answers from Italy mortality data.| vauthors = Modi C |date=21 April 2020 |website=Medium |access-date=23 April 2020}}</ref> In September 2020, the U.S. Centers for Disease Control and Prevention (CDC) reported preliminary estimates of age-specific IFRs for public health planning purposes.<ref name=":1">{{#invoke:Cite web||title=Coronavirus Disease 2019 (COVID-19) |url=https://www.cdc.gov/coronavirus/2019-ncov/hcp/planning-scenarios.html |website=U.S. ] (CDC) |access-date=9 December 2020 |date=10 September 2020}}</ref> | |||
| === Sex differences === | |||
| {{Main|Gendered impact of the COVID-19 pandemic}} | |||
| {| class="wikitable collapsible collapsed" style="margin-left:1em; font-size: 90%; float:right; clear:right" | |||
| |+ class="nowrap" | Estimated prognosis by age and sex<br />based on cases from ]<br />and ]<ref>{{#invoke:cite journal || vauthors = Salje H, Tran Kiem C, Lefrancq N, Courtejoie N, Bosetti P, Paireau J, Andronico A, Hozé N, Richet J, Dubost CL, Le Strat Y, Lessler J, Levy-Bruhl D, Fontanet A, Opatowski L, Boelle PY, Cauchemez S | title = Estimating the burden of SARS-CoV-2 in France | journal = Science | volume = 369 | issue = 6500 | pages = 208–211 | date = July 2020 | pmid = 32404476 | pmc = 7223792 | doi = 10.1126/science.abc3517 | title-link = doi | doi-access = free | bibcode = 2020Sci...369..208S }}</ref> | |||
| |- | |- | ||
| ! colspan=10| Percentage of infected people who are hospitalised | |||
| !Age | |||
| !0–19 | |||
| !20–44 | |||
| !45–54 | |||
| !55–64 | |||
| !65–74 | |||
| !75–84 | |||
| !85+ | |||
| |- | |- | ||
| ! 0–19 !! 20–29 !! 30–39 !! 40–49 !! 50–59 !! 60–69 !! 70–79 !! 80+ !! Total | |||
| |United States as of 16 March<ref name="CDCMMWR18Mar2020">{{cite journal|author=CDC COVID-19 Response Team|title=Severe Outcomes Among Patients with Coronavirus Disease 2019 (COVID-19)—United States, February 12 – March 16, 2020|url=https://www.cdc.gov/mmwr/volumes/69/wr/mm6912e2.htm|journal=]|volume=69|issue=12|pages=343–346|date=18 March 2020|publisher=]|doi=10.15585/mmwr.mm6912e2|pmid=32214079|access-date=22 March 2020|archive-url=https://web.archive.org/web/20200322021219/https://www.cdc.gov/mmwr/volumes/69/wr/mm6912e2.htm|archive-date=22 March 2020|url-status=live}}</ref> | |||
| |style="text-align:center;"|0.0 | |||
| |style="text-align:center;"|0.1–0.2 | |||
| |style="text-align:center;"|0.5–0.8 | |||
| |style="text-align:center;"|1.4–2.6 | |||
| |style="text-align:center;"|2.7–4.9 | |||
| |style="text-align:center;"|4.3–10.5 | |||
| |style="text-align:center;"|10.4–27.3 | |||
| |- | |- | ||
| ! Female | |||
| | colspan="8" |Note: The lower bound includes all cases. The upper bound excludes cases that were missing data. | |||
| | {{shade|0.1}}<br /><small>(0.07–0.2)</small> | |||
| |} | |||
| | {{shade|0.5}}<br /><small>(0.3–0.8)</small> | |||
| | {{shade|0.9}}<br /><small>(0.5–1.5)</small> | |||
| {| class="wikitable" | |||
| | {{shade|1.3}}<br /><small>(0.7–2.1)</small> | |||
| |+Estimate of infection fatality rates and probability of severe disease course (%) by age based on cases from China<ref>{{Cite journal|last=Verity|first=Robert|last2=Okell|first2=Lucy C|last3=Dorigatti|first3=Ilaria|last4=Winskill|first4=Peter|last5=Whittaker|first5=Charles|last6=Imai|first6=Natsuko|last7=Cuomo-Dannenburg|first7=Gina|last8=Thompson|first8=Hayley|last9=Walker|first9=Patrick G T|last10=Fu|first10=Han|last11=Dighe|first11=Amy|date=30 March 2020|title=Estimates of the severity of coronavirus disease 2019: a model-based analysis|journal=The Lancet Infectious Diseases|doi=10.1016/s1473-3099(20)30243-7|doi-access=free|issn=1473-3099}}</ref> | |||
| | {{shade|2.6}}<br /><small>(1.5–4.2)</small> | |||
| | {{shade|5.1}}<br /><small>(2.9–8.3)</small> | |||
| | {{shade|7.8}}<br /><small>(4.4–12.8)</small> | |||
| | {{shade|19.3}}<br /><small>(10.9–31.6)</small> | |||
| | {{shade|2.6}}<br /><small>(1.5–4.3)</small> | |||
| |- | |- | ||
| ! Male | |||
| ! | |||
| | {{shade|0.2}}<br /><small>(0.08–0.2)</small> | |||
| !0–9 | |||
| | {{shade|0.6}}<br /><small>(0.3–0.9)</small> | |||
| !10–19 | |||
| | {{shade|1.2}}<br /><small>(0.7–1.9)</small> | |||
| !20–29 | |||
| | {{shade|1.6}}<br /><small>(0.9–2.6)</small> | |||
| !30–39 | |||
| | {{shade|3.2}}<br /><small>(1.8–5.2)</small> | |||
| !40–49 | |||
| | {{shade|6.7}}<br /><small>(3.7–10.9)</small> | |||
| !50–59 | |||
| | {{shade|11.0}}<br /><small>(6.2–17.9)</small> | |||
| !60–69 | |||
| | {{shade|37.6}}<br /><small>(21.1–61.3)</small> | |||
| !70–79 | |||
| | {{shade|3.3}}<br /><small>(1.8–5.3)</small> | |||
| !80+ | |||
| |- | |- | ||
| ! Total | |||
| !Severe disease | |||
| | |
| {{shade|0.1}}<br /><small>(0.08–0.2)</small> | ||
| | |
| {{shade|0.5}}<br /><small>(0.3–0.8)</small> | ||
| | |
| {{shade|1.1}}<br /><small>(0.6–1.7)</small> | ||
| | |
| {{shade|1.4}}<br /><small>(0.8–2.3)</small> | ||
| | |
| {{shade|2.9}}<br /><small>(1.6–4.7)</small> | ||
| | |
| {{shade|5.8}}<br /><small>(3.3–9.5)</small> | ||
| | |
| {{shade|9.3}}<br /><small>(5.2–15.1)</small> | ||
| | |
| {{shade|26.2}}<br /><small>(14.8–42.7)</small> | ||
| | |
| {{shade|2.9}}<br /><small>(1.7–4.8)</small> | ||
| |- | |- | ||
| ! colspan=10| Percentage of hospitalised people who go to Intensive Care Unit | |||
| !Death | |||
| |style="text-align:center;"|0.0016<br /><small>(0.00016–0.025)</small> | |||
| |style="text-align:center;"|0.0070<br /><small>(0.0015–0.050)</small> | |||
| |style="text-align:center;"|0.031<br /><small>(0.014–0.092)</small> | |||
| |style="text-align:center;"|0.084<br /><small>(0.041–0.19)</small> | |||
| |style="text-align:center;"|0.16<br /><small>(0.076–0.32)</small> | |||
| |style="text-align:center;"|0.60<br /><small>(0.34–1.3)</small> | |||
| |style="text-align:center;"|1.9<br /><small>(1.1–3.9)</small> | |||
| |style="text-align:center;"|4.3<br /><small>(2.5–8.4)</small> | |||
| |style="text-align:center;"|7.8<br /><small>(3.8–13)</small> | |||
| |- | |- | ||
| ! 0–19 !! 20–29 !! 30–39 !! 40–49 !! 50–59 !! 60–69 !! 70–79 !! 80+ !! Total | |||
| |colspan=10| Total infection fatality rate is estimated to be 0.66% (0.39–1.3). Infection fatality rate is fatality per all infected individuals, regardless of whether they were diagnosed or had any symptoms. Numbers in parentheses are 95% ]s for the estimates. | |||
| |- | |||
| ! Female | |||
| | {{shade|16.7}}<br /><small>(14.3–19.3)</small> | |||
| | {{shade|8.7}}<br /><small>(7.5–9.9)</small> | |||
| | {{shade|11.9}}<br /><small>(10.9–13.0)</small> | |||
| | {{shade|16.6}}<br /><small>(15.6–17.7)</small> | |||
| | {{shade|20.7}}<br /><small>(19.8–21.6)</small> | |||
| | {{shade|23.1}}<br /><small>(22.2–24.0)</small> | |||
| | {{shade|18.7}}<br /><small>(18.0–19.5)</small> | |||
| | {{shade|4.2}}<br /><small>(4.0–4.5)</small> | |||
| | {{shade|14.3}}<br /><small>(13.9–14.7)</small> | |||
| |- | |||
| ! Male | |||
| | {{shade|26.9}}<br /><small>(23.1–31.1)</small> | |||
| | {{shade|14.0}}<br /><small>(12.2–16.0)</small> | |||
| | {{shade|19.2}}<br /><small>(17.6–20.9)</small> | |||
| | {{shade|26.9}}<br /><small>(25.4–28.4)</small> | |||
| | {{shade|33.4}}<br /><small>(32.0–34.8)</small> | |||
| | {{shade|37.3}}<br /><small>(36.0–38.6)</small> | |||
| | {{shade|30.2}}<br /><small>(29.1–31.3)</small> | |||
| | {{shade|6.8}}<br /><small>(6.5–7.2)</small> | |||
| | {{shade|23.1}}<br /><small>(22.6–23.6)</small> | |||
| |- | |||
| ! Total | |||
| | {{shade|22.2}}<br /><small>(19.1–25.7)</small> | |||
| | {{shade|11.6}}<br /><small>(10.1–13.2)</small> | |||
| | {{shade|15.9}}<br /><small>(14.5–17.3)</small> | |||
| | {{shade|22.2}}<br /><small>(21.0–23.5)</small> | |||
| | {{shade|27.6}}<br /><small>(26.5–28.7)</small> | |||
| | {{shade|30.8}}<br /><small>(29.8–31.8)</small> | |||
| | {{shade|24.9}}<br /><small>(24.1–25.8)</small> | |||
| | {{shade|5.6}}<br /><small>(5.3–5.9)</small> | |||
| | {{shade|19.0}}<br /><small>(18.7–19.44)</small> | |||
| |- | |||
| ! colspan=10| Percent of hospitalised people who die | |||
| |- | |||
| ! 0–19 !! 20–29 !! 30–39 !! 40–49 !! 50–59 !! 60–69 !! 70–79 !! 80+ !! Total | |||
| |- | |||
| ! Female | |||
| | {{shade|0.5}}<br /><small>(0.2–1.0)</small> | |||
| | {{shade|0.9}}<br /><small>(0.5–1.3)</small> | |||
| | {{shade|1.5}}<br /><small>(1.2–1.9)</small> | |||
| | {{shade|2.6}}<br /><small>(2.3–3.0)</small> | |||
| | {{shade|5.2}}<br /><small>(4.8–5.6)</small> | |||
| | {{shade|10.1}}<br /><small>(9.5–10.6)</small> | |||
| | {{shade|16.7}}<br /><small>(16.0–17.4)</small> | |||
| | {{shade|25.2}}<br /><small>(24.4–26.0)</small> | |||
| | {{shade|14.4}}<br /><small>(14.0–14.8)</small> | |||
| |- | |||
| ! Male | |||
| | {{shade|0.7}}<br /><small>(0.3–1.5)</small> | |||
| | {{shade|1.3}}<br /><small>(0.8–1.9)</small> | |||
| | {{shade|2.2}}<br /><small>(1.7–2.7)</small> | |||
| | {{shade|3.8}}<br /><small>(3.3–4.4)</small> | |||
| | {{shade|7.6}}<br /><small>(7.0–8.2)</small> | |||
| | {{shade|14.8}}<br /><small>(14.1–15.6)</small> | |||
| | {{shade|24.6}}<br /><small>(23.7–25.6)</small> | |||
| | {{shade|37.1}}<br /><small>(36.1–38.2)</small> | |||
| | {{shade|21.2}}<br /><small>(20.8–21.7)</small> | |||
| |- | |||
| ! Total | |||
| | {{shade|0.6}}<br /><small>(0.2–1.3)</small> | |||
| | {{shade|1.1}}<br /><small>(0.7–1.6)</small> | |||
| | {{shade|1.9}}<br /><small>(1.5–2.3)</small> | |||
| | {{shade|3.3}}<br /><small>(2.9–3.8)</small> | |||
| | {{shade|6.5}}<br /><small>(6.0–7.0)</small> | |||
| | {{shade|12.6}}<br /><small>(12.0–13.2)</small> | |||
| | {{shade|21.0}}<br /><small>(20.3–21.7)</small> | |||
| | {{shade|31.6}}<br /><small>(30.9–32.4)</small> | |||
| | {{shade|18.1}}<br /><small>(17.8–18.4)</small> | |||
| |- | |||
| ! colspan=10| Percent of infected people who die{{snd}}infection fatality rate (IFR) | |||
| |- | |||
| ! 0–19 !! 20–29 !! 30–39 !! 40–49 !! 50–59 !! 60–69 !! 70–79 !! 80+ !! Total | |||
| |- | |||
| ! Female | |||
| | {{shade|0.001}}<br /><small>(<0.001–0.002)</small> | |||
| | {{shade|0.004}}<br /><small>(0.002–0.007)</small> | |||
| | {{shade|0.01}}<br /><small>(0.007–0.02)</small> | |||
| | {{shade|0.03}}<br /><small>(0.02–0.06)</small> | |||
| | {{shade|0.1}}<br /><small>(0.08–0.2)</small> | |||
| | {{shade|0.5}}<br /><small>(0.3–0.8)</small> | |||
| | {{shade|1.3}}<br /><small>(0.7–2.1)</small> | |||
| | {{shade|4.9}}<br /><small>(2.7–8.0)</small> | |||
| | {{shade|0.4}}<br /><small>(0.2–0.6)</small> | |||
| |- | |||
| ! Male | |||
| | {{shade|0.001}}<br /><small>(<0.001–0.003)</small> | |||
| | {{shade|0.007}}<br /><small>(0.003–0.01)</small> | |||
| | {{shade|0.03}}<br /><small>(0.02–0.05)</small> | |||
| | {{shade|0.06}}<br /><small>(0.03–0.1)</small> | |||
| | {{shade|0.2}}<br /><small>(0.1–0.4)</small> | |||
| | {{shade|1.0}}<br /><small>(0.6–1.6)</small> | |||
| | {{shade|2.7}}<br /><small>(1.5–1.4)</small> | |||
| | {{shade|14.0}}<br /><small>(7.9–22.7)</small> | |||
| | {{shade|0.7}}<br /><small>(0.4–1.1)</small> | |||
| |- | |||
| ! Total | |||
| | {{shade|0.001}}<br /><small>(<0.001–0.002)</small> | |||
| | {{shade|0.005}}<br /><small>(0.003–0.01)</small> | |||
| | {{shade|0.02}}<br /><small>(0.01–0.03)</small> | |||
| | {{shade|0.05}}<br /><small>(0.03–0.08)</small> | |||
| | {{shade|0.2}}<br /><small>(0.1–0.3)</small> | |||
| | {{shade|0.7}}<br /><small>(0.4–1.2)</small> | |||
| | {{shade|1.9}}<br /><small>(1.1–3.2)</small> | |||
| | {{shade|8.3}}<br /><small>(4.7–13.5)</small> | |||
| | {{shade|0.5}}<br /><small>(0.3–0.9)</small> | |||
| |- | |||
| | colspan=10| Numbers in parentheses are 95% ]s for the estimates. | |||
| |} | |} | ||
| COVID‑19 ]s are higher among men than women in most countries. However, in a few countries like India, Nepal, Vietnam, and Slovenia the fatality cases are higher in women than men.<ref name="Dehingia-2021" /> Globally, men are more likely to be admitted to the ] and more likely to die.<ref>{{#invoke:Cite web|| vauthors = McIntosh K |title=Covid 19 Clinical Features|url=https://www.uptodate.com/contents/covid-19-clinical-features|access-date=12 May 2021|website=]|publication-date=April 2021}}</ref><ref>{{#invoke:cite journal || vauthors = Peckham H, de Gruijter NM, Raine C, Radziszewska A, Ciurtin C, Wedderburn LR, Rosser EC, Webb K, Deakin CT | title = Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission | journal = Nature Communications | volume = 11 | issue = 1 | pages = 6317 | date = December 2020 | pmid = 33298944 | doi = 10.1038/s41467-020-19741-6 | pmc = 7726563 | bibcode = 2020NatCo..11.6317P }}</ref> One meta-analysis found that globally, men were more likely to get COVID‑19 than women; there were approximately 55 men and 45 women per 100 infections (]: 51.43–56.58).<ref>{{#invoke:cite journal || vauthors = Abate BB, Kassie AM, Kassaw MW, Aragie TG, Masresha SA | title = Sex difference in coronavirus disease (COVID-19): a systematic review and meta-analysis | journal = BMJ Open | volume = 10 | issue = 10 | pages = e040129 | date = October 2020 | pmid = 33028563 | doi = 10.1136/bmjopen-2020-040129 | pmc = 7539579 }}</ref> | |||
| The ] reported the death rate was 2.8% for men and 1.7% for women.<ref name="Epidemiology17Feb2020"/> Later reviews in June 2020 indicated that there is no significant difference in susceptibility or in CFR between genders.<ref>{{#invoke:cite journal || vauthors = Hu Y, Sun J, Dai Z, Deng H, Li X, Huang Q, Wu Y, Sun L, Xu Y | title = Prevalence and severity of corona virus disease 2019 (COVID-19): A systematic review and meta-analysis | journal = Journal of Clinical Virology | volume = 127 | pages = 104371 | date = June 2020 | pmid = 32315817 | pmc = 7195434 | doi = 10.1016/j.jcv.2020.104371 }}</ref><ref>{{#invoke:cite journal || vauthors = Fu L, Wang B, Yuan T, Chen X, Ao Y, Fitzpatrick T, Li P, Zhou Y, Lin YF, Duan Q, Luo G, Fan S, Lu Y, Feng A, Zhan Y, Liang B, Cai W, Zhang L, Du X, Li L, Shu Y, Zou H | title = Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: A systematic review and meta-analysis | journal = The Journal of Infection | volume = 80 | issue = 6 | pages = 656–665 | date = June 2020 | pmid = 32283155 | pmc = 7151416 | doi = 10.1016/j.jinf.2020.03.041 }}</ref> One review acknowledges the different mortality rates in Chinese men, suggesting that it may be attributable to lifestyle choices such as smoking and drinking alcohol rather than genetic factors.<ref>{{#invoke:cite journal || vauthors = Yuki K, Fujiogi M, Koutsogiannaki S | title = COVID-19 pathophysiology: A review | journal = Clinical Immunology | volume = 215 | pages = 108427 | date = June 2020 | pmid = 32325252 | pmc = 7169933 | doi = 10.1016/j.clim.2020.108427 | s2cid = 216028003 }}</ref> Smoking, which in some countries like China is mainly a male activity, is a habit that contributes to increasing significantly the case fatality rates among men.<ref name="Dehingia-2021" /> Sex-based immunological differences, lesser prevalence of smoking in women and men developing co-morbid conditions such as hypertension at a younger age than women could have contributed to the higher mortality in men.<ref name="nyt-italy">{{#invoke:cite news || vauthors = Rabin RC | title = In Italy, Coronavirus Takes a Higher Toll on Men |url=https://www.nytimes.com/2020/03/20/health/coronavirus-italy-men-risk.html |archive-url=https://web.archive.org/web/20200320214013/https://www.nytimes.com/2020/03/20/health/coronavirus-italy-men-risk.html |archive-date=20 March 2020 |url-access=subscription |url-status=live |access-date=7 April 2020 |work=The New York Times |date=20 March 2020}}</ref> In Europe as of February 2020, 57% of the infected people were men and 72% of those died with COVID‑19 were men.<ref>{{#invoke:Cite web||title=COVID-19 weekly surveillance report |url=https://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/weekly-surveillance-report |archive-url=https://web.archive.org/web/20200315074508/https://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/weekly-surveillance-report |url-status=dead |archive-date=15 March 2020 |website=] (WHO) |access-date=7 April 2020}}</ref> As of April 2020, the US government is not tracking sex-related data of COVID‑19 infections.<ref name="nytimesus">{{#invoke:cite news || vauthors = Gupta AH | title = Does Covid-19 Hit Women and Men Differently? U.S. Isn't Keeping Track |url=https://www.nytimes.com/2020/04/03/us/coronavirus-male-female-data-bias.html |archive-url=https://web.archive.org/web/20200403135013/https://www.nytimes.com/2020/04/03/us/coronavirus-male-female-data-bias.html |archive-date=3 April 2020 |url-access=subscription |url-status=live |access-date=7 April 2020 |work=The New York Times |date=3 April 2020}}</ref> Research has shown that viral illnesses like Ebola, HIV, influenza and SARS affect men and women differently.<ref name="nytimesus" /> | |||
| ===Reinfection=== | |||
| === Ethnic differences === | |||
| As of March 2020, it was unknown if past infection provides effective and long-term ] in people who recover from the disease.<ref>{{cite web|url=https://www.immunology.org/news/bsi-open-letter-government-sars-cov-2-outbreak-response|title=BSI open letter to Government on SARS-CoV-2 outbreak response | publisher= British Society for Immunology|website= immunology.org|access-date=15 March 2020|archive-url= https://web.archive.org/web/20200314221816/https://www.immunology.org/news/bsi-open-letter-government-sars-cov-2-outbreak-response|archive-date=14 March 2020 |url-status=live}}</ref> Immunity is seen as likely, based on the behaviour of other coronaviruses,<ref>{{cite news| url= https://www.independent.co.uk/life-style/health-and-families/coronavirus-immunity-reinfection-get-covid-19-twice-sick-spread-relapse-a9400691.html |title= Can you get coronavirus twice or does it cause immunity?|date=13 March 2020|website=The Independent|language=en|access-date=15 March 2020|archive-url= https://web.archive.org/web/20200314211439/https://www.independent.co.uk/life-style/health-and-families/coronavirus-immunity-reinfection-get-covid-19-twice-sick-spread-relapse-a9400691.html|archive-date=14 March 2020|url-status=live}}</ref> but cases in which recovery from COVID-19 have been followed by positive tests for coronavirus at a later date have been reported.<ref>{{cite web|url=https://www.latimes.com/world-nation/story/2020-03-13/china-japan-korea-coronavirus-reinfection-test-positive|title=They survived the coronavirus. Then they tested positive again. Why?|date=13 March 2020|website=Los Angeles Times|language=en-US|access-date=15 March 2020|archive-url=https://web.archive.org/web/20200314220822/https://www.latimes.com/world-nation/story/2020-03-13/china-japan-korea-coronavirus-reinfection-test-positive|archive-date=14 March 2020|url-status=live}}</ref><ref>{{cite web |url= https://www.caixinglobal.com/2020-02-26/14-of-recovered-covid-19-patients-in-guangdong-tested-positive-again-101520415.html|title=14% of Recovered Covid-19 Patients in Guangdong Tested Positive Again| publisher= Caixin Global|website= caixinglobal.com|language=en|access-date=15 March 2020|archive-url= https://web.archive.org/web/20200303181249/https://www.caixinglobal.com/2020-02-26/14-of-recovered-covid-19-patients-in-guangdong-tested-positive-again-101520415.html|archive-date=3 March 2020|url-status=live}}</ref><ref name="Omer2020">{{cite journal |last1=Omer |first1=SB |last2=Malani |first2=P |last3=del Rio |first3=C |title=The COVID-19 Pandemic in the US A Clinical Update |journal=JAMA |date= 6 April 2020 |doi=10.1001/jama.2020.5788}}</ref> These cases are believed to be worsening of a lingering infection rather than re-infection.<ref name="Omer2020"/> | |||
| In the US, a greater proportion of deaths due to COVID‑19 have occurred among African Americans and other minority groups.<ref name="AVD">{{#invoke:cite journal || vauthors = Dorn AV, Cooney RE, Sabin ML | title = COVID-19 exacerbating inequalities in the US | journal = Lancet | volume = 395 | issue = 10232 | pages = 1243–1244 | date = April 2020 | pmid = 32305087 | pmc = 7162639 | doi = 10.1016/S0140-6736(20)30893-X }}</ref> Structural factors that prevent them from practising social distancing include their concentration in crowded substandard housing and in "essential" occupations such as retail grocery workers, public transit employees, health-care workers and custodial staff. Greater prevalence of lacking ] and care of underlying conditions such as ],<ref name="Shauly-Aharonov-2021">{{#invoke:cite journal ||last1=Shauly-Aharonov |first1=Michal |last2=Shafrir |first2=Asher |last3=Paltiel |first3=Ora |last4=Calderon-Margalit |first4=Ronit |last5=Safadi |first5=Rifaat |last6=Bicher |first6=Roee |last7=Barenholz-Goultschin |first7=Orit |last8=Stokar |first8=Joshua |date=22 July 2021 |title=Both high and low pre-infection glucose levels associated with increased risk for severe COVID-19: New insights from a population-based study |journal=PLOS ONE |volume=16 |issue=7 |pages=e0254847 |doi=10.1371/journal.pone.0254847 |issn=1932-6203 |pmc=8297851 |pmid=34293038|bibcode=2021PLoSO..1654847S |doi-access=free }}</ref> hypertension, and ] also increase their risk of death.<ref>{{#invoke:cite journal || vauthors = Adams ML, Katz DL, Grandpre J | title = Population-Based Estimates of Chronic Conditions Affecting Risk for Complications from Coronavirus Disease, United States | journal = Emerging Infectious Diseases | volume = 26 | issue = 8 | pages = 1831–1833 | date = August 2020 | pmid = 32324118 | pmc = 7392427 | doi = 10.3201/eid2608.200679 | title-link = doi | doi-access = free }}</ref> Similar issues affect ] and ] communities.<ref name="AVD" /> On the one hand, in the Dominican Republic there is a clear example of both gender and ethnic inequality. In this Latin American territory, there is great inequality and precariousness that especially affects Dominican women, with greater emphasis on those of Haitian descent.<ref name="Batthyany-2020">{{#invoke:Cite web|| vauthors = Batthyány K |title=Coronavirus y Desigualdades preexistentes: Género y Cuidados|url=https://www.clacso.org/coronavirus-y-desigualdades-preexistentes-genero-y-cuidados/|access-date=22 April 2021|website=CLACSO (Consejo Latinoamericano de Ciencias Sociales)|date=13 October 2020}}</ref> According to a US health policy non-profit, 34% of American Indian and Alaska Native People (AIAN) non-elderly adults are at risk of serious illness compared to 21% of white non-elderly adults.<ref name=":2">{{#invoke:Cite web||url=https://www.kff.org/coronavirus-covid-19/issue-brief/covid-19-presents-significant-risks-for-american-indian-and-alaska-native-people/|title=COVID-19 Presents Significant Risks for American Indian and Alaska Native People|date=14 May 2020}}</ref> The source attributes it to disproportionately high rates of many health conditions that may put them at higher risk as well as living conditions like lack of access to clean water.<ref name=":2" /> | |||
| Leaders have called for efforts to research and address the disparities.<ref>{{#invoke:cite journal || vauthors = Laurencin CT, McClinton A | title = The COVID-19 Pandemic: a Call to Action to Identify and Address Racial and Ethnic Disparities | journal = Journal of Racial and Ethnic Health Disparities | volume = 7 | issue = 3 | pages = 398–402 | date = June 2020 | pmid = 32306369 | pmc = 7166096 | doi = 10.1007/s40615-020-00756-0 }}</ref> In the UK, a greater proportion of deaths due to COVID‑19 have occurred in those of a ], ], and other ethnic minority background.<ref>{{#invoke:Cite web||date=9 June 2020|title=How coronavirus deaths in the UK compare by race and ethnicity|url=https://www.independent.co.uk/news/uk/home-news/coronavirus-death-toll-uk-race-white-black-asian-bame-ethnicity-cases-a9557076.html|access-date=10 June 2020|website=The Independent}}</ref><ref>{{#invoke:Cite web||title=Emerging findings on the impact of COVID-19 on black and minority ethnic people|url=https://www.health.org.uk/news-and-comment/charts-and-infographics/emerging-findings-on-the-impact-of-covid-19-on-black-and-min|access-date=10 June 2020|publisher=The Health Foundation}}</ref><ref>{{#invoke:cite news || vauthors = Butcher B, Massey J |date=9 June 2020|title=Why are more BAME people dying from coronavirus?|work=BBC News |url=https://www.bbc.com/news/uk-52219070 |access-date=10 June 2020}}</ref> More severe impacts upon patients including the relative incidence of the necessity of hospitalisation requirements, and vulnerability to the disease has been associated via DNA analysis to be expressed in genetic variants at chromosomal region 3, features that are associated with European ] heritage. That structure imposes greater risks that those affected will develop a more severe form of the disease.<ref name=Neanderthal>{{#invoke:Cite web|| title=The ancient Neanderthal hand in severe COVID-19 | website=ScienceDaily | date=30 September 2020 | url=https://www.sciencedaily.com/releases/2020/09/200930094758.htm | access-date=13 December 2020}}</ref> The findings are from Professor Svante Pääbo and researchers he leads at the ] and the ].<ref name=Neanderthal /> This admixture of modern human and Neanderthal genes is estimated to have occurred roughly between 50,000 and 60,000 years ago in Southern Europe.<ref name=Neanderthal /> | |||
| ==History== | |||
| === Comorbidities === | |||
| The virus is thought to be natural and have an ],<ref name="NM-20200317">{{cite journal |vauthors=Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF |title=The proximal origin of SARS-CoV-2 |url=https://www.nature.com/articles/s41591-020-0820-9 |date=17 March 2020 |journal=] |pages=1–3 |doi=10.1038/s41591-020-0820-9 |issn=1546-170X |access-date=18 March 2020 |archive-url=https://web.archive.org/web/20200318001738/https://www.nature.com/articles/s41591-020-0820-9 |archive-date=18 March 2020 |url-status=live }}</ref> through ].<ref>{{cite web|url=http://nautil.us/issue/83/intelligence/the-man-who-saw-the-pandemic-coming|title=The Man Who Saw the Pandemic Coming|last=Berger|first=Kevin|name-list-format=vanc|date=12 March 2020|website=Nautilus|access-date=16 March 2020|archive-url=https://web.archive.org/web/20200315180124/http://nautil.us/issue/83/intelligence/the-man-who-saw-the-pandemic-coming|archive-date=15 March 2020|url-status=live}}</ref> The origin is unknown but by December 2019 the spread of infection was almost entirely driven by human-to-human transmission.<ref name="Epidemiology2020Feb17">{{cite journal|vauthors=Yanping Z, et al.|collaboration=The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team|title=The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID-19)—China, 2020|url=http://weekly.chinacdc.cn/en/article/id/e53946e2-c6c4-41e9-9a9b-fea8db1a8f51|journal=China CDC Weekly|volume=2|issue=8|pages=113–122|date=17 February 2020|publisher=]|access-date=18 March 2020|archive-url=https://web.archive.org/web/20200219142101/http://weekly.chinacdc.cn/en/article/id/e53946e2-c6c4-41e9-9a9b-fea8db1a8f51|archive-date=19 February 2020|url-status=live}}</ref><ref name="Heymann Shindo 2020 pp. 542–45">{{cite journal | vauthors=Heymann DL, Shindo N | title=COVID-19: what is next for public health? | journal=Lancet | volume=395 | issue=10224 | date=February 2020 | pmid=32061313 | doi=10.1016/S0140-6736(20)30374-3 | doi-access=free | pages=542–45 }}</ref> The earliest reported infection has been unofficially reported to have occurred on 17 November 2019 in ].<ref name=Davidson13March2020>{{cite news |author= Davidson, Helen |date= 13 March 2020 |title= First Covid-19 case happened in November, China government records show—report |url= https://www.theguardian.com/world/2020/mar/13/first-covid-19-case-happened-in-november-china-government-records-show-report |work= The Guardian |access-date= 21 March 2020 |archive-url= https://web.archive.org/web/20200320235432/https://www.theguardian.com/world/2020/mar/13/first-covid-19-case-happened-in-november-china-government-records-show-report |archive-date= 20 March 2020 |url-status= live }}</ref> A study of the first 41 cases of confirmed COVID-19, published in January 2020 in ''The Lancet'', revealed the earliest date of onset of symptoms as 1{{nbsp}}December 2019.<ref name=WuMarch2020>{{Cite journal|last=Wu|first=Yi-Chi|last2=Chen|first2=Ching-Sung|last3=Chan|first3=Yu-Jiun|date=March 2020|title=The outbreak of COVID-19: An overview|journal=Journal of the Chinese Medical Association|language=en-US|volume=83|issue=3|pages=217–220|doi=10.1097/JCMA.0000000000000270|issn=1726-4901}}</ref><ref name="Wang24Jan2020">{{cite journal |last1=Wang |first1=C. |last2=Horby |first2=P. W. |last3=Hayden |first3=F. G. |last4=Gao |first4=G. F. |title=A novel coronavirus outbreak of global health concern |journal=] |volume=395 |issue=10223 |pages=470–473 |date=February 2020 |pmid=31986257 |doi=10.1016/S0140-6736(20)30185-9 |url=https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30185-9/abstract}} {{free access}}</ref><ref name="AutoDW-67">{{Cite journal |last=Cohen |first=Jon |date=January 2020 |title=Wuhan seafood market may not be source of novel virus spreading globally |url=https://www.sciencemag.org/news/2020/01/wuhan-seafood-market-may-not-be-source-novel-virus-spreading-globally |journal=] |doi=10.1126/science.abb0611}}</ref> Official publications from the WHO reported the earliest onset of symptoms as 8{{nbsp}}December 2019.<ref name=Davidson13March2020/> | |||
| ] factors (immune response) and the general behaviour (habits) can strongly determine the consequences of COVID‑19.<ref name="Dehingia-2021" /> Most of those who die of COVID‑19 have ]s, including hypertension, ],<ref name="Shauly-Aharonov-2021" /> and ].<ref name="WHO-2020a">{{#invoke:Cite web||url=https://www.who.int/dg/speeches/detail/who-director-general-s-statement-on-the-advice-of-the-ihr-emergency-committee-on-novel-coronavirus |title=WHO Director-General's statement on the advice of the IHR Emergency Committee on Novel Coronavirus |website=] (WHO)}}</ref> According to March data from the United States, 89% of those hospitalised had preexisting conditions.<ref>{{#invoke:cite journal || vauthors = Garg S, Kim L, Whitaker M, O'Halloran A, Cummings C, Holstein R, Prill M, Chai SJ, Kirley PD, Alden NB, Kawasaki B, Yousey-Hindes K, Niccolai L, Anderson EJ, Openo KP, Weigel A, Monroe ML, Ryan P, Henderson J, Kim S, Como-Sabetti K, Lynfield R, Sosin D, Torres S, Muse A, Bennett NM, Billing L, Sutton M, West N, Schaffner W, Talbot HK, Aquino C, George A, Budd A, Brammer L, Langley G, Hall AJ, Fry A | title = Hospitalization Rates and Characteristics of Patients Hospitalized with Laboratory-Confirmed Coronavirus Disease 2019 – COVID-NET, 14 States, March 1–30, 2020 | journal = MMWR. Morbidity and Mortality Weekly Report | volume = 69 | issue = 15 | pages = 458–464 | date = April 2020 | pmid = 32298251 | pmc = 7755063 | doi = 10.15585/mmwr.mm6915e3 | title-link = doi | doi-access = free }}</ref> The Italian Istituto Superiore di Sanità reported that out of 8.8% of deaths where ]s were available, 96.1% of people had at least one ] with the average person having 3.4 diseases.<ref name="ISSCharacteristics">{{#invoke:cite report||url=https://www.epicentro.iss.it/en/coronavirus/bollettino/Report-COVID-2019_22_July_2020.pdf|title=Characteristics of SARS-CoV-2 patients dying in Italy Report based on available data on July 22nd, 2020|date=22 July 2020|publisher=]|access-date=4 October 2020|vauthors=Palmieri L, Andrianou X, Barbariol P, Bella A, Bellino S, Benelli E, Bertinato L, Boros S, Brambilla G, Calcagnini G, Canevelli M, Castrucci MR, Censi F, Ciervo A, Colaizzo E, D'Ancona F, Del Manso M, Donfrancesco C, Fabiani M, Filia A, Floridia M, Giuliano M, Grisetti T, Langer M, Lega I, Lo Noce C, Maiozzi P, Malchiodi Albedi F, Manno V, Martini M, Mateo Urdiales A, Mattei E, Meduri C, Meli P, Minelli G, Nebuloni M, Nisticò L, Nonis M, Onder G, Palmisano L, Petrosillo N, Pezzotti P, Pricci F, Punzo O, Puro V, Raparelli V, Rezza G, Riccardo F, Rota MC, Salerno P, Serra D, Siddu A, Stefanelli P, Tamburo De Bella M, Tiple D, Unim B, Vaianella L, Vanacore N, Vichi M, Villani ER, Brusaferro S}}</ref> According to this report the most common comorbidities are hypertension (66% of deaths), ] (29.8% of deaths), ] (27.6% of deaths), ] (23.1% of deaths) and ] (20.2% of deaths). | |||
| Most critical respiratory comorbidities according to the US Centers for Disease Control and Prevention (CDC), are: moderate or severe ], pre-existing ], ], ].<ref name=":3">{{#invoke:Cite web||date=11 February 2020|title=Coronavirus Disease 2019 (COVID-19)|url=https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/groups-at-higher-risk.html|access-date=19 June 2020|website=U.S. ] (CDC) }}</ref> Evidence stemming from ] of several smaller research papers also suggests that smoking can be associated with worse outcomes.<ref>{{#invoke:cite journal || vauthors = Zhao Q, Meng M, Kumar R, Wu Y, Huang J, Lian N, Deng Y, Lin S | title = The impact of COPD and smoking history on the severity of COVID-19: A systemic review and meta-analysis | journal = Journal of Medical Virology | volume = 92 | issue = 10 | pages = 1915–1921 | date = October 2020 | pmid = 32293753 | pmc = 7262275 | doi = 10.1002/jmv.25889 }}</ref><ref>{{#invoke:Cite web||title=Smoking and COVID-19|url=https://www.who.int/news-room/commentaries/detail/smoking-and-covid-19|access-date=19 June 2020|website=] (WHO)}}</ref> When someone with existing respiratory problems is infected with COVID‑19, they might be at greater risk for severe symptoms.<ref name=":3" /> COVID‑19 also poses a greater risk to people who ]s and ]s, insofar as their drug use may have caused lung damage.<ref>{{#invoke:Cite web||title=People who use drugs are more vulnerable to coronavirus. Here's what clinics are doing to help.|url=https://www.theadvocate.com/baton_rouge/news/coronavirus/article_f80cf77e-84fa-11ea-88d5-2b37dc9dd966.html| vauthors = DeRobertis J |date=3 May 2020|website=The Advocate (Louisiana)|access-date=4 May 2020}}</ref> | |||
| ==Epidemiology== | |||
| In August 2020, the CDC issued a caution that ] (TB) infections could increase the risk of severe illness or death. The WHO recommended that people with respiratory symptoms be screened for both diseases, as testing positive for COVID‑19 could not rule out co-infections. Some projections have estimated that reduced TB detection due to the pandemic could result in 6.3 million additional TB cases and 1.4 million TB-related deaths by 2025.<ref>{{#invoke:Cite web||url=https://www.cdc.gov/coronavirus/2019-ncov/global-covid-19/TB-non-us-settings.html|title=Coronavirus Disease 2019 (COVID-19)|date=11 February 2020|website=U.S. ] (CDC) }}</ref> | |||
| {{main|2019–20 coronavirus pandemic}} | |||
| == History == | |||
| Several measures are commonly used to quantify mortality.<ref>{{cite web|url=https://www.cdc.gov/csels/dsepd/ss1978/lesson3/section3.html|title=Principles of Epidemiology {{!}} Lesson 3—Section 3|date=18 February 2019|website=www.cdc.gov|language=en-us|access-date=28 March 2020|archive-url=https://web.archive.org/web/20200228150607/https://www.cdc.gov/csels/dsepd/ss1978/lesson3/section3.html|archive-date=28 February 2020|url-status=live}}</ref> These numbers vary by region and over time and are influenced by the volume of testing, healthcare system quality, treatment options, time since initial outbreak and population characteristics such as age, sex and overall health.<ref>{{cite web |url=https://ourworldindata.org/covid-mortality-risk |title=What do we know about the risk of dying from COVID-19? |last=Ritchie |first=Hannah |last2=Roser |first2=Max |date=25 March 2020 |editor-last=Chivers |editor-first=Tom |website=] |url-status=live |access-date=28 March 2020 |archive-url=https://web.archive.org/web/20200328192730/https://ourworldindata.org/covid-mortality-risk |archive-date=28 March 2020 }}</ref> In late 2019, WHO assigned the emergency ] disease codes ] for deaths from lab-confirmed SARS-CoV-2 infection and U07.2 for deaths from clinically or epidemiologically diagnosed COVID-19 without lab-confirmed SARS-CoV-2 infection.<ref name="ICD10_2019_U07p2">{{cite web | title = ICD-10 Version:2019 | website = ] | quote = U07.2—COVID-19, virus not identified—COVID-19 NOS—Use this code when COVID-19 is diagnosed clinically or epidemiologically but laboratory testing is inconclusive or not available. Use additional code, if desired, to identify pneumonia or other manifestations | year = 2019 | url = https://icd.who.int/browse10/2019/en#/U07.1 | accessdate = 2020-03-31 | archiveurl = https://archive.today/20200331004754/https://icd.who.int/browse10/2019/en%23/U07.1#/U07.1 | archivedate = 31 March 2020 | url-status = live | url-access = <!--(subscription/registration/limited) default=free--> }}</ref> | |||
| {{update section|reason=excessive detail about the very early pandemic while missing an overview of the later pandemic|date=July 2023}} | |||
| {{Main|Timeline of the COVID-19 pandemic|Investigations into the origin of COVID-19}} | |||
| {{COVID-19 pandemic sidebar}} | |||
| The virus is thought to be of natural animal origin, most likely through ].<ref name="NM-20200317">{{#invoke:cite journal || vauthors = Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF | title = The proximal origin of SARS-CoV-2 | journal = Nature Medicine | volume = 26 | issue = 4 | pages = 450–452 | date = April 2020 | pmid = 32284615 | pmc = 7095063 | doi = 10.1038/s41591-020-0820-9 }}</ref><ref name="PMC7969828">{{#invoke:cite journal || vauthors = Frutos R, Gavotte L, Devaux CA | title = Understanding the origin of COVID-19 requires to change the paradigm on zoonotic emergence from the spillover to the circulation model | journal = Infection, Genetics and Evolution | volume = 95 | pages = 104812 | date = November 2021 | pmid = 33744401 | pmc = 7969828 | doi = 10.1016/j.meegid.2021.104812 | bibcode = 2021InfGE..9504812F }}</ref><ref>{{#invoke:cite journal || vauthors = Holmes EC, Goldstein SA, Rasmussen AL, Robertson DL, Crits-Christoph A, Wertheim JO, Anthony SJ, Barclay WS, Boni MF, Doherty PC, Farrar J, Geoghegan JL, Jiang X, Leibowitz JL, Neil SJ, Skern T, Weiss SR, Worobey M, Andersen KG, Garry RF, Rambaut A | title = The origins of SARS-CoV-2: A critical review | journal = Cell | volume = 184 | issue = 19 | pages = 4848–4856 | date = September 2021 | pmid = 34480864 | pmc = 8373617 | doi = 10.1016/j.cell.2021.08.017 }}</ref> A joint-study conducted in early 2021 by the People's Republic of China and the World Health Organization indicated that the virus descended from a coronavirus that infects wild bats, and likely spread to humans through an intermediary wildlife host.<ref>{{#invoke:Cite web||url=https://www.who.int/publications/i/item/who-convened-global-study-of-origins-of-sars-cov-2-china-part|work=] (WHO) |title=WHO-convened Global Study of Origins of SARS-CoV-2: China Part|date=30 March 2021|access-date=29 July 2022}}</ref> There are several theories about where the ] originated and ] are ongoing.<ref name="patientZero">{{#invoke:cite news|| vauthors = Duarte F | date=24 February 2020|title=As the cases of coronavirus increase in China and around the world, the hunt is on to identify "patient zero".|work=BBC News|url=https://www.bbc.com/future/article/20200221-coronavirus-the-harmful-hunt-for-covid-19s-patient-zero|access-date=22 March 2020}}</ref> According to articles published in July 2022 in '']'', virus transmission into humans occurred through two spillover events in November 2019 and was likely due to live wildlife trade on the ] in the city of ] (Hubei, China).<ref>{{#invoke:cite journal||title=The molecular epidemiology of multiple zoonotic origins of SARS-CoV-2 |date=26 July 2022 |journal=]|vauthors=Pekar JE, Magee P, Parker E, Moshiri N, Izhikevich K, Havens JL, Gangavarapu K, Serrano LM, Crits-Christoph A, Matteson NL, Zeller M, Levy JI, Wang JC, Hughes S, Lee JM, Park H, Park MS, Ching ZY, Lin TP, Isa NM, Noor YM, Vasylyeva TI, Garry RF, Holmes EC, Rambaut A, Suchard MA, Andersen KG, Worobey M, Wertheim JO|pages=960–966 |doi-access = free | title-link = doi |volume=377 |issue=6609 |doi=10.1126/science.abp8337|pmid=35881005 |pmc=9348752 |bibcode=2022Sci...377..960P }}</ref><ref>{{#invoke:Cite news ||last=Gill |first=Victoria |date=26 July 2022 |title=Covid origin studies say evidence points to Wuhan market |work=] |publisher=] |url=https://www.bbc.com/news/science-environment-62307383 |url-status=live |access-date=31 August 2023 |archive-url=https://web.archive.org/web/20220726153445/https://www.bbc.com/news/science-environment-62307383 |archive-date=26 July 2022}}</ref><ref>{{#invoke:cite journal ||title=The Huanan Seafood Wholesale Market in Wuhan was the early epicenter of the COVID-19 pandemic |date=July 2022 |journal=Science |doi=10.1126/science.abp8715 | vauthors = Worobey M, Levy JI, Serrano LM, Crits-Christoph A, Pekar JE, Goldstein SA, Rasmussen AL, Kraemer MU, Newman C, Koopmans MP, Suchard MA, Wertheim JO, Lemey P, Robertson DL, Garry RF, Holmes EC, Rambaut A, Andersen KG | title-link=doi |volume=377 |issue=6609 |pages=951–959 |pmid=35881010 |pmc=9348750 |bibcode=2022Sci...377..951W |s2cid=251067542 }}</ref> Doubts about the conclusions have mostly centered on the precise site of spillover.<ref>{{#invoke:cite news ||url=https://www.nationalgeographic.com/magazine/article/debate-deepens-over-wuhan-wet-markets-role-in-kickstarting-the-pandemic |title=Debate deepens over Wuhan wet market's role in kickstarting the pandemic |date=27 July 2022 |work=]}}</ref> Earlier ] estimated that SARS-CoV-2 arose in October or November 2019.<ref name="evolutionary">{{#invoke:cite journal || vauthors = Li X, Zai J, Zhao Q, Nie Q, Li Y, Foley BT, Chaillon A | title = Evolutionary history, potential intermediate animal host, and cross-species analyses of SARS-CoV-2 | journal = Journal of Medical Virology | volume = 92 | issue = 6 | pages = 602–611 | date = June 2020 | pmid = 32104911 | pmc = 7228310 | doi = 10.1002/jmv.25731 }}</ref><ref name="zoonotic">{{#invoke:cite journal || vauthors = Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF | title = The proximal origin of SARS-CoV-2 | journal = Nature Medicine | volume = 26 | issue = 4 | pages = 450–452 | date = April 2020 | pmid = 32284615 | pmc = 7095063 | doi = 10.1038/s41591-020-0820-9 | title-link = doi | doi-access = free }}</ref><ref name="dorp_evo">{{#invoke:cite journal || vauthors = van Dorp L, Acman M, Richard D, Shaw LP, Ford CE, Ormond L, Owen CJ, Pang J, Tan CC, Boshier FA, Ortiz AT, Balloux F | title = Emergence of genomic diversity and recurrent mutations in SARS-CoV-2 | journal = Infection, Genetics and Evolution | volume = 83 | pages = 104351 | date = September 2020 | pmid = 32387564 | pmc = 7199730 | doi = 10.1016/j.meegid.2020.104351 | bibcode = 2020InfGE..8304351V }}</ref> A phylogenetic algorithm analysis suggested that the virus may have been circulating in ] before Wuhan.<ref>{{#invoke:cite news || vauthors = Grose TK | |||
| |url= https://www.usnews.com/news/best-countries/articles/2020-05-13/scientist-suggests-coronavirus-originated-outside-of-wuhan |title=Did the Coronavirus Originate Outside of Wuhan? |work=U.S. News & World Report |date=13 May 2020}}</ref> | |||
| Most scientists believe the virus spilled into human populations through natural ], similar to the ] and ] outbreaks, and consistent with other pandemics in human history.<ref name="pekar"/><ref name="jiang_wang"/> According to the ] several social and environmental factors including ], ] and ] increased the likelihood of such ].<ref name="IPCC-2022a"/><ref name="IPCC-2022b"/> One study made with the support of the ] found ] increased the likelihood of the pandemic by influencing distribution of bat species.<ref name="University-of-Cambridge-2021"/><ref name="European-Commission"/> | |||
| The death-to-case ratio reflects the number of deaths divided by the number of diagnosed cases within a given time interval. Based on Johns Hopkins University statistics, the global death-to-case ratio is {{Cases in 2019–20 coronavirus pandemic|ratio|editlink=|ref=no}} ({{Cases in 2019–20 coronavirus pandemic|deaths|editlink=|ref=no}}/{{Cases in 2019–20 coronavirus pandemic|confirmed|editlink=|ref=no}}) as of {{Cases in 2019–20 coronavirus pandemic|date|editlink=|ref=no}}.{{Cases in 2019–20 coronavirus pandemic|ref=yes}} The number varies by region.<ref>{{Cite journal |last=Lazzerini |first=Marzia |last2=Putoto |first2=Giovanni |date=18 March 2020 |title=COVID-19 in Italy: momentous decisions and many uncertainties |url=https://www.thelancet.com/journals/langlo/article/PIIS2214-109X(20)30110-8/abstract |journal=The Lancet Global Health |language=English |volume=0 |doi=10.1016/S2214-109X(20)30110-8 |issn=2214-109X |pmid=32199072|pmc=7104294 }}</ref> | |||
| Available evidence suggests that the SARS-CoV-2 virus was originally harboured by bats, and spread to humans multiple times from infected wild animals at the ] in ] in December 2019.<ref name="pekar" /><ref name="jiang_wang" /> A minority of scientists and some members of the ] believe the virus may have been ] from a laboratory such as the ].<ref name="NYT_DoE_Feb2023">{{#invoke:cite web ||last1=Barnes |first1=Julian E. |title=Lab Leak Most Likely Caused Pandemic, Energy Dept. Says |url=https://www.nytimes.com/2023/02/26/us/politics/china-lab-leak-coronavirus-pandemic.html |website=The New York Times |access-date=27 February 2023 |date=26 February 2023}}</ref><ref name="Hill_Feb_2023_DoE">{{#invoke:cite news ||last1=Mueller |first1=Julia |title=Energy Department's COVID lab leak conclusion: What we know |url=https://thehill.com/policy/healthcare/3874965-what-we-know-about-energy-departments-lab-leak-conclusion/ |access-date=26 March 2023 |work=The Hill |date=26 February 2023}}</ref> The US intelligence community has mixed views on the issue,<ref name="CNN_Feb2023_LeBlanc">{{#invoke:cite news ||last1=LeBlanc |first1=Paul |title=New assessment on the origins of Covid-19 adds to the confusion {{!}} CNN Politics |url=https://www.cnn.com/2023/02/27/politics/covid-origins-doe-assessment-what-matters/index.html |access-date=27 February 2023 |work=CNN |date=27 February 2023}}</ref><ref name="Guardian_Feb2023">{{#invoke:cite news ||last1=Davis |first1=Nicola |last2=Hawkins |first2=Amy |title=How seriously should we take the US DoE's Covid lab leak theory? |url=https://www.theguardian.com/world/2023/feb/27/how-seriously-should-we-take-the-us-does-covid-lab-leak-theory |access-date=27 February 2023 |work=The Guardian |date=27 February 2023}}</ref> but overall agrees with the scientific consensus that the virus was not developed as a ] and is unlikely to have been ].<ref>{{#invoke:Cite web|| vauthors = Wolf ZB |title=Analysis: Why scientists are suddenly more interested in the lab-leak theory of Covid's origin |url=https://www.cnn.com/2021/05/25/politics/wuhan-lab-covid-origin-theory/index.html|access-date=26 May 2021 |publisher=CNN|date=25 May 2021}}</ref><ref>{{#invoke:cite journal ||vauthors=Maxmen A |title=US COVID origins report: researchers pleased with scientific approach |journal=Nature |volume=597 |issue=7875 |pages=159–160 |date=September 2021 |pmid=34465917 |doi=10.1038/d41586-021-02366-0 |s2cid=237373547 |bibcode=2021Natur.597..159M}}</ref><ref>{{#invoke:Cite news||url=https://www.politico.com/newsletters/future-pulse/2022/11/04/cross-examining-the-lab-leak-theorists-00065103|title=Cross-examining the lab-leak theorists|date=4 November 2022|work=] |vauthors=Paun C, Zeller S, Reader R, Leonard B, Scullion G | access-date=21 November 2022 }}</ref><ref>{{#invoke:Cite news||url=https://www.reuters.com/world/us-intelligence-releases-report-covid-19-origins-2021-10-29/|title=U.S. spy agencies say origins of COVID-19 may never be known |vauthors=Hosenball M, Zengerle P|date=30 October 2021 |work=]|access-date=21 November 2022}}</ref> There is no evidence SARS-CoV-2 existed in any laboratory prior to the pandemic.<ref name=critical>{{#invoke:cite journal ||vauthors=Holmes EC, Goldstein SA, Rasmussen AL, Robertson DL, Crits-Christoph A, Wertheim JO, Anthony SJ, Barclay WS, Boni MF, Doherty PC, Farrar J, Geoghegan JL, Jiang X, Leibowitz JL, Neil SJ, Skern T, Weiss SR, Worobey M, Andersen KG, Garry RF, Rambaut A |title=The origins of SARS-CoV-2: A critical review |journal=Cell |volume=184 |issue=19 |pages=4848–4856 |date=September 2021 |pmid=34480864 |pmc=8373617 |doi=10.1016/j.cell.2021.08.017 |type=Review |quote=Under any laboratory escape scenario, SARS-CoV-2 would have to have been present in a laboratory prior to the pandemic, yet no evidence exists to support such a notion and no sequence has been identified that could have served as a precursor.}}</ref><ref name="Gorski">{{#invoke:cite web ||last=Gorski |first=David |date=31 May 2021 |title=The origin of SARS-CoV-2, revisited |url=https://sciencebasedmedicine.org/the-origin-of-sars-cov-2-revisited/ |url-status=live |archive-url=https://web.archive.org/web/20210601072923/https://sciencebasedmedicine.org/the-origin-of-sars-cov-2-revisited/ |archive-date=1 June 2021 |access-date=19 July 2021 |publisher=] |quote=The second is the version that "reasonable" people consider plausible, but there is no good evidence for either version.}}</ref><ref name="HolmesConversationDead">{{#invoke:cite web ||last=Holmes |first=Edward C. |date=14 August 2022 |title=The COVID lab leak theory is dead. Here's how we know the virus came from a Wuhan market |url=http://theconversation.com/the-covid-lab-leak-theory-is-dead-heres-how-we-know-the-virus-came-from-a-wuhan-market-188163 |access-date=4 September 2022 |website=The Conversation |quote=For the lab leak theory to be true, SARS-CoV-2 must have been present in the Wuhan Institute of Virology before the pandemic started. This would convince me. But the inconvenient truth is there's not a single piece of data suggesting this. There's no evidence for a genome sequence or isolate of a precursor virus at the Wuhan Institute of Virology. Not from gene sequence databases, scientific publications, annual reports, student theses, social media, or emails. Even the intelligence community has found nothing. Nothing. And there was no reason to keep any work on a SARS-CoV-2 ancestor secret before the pandemic.}}</ref> | |||
| Other measures include the ] (CFR), which reflects the percent of diagnosed individuals who die from a disease, and the infection fatality rate (IFR), which reflects the percent of infected individuals (diagnosed and undiagnosed) who die from a disease. These statistics are not time bound and follow a specific population from infection through case resolution. A number of academics have attempted to calculate these numbers for specific populations.<ref>{{cite web |url=https://ourworldindata.org/covid-mortality-risk |title=What do we know about the risk of dying from COVID-19? |website=Our World in Data |access-date=28 March 2020 |archive-url=https://web.archive.org/web/20200328192730/https://ourworldindata.org/covid-mortality-risk |archive-date=28 March 2020 |url-status=live }}</ref> In the epicentre of the outbreak in Italy, Castiglione d'Adda, a small village of 4500, 80 (1.8%) are already dead. Most people in the village appear to have developed ] and plausible immunity, most did so without being diagnosed, and many did not have symptoms.<ref>{{cite web|url=http://www.ilcittadino.it/cronaca/2020/04/02/contagiati-senza-saperlo-all-avis-sono-ben-40-su-60/KYwVaGiHShP3odU4dY4zx3/index.html|title=Castiglione: contagiati senza saperlo, all'Avis sono ben 40 donatori su 60|website=Il Cittadino di Lodi|language=it|access-date=2020-04-05}}</ref><ref>{{cite web|url=https://www.ilgiornale.it/news/cronache/asintomatici-anticorpi-loro-plasma-cura-virus-1848999.html|title=Asintomatici, ma con anticorpi: dal loro plasma arriva la cura?|last=Bernasconi|first=Francesca|date=2020-04-02|website=ilGiornale.it|language=it|access-date=2020-04-05}}</ref> An investigation is underway to test the entire population to learn more about the disease.<ref>{{cite web|url=https://www.adnkronos.com/fatti/cronaca/2020/04/02/coronavirus-galli-pronti-test-tappeto-castiglione-adda_p1pDUsh8cd1En9PvyqTRWM.html|title=Coronavirus, Galli: "Pronti a test a tappeto a Castiglione d'Adda"|website=Adnkronos|access-date=2020-04-05}}</ref> | |||
| <gallery mode="packed" heights="210"> | |||
| File:Total-cases-covid-19-who.png|Total confirmed cases over time | |||
| File:Total-deaths-covid-19-who (1).png|Total deaths over time | |||
| File:Total-confirmed-cases-of-covid-19-per-million-people.png|Total confirmed cases of COVID-19 per million people, 20 March 2020<ref>{{cite web |title=Total confirmed cases of COVID-19 per million people |url=https://ourworldindata.org/grapher/total-confirmed-cases-of-covid-19-per-million-people |website=Our World in Data |access-date=20 March 2020 |archive-url=https://web.archive.org/web/20200319163452/https://ourworldindata.org/grapher/total-confirmed-cases-of-covid-19-per-million-people |archive-date=19 March 2020 |url-status=live }}</ref> | |||
| File:Total-covid-deaths-per-million.png|Total confirmed deaths due to COVID-19 per million people, 24 March 2020<ref>{{cite web |title=Total confirmed deaths due to COVID-19 per million people |url=https://ourworldindata.org/grapher/total-covid-deaths-per-million |website=Our World in Data |access-date=24 March 2020 |archive-url=https://web.archive.org/web/20200319163452/https://ourworldindata.org/grapher/total-covid-deaths-per-million |archive-date=19 March 2020 |url-status=live }}</ref> | |||
| </gallery> | |||
| The first confirmed human infections were in Wuhan. A study of the first 41 cases of confirmed COVID‑19, published in January 2020 in ''The Lancet'', reported the earliest date of onset of symptoms as 1{{spaces}}December 2019.<ref name="WuMarch2020">{{#invoke:cite journal ||vauthors=Wu YC, Chen CS, Chan YJ |title=The outbreak of COVID-19: An overview |journal=Journal of the Chinese Medical Association |volume=83 |issue=3 |pages=217–220 |date=March 2020 |pmid=32134861 |pmc=7153464 |doi=10.1097/JCMA.0000000000000270}}</ref><ref name="Wang24Jan2020">{{#invoke:cite journal ||vauthors=Wang C, Horby PW, Hayden FG, Gao GF |title=A novel coronavirus outbreak of global health concern |journal=Lancet |volume=395 |issue=10223 |pages=470–473 |date=February 2020 |pmid=31986257 |pmc=7135038 |doi=10.1016/S0140-6736(20)30185-9 |title-link=doi |doi-access=free}}</ref><ref name="AutoDW-67">{{#invoke:cite journal|| vauthors=Cohen J | date=January 2020|title=Wuhan seafood market may not be source of novel virus spreading globally |url=https://www.science.org/content/article/wuhan-seafood-market-may-not-be-source-novel-virus-spreading-globally|journal=] |doi=10.1126/science.abb0611 |doi-access=free |title-link=doi}}</ref> Official publications from the WHO reported the earliest onset of symptoms as 8{{spaces}}December 2019.<ref>{{#invoke:Cite web||date=12 January 2020|title=Novel Coronavirus – China|website=] (WHO) |url=https://www.who.int/csr/don/12-january-2020-novel-coronavirus-china/en/|url-status=dead|archive-date=14 January 2020|archive-url=https://web.archive.org/web/20200114185815/https://www.who.int/csr/don/12-january-2020-novel-coronavirus-china/en/}}</ref> Human-to-human transmission was confirmed by the WHO and Chinese authorities by 20 January 2020.<ref>{{#invoke:cite news|| vauthors=Kessler G |date=17 April 2020|title=Trump's false claim that the WHO said the coronavirus was 'not communicable' |newspaper=] |url=https://www.washingtonpost.com/politics/2020/04/17/trumps-false-claim-that-who-said-coronavirus-was-not-communicable/|url-status=live |access-date=17 April 2020|archive-date=17 April 2020|archive-url=https://archive.today/20200417193804/https://www.washingtonpost.com/politics/2020/04/17/trumps-false-claim-that-who-said-coronavirus-was-not-communicable/}}</ref><ref>{{#invoke:cite news ||vauthors=Kuo L |date=21 January 2020 |title=China confirms human-to-human transmission of coronavirus |work=] |url=https://www.theguardian.com/world/2020/jan/20/coronavirus-spreads-to-beijing-as-china-confirms-new-cases|access-date=18 April 2020}}</ref> According to official Chinese sources, these were mostly linked to the ], which also sold live animals.<ref name="characteristicsZH">{{#invoke:cite journal ||title= |language=zh |journal=Zhonghua Liu Xing Bing Xue Za Zhi = Zhonghua Liuxingbingxue Zazhi |volume=41 |issue=2 |pages=145–151 |date=February 2020 |pmid=32064853 |doi=10.3760/cma.j.issn.0254-6450.2020.02.003 |s2cid=211133882 |author1=Epidemiology Working Group For Ncip Epidemic Response |author2=Chinese Center for Disease Control Prevention}}</ref> In May 2020, ], the director of the CDC, said animal samples collected from the seafood market had tested negative for the virus, indicating that the market was the site of an early ], but that it was not the site of the initial outbreak.<ref>{{#invoke:cite news|| vauthors = Areddy JT |date=26 May 2020|title=China Rules Out Animal Market and Lab as Coronavirus Origin|work=] |url=https://www.wsj.com/articles/china-rules-out-animal-market-and-lab-as-coronavirus-origin-11590517508|access-date=29 May 2020|url-access=subscription}}</ref> Traces of the virus have been found in wastewater samples that were collected in ] and ], Italy, on 18 December 2019.<ref>{{#invoke:cite news|| vauthors=Kelland K |date=19 June 2020 |title=Italy sewage study suggests COVID-19 was there in December 2019|work=Reuters |url=https://www.reuters.com/article/us-health-coronavirus-italy-sewage/italy-sewage-study-suggests-covid-19-was-there-in-december-2019-idUSKBN23Q1J9|access-date=23 June 2020}}</ref> | |||
| ==Society and culture== | |||
| ===Nomenclature=== | |||
| By December 2019, the spread of infection was almost entirely driven by human-to-human transmission.<ref name="Epidemiology17Feb2020"/><ref>{{#invoke:cite journal ||vauthors=Heymann DL, Shindo N |title=COVID-19: what is next for public health? |journal=Lancet |volume=395 |issue=10224 |pages=542–545 |date=February 2020 |pmid=32061313 |pmc=7138015 |doi=10.1016/S0140-6736(20)30374-3 |title-link=doi |doi-access=free}}</ref> The number of COVID-19 cases in Hubei gradually increased, reaching sixty by 20 December,<ref>{{#invoke:Cite web|| date=14 March 2020 |vauthors=Bryner J |title=1st known case of coronavirus traced back to November in China |website=livescience.com |url=https://www.livescience.com/first-case-coronavirus-found.html|access-date=31 May 2020}}</ref> and at least 266 by 31 December.<ref>{{#invoke:cite news ||author=Canadian Politics |date=8 April 2020 |title=The birth of a pandemic: How COVID-19 went from Wuhan to Toronto |newspaper=National Post|url=https://nationalpost.com/news/politics/the-birth-of-a-pandemic-how-covid-19-went-from-wuhan-to-toronto |access-date=31 May 2020}}</ref> On 24 December, ] sent a ] (BAL) sample from an unresolved clinical case to sequencing company Vision Medicals. On 27 and 28 December, Vision Medicals informed the Wuhan Central Hospital and the Chinese CDC of the results of the test, showing a new coronavirus.<ref>{{#invoke:cite news||author=高昱|date=26 February 2020|title=独家 {{!}} 新冠病毒基因测序溯源: 警报是何时拉响的|language=zh|trans-title=Exclusive {{!}} Tracing the New Coronavirus gene sequencing: when did the alarm sound|work=]|url=https://china.caixin.com/2020-02-26/101520972.html|access-date=1 March 2020|archive-url=https://web.archive.org/web/20200227094018/https://china.caixin.com/2020-02-26/101520972.html|archive-date=27 February 2020|url-status=dead}}</ref> A pneumonia cluster of unknown cause was observed on 26 December and treated by the doctor ] in Hubei Provincial Hospital, who informed the Wuhan Jianghan CDC on 27 December.<ref>{{#invoke:Cite web||author1=路子康 |title=最早上报疫情的她, 怎样发现这种不一样的肺炎|website=中国网新闻 |location=北京|language=zh-cn |url=https://news.china.com/zw/news/13000776/20200209/37780703.html |archive-url=https://web.archive.org/web/20200302165302/https://news.china.com/zw/news/13000776/20200209/37780703.html|archive-date=2 March 2020|access-date=11 February 2020}}</ref> On 30 December, a test report addressed to Wuhan Central Hospital, from company CapitalBio Medlab, stated an erroneous positive result for ], causing a group of doctors at Wuhan Central Hospital to alert their colleagues and relevant hospital authorities of the result. The Wuhan Municipal Health Commission issued a notice to various medical institutions on "the treatment of pneumonia of unknown cause" that same evening.<ref>{{#invoke:Cite web||title=Undiagnosed pneumonia – China (HU): RFI|url=https://promedmail.org/promed-post/?id=6864153|access-date=7 May 2020|website=ProMED Mail |publisher=ProMED}}</ref> Eight of these doctors, including ] (punished on 3{{spaces}}January),<ref>{{#invoke:cite news||date=7 February 2020|title='Hero who told the truth': Chinese rage over coronavirus death of whistleblower doctor|work=]|url=https://www.theguardian.com/global-development/2020/feb/07/coronavirus-chinese-rage-death-whistleblower-doctor-li-wenliang}}</ref> were later admonished by the police for spreading false rumours and another, ], was reprimanded by her superiors for raising the alarm.<ref>{{#invoke:cite news ||vauthors=Kuo L |date=11 March 2020 |title=Coronavirus: Wuhan doctor speaks out against authorities |work=The Guardian |location=London|url=https://www.theguardian.com/world/2020/mar/11/coronavirus-wuhan-doctor-ai-fen-speaks-out-against-authorities}}</ref> | |||
| The ] announced in February 2020 that COVID-19 is the official name of the disease. World Health Organisation chief ] explained that ''CO'' stands for {{Em|corona}}, ''VI'' for {{Em|virus}} and ''D'' for {{Em|disease}}, while ''19'' is for when the outbreak was first identified: 31 December 2019.<ref>{{cite news |title=Novel coronavirus disease 2019 (COVID-19) pandemic: increased transmission in the EU/EEA and the UK—sixth update |url=https://www.ecdc.europa.eu/sites/default/files/documents/RRA-sixth-update-Outbreak-of-novel-coronavirus-disease-2019-COVID-19.pdf |access-date=26 March 2020 |publisher=ecdc |archive-url=https://web.archive.org/web/20200314223709/https://www.ecdc.europa.eu/sites/default/files/documents/RRA-sixth-update-Outbreak-of-novel-coronavirus-disease-2019-COVID-19.pdf |archive-date=14 March 2020 |url-status=live }}</ref> The name had been chosen to avoid references to a specific geographical location (e.g. China), animal species or group of people, in line with international recommendations for naming aimed at preventing ].<ref>{{cite news |title=Novel coronavirus named 'Covid-19': WHO |url=https://www.todayonline.com/world/wuhan-novel-coronavirus-named-covid-19-who |access-date=11 February 2020 |publisher=TODAYonline |name-list-format=vanc |archive-url=https://archive.today/20200321085608/https://www.todayonline.com/world/wuhan-novel-coronavirus-named-covid-19-who |archive-date=21 March 2020 |url-status=live }}</ref><ref name="veconomist">{{cite news|author= |title= The coronavirus spreads racism against—and among—ethnic Chinese|url= https://www.economist.com/china/2020/02/17/the-coronavirus-spreads-racism-against-and-among-ethnic-chinese|work= ]|date= 17 February 2020|access-date= 17 February 2020|archive-url= https://web.archive.org/web/20200217223902/https://www.economist.com/china/2020/02/17/the-coronavirus-spreads-racism-against-and-among-ethnic-chinese|archive-date= 17 February 2020|url-status= live| name-list-format = vanc}}</ref> | |||
| The |
The Wuhan Municipal Health Commission made the first public announcement of a pneumonia outbreak of unknown cause on 31 December, confirming 27 cases<ref name="AutoDW-69">{{#invoke:Cite web||title=Novel Coronavirus |url=https://www.who.int/westernpacific/emergencies/novel-coronavirus|access-date=6 February 2020|url-status=live|archive-url=https://web.archive.org/web/20200202151307/https://www.who.int/westernpacific/emergencies/novel-coronavirus|archive-date=2 February 2020|work=] (WHO)}}</ref><ref>{{#invoke:cite news||date=31 December 2019|title=武汉现不明原因肺炎 官方确认属实: 已经做好隔离|publisher=Xinhua Net 新華網 |url=https://news.163.com/19/1231/10/F1NGTJNJ00019K82.html|access-date=31 March 2020}}</ref><ref name="AutoDW-68">{{#invoke:Cite web||date=31 December 2019|script-title=zh:武汉市卫健委关于当前我市肺炎疫情的情况通报 |url=https://wjw.wuhan.gov.cn/front/web/showDetail/2019123108989|url-status=dead|archive-url=https://web.archive.org/web/20200109215413/https://wjw.wuhan.gov.cn/front/web/showDetail/2019123108989|archive-date=9 January 2020 |access-date=8 February 2020|work=WJW.Wuhan.gov.cn|publisher=Wuhan Municipal Health Commission|language=zh}}</ref>{{snd}}enough to trigger an investigation.<ref name="bbc50984025">{{#invoke:cite news||date=3 January 2020|title=Mystery pneumonia virus probed in China|work=]|url=https://www.bbc.com/news/world-asia-china-50984025|url-status=live|access-date=29 January 2020|archive-url=https://web.archive.org/web/20200105051949/https://www.bbc.com/news/world-asia-china-50984025|archive-date=5 January 2020}}</ref> | ||
| During the early stages of the outbreak, the number of cases doubled approximately every seven and a half days.<ref name="Qun29Jan2020">{{#invoke:cite journal || vauthors = Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung KS, Lau EH, Wong JY, Xing X, Xiang N, Wu Y, Li C, Chen Q, Li D, Liu T, Zhao J, Liu M, Tu W, Chen C, Jin L, Yang R, Wang Q, Zhou S, Wang R, Liu H, Luo Y, Liu Y, Shao G, Li H, Tao Z, Yang Y, Deng Z, Liu B, Ma Z, Zhang Y, Shi G, Lam TT, Wu JT, Gao GF, Cowling BJ, Yang B, Leung GM, Feng Z | title = Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia | journal = The New England Journal of Medicine | volume = 382 | issue = 13 | pages = 1199–1207 | date = March 2020 | pmid = 31995857 | pmc = 7121484 | doi = 10.1056/NEJMoa2001316 | title-link = doi | doi-access = free }}</ref> In early and mid-January 2020, the virus spread to other ], helped by the ] and Wuhan being a transport hub and major rail interchange.<ref name="WHOReport24Feb2020" /> On 20 January, China reported nearly 140 new cases in one day, including two people in Beijing and one in ].<ref name="france2420200120">{{#invoke:cite news||date=20 January 2020|title=China confirms sharp rise in cases of SARS-like virus across the country|url=https://www.france24.com/en/20200120-china-confirms-sharp-rise-in-cases-of-sars-like-virus-across-the-country|url-status=live|access-date=20 January 2020|archive-url=https://web.archive.org/web/20200120055618/https://www.france24.com/en/20200120-china-confirms-sharp-rise-in-cases-of-sars-like-virus-across-the-country|archive-date=20 January 2020}}</ref> Later official data shows 6,174 people had already developed symptoms by then,<ref name="Epidemiology17Feb2020">{{#invoke:cite journal||vauthors=((The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team)) |date=February 2020|title=The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID-19) – China, 2020 |journal=China CDC Weekly|volume=2|issue=8|pages=113–122|doi=10.46234/ccdcw2020.032 |pmid=34594836|pmc=839292|doi-access=free |title-link=doi}}</ref> and more may have been infected.<ref name="flattery">{{#invoke:cite news||date=25 April 2020|title=Flattery and foot dragging: China's influence over the WHO under scrutiny|work=] |url=https://www.theglobeandmail.com/world/article-flattery-and-foot-dragging-chinas-influence-over-the-who-under/}}</ref> A report in ''The Lancet'' on 24 January indicated human transmission, strongly recommended personal protective equipment for health workers, and said testing for the virus was essential due to its "pandemic potential".<ref name="Huang24Jan2020">{{#invoke:cite journal ||vauthors=Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B |title=Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China |journal=Lancet |volume=395 |issue=10223 |pages=497–506 |date=February 2020 |pmid=31986264 |pmc=7159299 |doi=10.1016/S0140-6736(20)30183-5 |doi-access=free |title-link=doi}}</ref><ref name="Horton 18 March">{{#invoke:Cite web|| vauthors=Horton R |author-link=Richard Horton (editor)|date=18 March 2020 |title=Scientists have been sounding the alarm on coronavirus for months. Why did Britain fail to act?|url=https://www.theguardian.com/commentisfree/2020/mar/18/coronavirus-uk-expert-advice-wrong |access-date=23 April 2020|website=The Guardian}}</ref> On 30 January, the WHO declared COVID-19 a ].<ref name="flattery" /> By this time, the outbreak spread by a factor of 100 to 200 times.<ref>{{#invoke:Cite web||date=2 June 2020|title=China delayed releasing coronavirus info, frustrating WHO |work=Associated Press |url=https://apnews.com/3c061794970661042b18d5aeaaed9fae|access-date=3 June 2020}}</ref> | |||
| During the initial outbreak in Wuhan, China, the virus and disease were commonly referred to as "coronavirus" and "Wuhan coronavirus".<ref>{{cite web|url=https://www.npr.org/sections/health-shots/2020/01/24/799208865/a-second-u-s-case-of-wuhan-coronavirus-is-confirmed|title=2nd U.S. Case Of Wuhan Coronavirus Confirmed|website=NPR.org|language=en|access-date=2020-04-04}}</ref><ref>{{Cite news|last=Jr|first=Donald G. McNeil|url=https://www.nytimes.com/2020/02/02/health/coronavirus-pandemic-china.html|title=Wuhan Coronavirus Looks Increasingly Like a Pandemic, Experts Say|date=2020-02-02|work=The New York Times|access-date=2020-04-04|language=en-US|issn=0362-4331}}</ref><ref>{{cite news|url=https://www.cnn.com/2020/02/05/asia/wuhan-coronavirus-update-death-toll-spike-intl-hnk/index.html|title=Wuhan coronavirus deaths spike again as outbreak shows no signs of slowing|last=Griffiths|first=James|website=CNN|access-date=2020-04-04}}</ref> In January 2020, WHO recommended 2019-nCov<ref>{{cite web|url=https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200121-sitrep-1-2019-ncov.pdf|title=Novel Coronavirus (2019-nCoV) SITUATION REPORT—1|last=|first=|date=21 January 2020|website=WHO|url-status=live|archive-url=|archive-date=|access-date=}}</ref> and 2019-nCoV acute respiratory disease<ref>{{cite web|url=https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200130-sitrep-10-ncov.pdf|title=Novel Coronavirus(2019-nCoV) Situation Report—10|last=|first=|date=30 January 2020|website=WHO|url-status=live|archive-url=|archive-date=|access-date=}}</ref> as interim names for the virus and disease in accordance with 2015 guidance against using locations in disease and virus names.<ref>{{cite web|url=https://apps.who.int/iris/bitstream/handle/10665/163636/WHO_HSE_FOS_15.1_eng.pdf|title=World Health Organization Best Practices for the Naming of New Human Infectious Diseases|last=|first=|date=May 2015|website=WHO|url-status=live|archive-url=|archive-date=|access-date=}}</ref> The official names COVID-19 and SARS-CoV-2 were issued on 11 February 2020.<ref>{{cite web|url=https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it|title=Naming the coronavirus disease (COVID-19) and the virus that causes it|website=www.who.int|language=en|access-date=2020-04-04}}</ref><ref>{{cite web|url=https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200130-sitrep-10-ncov.pdf?sfvrsn=d0b2e480_2|title=Novel Coronavirus(2019-nCoV) Situation Report—10|date=30 January 2020|website=] (WHO)|url-status=live|archive-url=https://web.archive.org/web/20200131005409/https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200130-sitrep-10-ncov.pdf|archive-date=31 January 2020|access-date=15 March 2020}}</ref> | |||
| Italy had its first confirmed cases on 31 January 2020, two tourists from China.<ref name="Corriere_20Jan">{{#invoke:Cite web||date=31 January 2020|title=Coronavirus: Primi due casi in Italia|trans-title=Coronavirus: First two cases in Italy|url=https://www.corriere.it/cronache/20_gennaio_30/coronavirus-italia-corona-9d6dc436-4343-11ea-bdc8-faf1f56f19b7.shtml|access-date=31 January 2020|work=Corriere della sera|language=it}}</ref> Italy overtook China as the country with the most deaths on 19 March 2020.<ref name="sky11960412">{{#invoke:Cite news||title=Coronavirus: Number of COVID-19 deaths in Italy surpasses China as total reaches 3,405|url=https://news.sky.com/story/coronavirus-number-of-covid-19-deaths-in-italy-surpasses-china-as-total-reaches-3-405-11960412|access-date=7 May 2020|newspaper=Sky News}}</ref> By 26 March the United States had overtaken China and Italy with the highest number of confirmed cases in the world.<ref name="NYT-20200326">{{#invoke:cite news|| vauthors = McNeil Jr DG |author-link=Donald McNeil Jr.|date=26 March 2020|title=The U.S. Now Leads the World in Confirmed Coronavirus Cases|work=The New York Times|url=https://www.nytimes.com/2020/03/26/health/usa-coronavirus-cases.html |archive-url=https://web.archive.org/web/20200326211527/https://www.nytimes.com/2020/03/26/health/usa-coronavirus-cases.html |archive-date=26 March 2020 |url-access=subscription |url-status=live|access-date=27 March 2020}}</ref> Research on coronavirus genomes indicates the majority of COVID-19 cases in ] came from European travellers, rather than directly from China or any other Asian country.<ref name="20200408nytimes">{{#invoke:cite news||date=8 April 2020|title=Studies Show N.Y. Outbreak Originated in Europe|work=The New York Times|url=https://www.nytimes.com/2020/04/08/us/coronavirus-live-updates.html |archive-url=https://web.archive.org/web/20200408185016/https://www.nytimes.com/2020/04/08/us/coronavirus-live-updates.html |archive-date=8 April 2020 |url-access=subscription |url-status=live}}</ref> Retesting of prior samples found a person in France who had the virus on 27 December 2019,<ref name="France-retest">{{#invoke:cite news|| vauthors = Irish J |date=4 May 2020|title=After retesting samples, French hospital discovers COVID-19 case from December|work=]| veditors = Lough RM, Graff P |url=https://www.reuters.com/article/us-health-coronavirus-france-idUSKBN22G20L|access-date=4 May 2020}}</ref><ref name="Deslandes-2020">{{#invoke:cite journal || vauthors = Deslandes A, Berti V, Tandjaoui-Lambotte Y, Alloui C, Carbonnelle E, Zahar JR, Brichler S, Cohen Y | title = SARS-CoV-2 was already spreading in France in late December 2019 | journal = International Journal of Antimicrobial Agents | volume = 55 | issue = 6 | pages = 106006 | date = June 2020 | pmid = 32371096 | pmc = 7196402 | doi = 10.1016/j.ijantimicag.2020.106006 }}</ref> and a person in the United States who died from the disease on 6{{spaces}}February 2020.<ref name="PBS-2wks">{{#invoke:Cite web||date=22 April 2020|title=2 died with coronavirus weeks before 1st U.S. virus death|url=https://www.pbs.org/newshour/nation/2-died-with-coronavirus-weeks-before-1st-u-s-virus-death|access-date=23 April 2020|website=PBS NewsHour}}</ref> | |||
| ===Manufacturing=== | |||
| RT-PCR testing of untreated wastewater samples from Brazil and Italy have suggested detection of SARS-CoV-2 as early as November and December 2019, respectively, but the methods of such sewage studies have not been optimised, many have not been peer-reviewed, details are often missing, and there is a risk of false positives due to contamination or if only one gene target is detected.<ref>{{#invoke:cite journal || vauthors = Michael-Kordatou I, Karaolia P, Fatta-Kassinos D | title = Sewage analysis as a tool for the COVID-19 pandemic response and management: the urgent need for optimised protocols for SARS-CoV-2 detection and quantification | journal = Journal of Environmental Chemical Engineering | volume = 8 | issue = 5 | pages = 104306 | date = October 2020 | pmid = 32834990 | pmc = 7384408 | doi = 10.1016/j.jece.2020.104306 }}</ref> A September 2020 review journal article said, "The possibility that the COVID‑19 infection had already spread to Europe at the end of last year is now indicated by abundant, even if partially circumstantial, evidence", including pneumonia case numbers and radiology in France and Italy in November and December.<ref name=platto>{{#invoke:cite journal || vauthors = Platto S, Xue T, Carafoli E | title = COVID19: an announced pandemic | journal = Cell Death & Disease | volume = 11 | issue = 9 | pages = 799 | date = September 2020 | pmid = 32973152 | pmc = 7513903 | doi = 10.1038/s41419-020-02995-9 }}</ref> | |||
| Due to failures in the ]s, digital manufacturers are working to make healthcare material such as nasal swabs and ventilator parts.<ref>{{cite news |last1=Temple |first1=James |title=How 3D printing could save lives in the coronavirus outbreak |url=https://www.technologyreview.com/s/615420/3d-printing-coronavirus-covid-19-medical-supplies-devices/ |accessdate=5 April 2020 |work=MIT Technology Review}}</ref><ref>{{cite news |last1=Tibken |first1=Shara |title=3D printing may help supply more essential coronavirus medical gear |url=https://www.cnet.com/news/3d-printing-may-help-supply-more-essential-coronavirus-medical-gear/ |accessdate=5 April 2020 |work=CNET |language=en}}</ref> An Italian startup employed ] technology to produce valves for ventilators.<ref>{{cite news |title= Italian hospital saves Covid-19 patients lives by 3D printing valves for reanimation devices |url=https://www.3dprintingmedia.network/covid-19-3d-printed-valve-for-reanimation-device/ |accessdate=20 March 2020 |work=3D Printing Media Network |date=14 March 2020}}</ref> 3D printed valves costed $1 instead of $10,000 and were ready overnight.<ref>{{cite news |last1=Peters |first1=Jay |title=Volunteers produce 3D-printed valves for life-saving coronavirus treatments |url=https://www.theverge.com/2020/3/17/21184308/coronavirus-italy-medical-3d-print-valves-treatments |accessdate=20 March 2020 |work=The Verge |date=17 March 2020 |language=en}}</ref> | |||
| {{As of|2021|10|1}}, '']'' reported that it had estimated the worldwide total number of deaths due to COVID‑19 to have exceeded five million.<ref>{{#invoke:cite news||url=https://www.reuters.com/world/global-covid-19-deaths-hit-5-million-delta-variant-sweeps-world-2021-10-02/|title=Global COVID-19 deaths hit 5 million as Delta variant sweeps the world| vauthors = Kavya B, Abraham R |agency=Reuters| veditors = Shumaker L, Wardell J |date=3 October 2021|work=Reuters.com}}</ref> | |||
| ===Misinformation=== | |||
| The Public Health Emergency of International Concern for COVID-19 ended on May 5, 2023. By this time, everyday life in most countries had returned to how it was before the pandemic.<ref>{{#invoke:cite web||title=From emergency response to long-term COVID-19 disease management: sustaining gains made during the COVID-19 pandemic|url=https://www.who.int/publications/i/item/WHO-WHE-SPP-2023.1|publisher=] (WHO)|access-date=9 May 2023}}</ref><ref>{{#invoke:cite web||date=5 May 2023|title=WHO ends global health emergency declaration for COVID-19|url=https://www.npr.org/sections/goatsandsoda/2023/05/05/1174269442/who-ends-global-health-emergency-declaration-for-covid-19|website=NPR|first1=Giulia|last1=Heyward|first2=Marc|last2=Silver|access-date=9 May 2023}}</ref> | |||
| {{main|Misinformation related to the 2019–20 coronavirus pandemic}} | |||
| == Misinformation == | |||
| After the initial ] of COVID-19, conspiracy theories, ] and ] emerged regarding the origin, scale, prevention, treatment and other aspects of the disease and rapidly spread online.<ref name="bbc_misinfo">{{Cite news |url=https://www.bbc.com/news/blogs-trending-51271037 |title=China coronavirus: Misinformation spreads online about origin and scale |date=30 January 2020 |work=] |access-date=10 February 2020 |archive-url= https://web.archive.org/web/20200204163412/https://www.bbc.com/news/blogs-trending-51271037 |archive-date=4 February 2020 |url-status=live}}</ref><ref name=GUAR>{{cite newspaper | url=https://www.theguardian.com/world/2020/jan/31/bat-soup-dodgy-cures-and-diseasology-the-spread-of-coronavirus-bunkum | title=Bat soup, dodgy cures and 'diseasology': the spread of coronavirus misinformation | date=January 31, 2020 | accessdate=February 3, 2020 | first=Josh | last=Taylor | newspaper=] | archive-url=https://web.archive.org/web/20200202141231/https://www.theguardian.com/world/2020/jan/31/bat-soup-dodgy-cures-and-diseasology-the-spread-of-coronavirus-bunkum | archive-date=February 2, 2020 | url-status=live }}</ref><ref name=Lowy>{{Cite news|first=Natasha|last=Kassam|url=https://www.lowyinstitute.org/the-interpreter/disinformation-and-coronavirus|work=The Interpreter|publisher=Lowy Institute|title=Disinformation and coronavirus|date=March 25, 2020}}</ref><ref name=RunningList>{{cite web |url=https://www.buzzfeednews.com/article/janelytvynenko/coronavirus-disinformation-spread |title=Here's A Running List Of Disinformation Spreading About The Coronavirus |website=Buzzfeed News |access-date=February 8, 2020 |archive-url=https://web.archive.org/web/20200206212717/https://www.buzzfeednews.com/article/janelytvynenko/coronavirus-disinformation-spread |archive-date=February 6, 2020 |url-status=live }}</ref> | |||
| {{Main|COVID-19 misinformation}} | |||
| After the initial outbreak of COVID{{nbhyph}}19, ] and ] regarding the origin, scale, prevention, treatment, and other aspects of the disease rapidly spread online.<ref name="bbc_misinfo">{{#invoke:cite news ||url=https://www.bbc.com/news/blogs-trending-51271037 |title=China coronavirus: Misinformation spreads online about origin and scale |date=30 January 2020 |work=] |access-date=10 February 2020 |archive-url=https://web.archive.org/web/20200204163412/https://www.bbc.com/news/blogs-trending-51271037 |archive-date=4 February 2020 |url-status=live}}</ref><ref name="GUAR">{{#invoke:cite news ||url=https://www.theguardian.com/world/2020/jan/31/bat-soup-dodgy-cures-and-diseasology-the-spread-of-coronavirus-bunkum |title=Bat soup, dodgy cures and 'diseasology': the spread of coronavirus misinformation |date=31 January 2020 |access-date=3 February 2020 | vauthors = Taylor J |newspaper=] |archive-url=https://web.archive.org/web/20200202141231/https://www.theguardian.com/world/2020/jan/31/bat-soup-dodgy-cures-and-diseasology-the-spread-of-coronavirus-bunkum |archive-date=2 February 2020 |url-status=live}}</ref><ref name="RunningList">{{#invoke:Cite web||url=https://www.buzzfeednews.com/article/janelytvynenko/coronavirus-disinformation-spread |title=Here's A Running List Of Disinformation Spreading About The Coronavirus |website=Buzzfeed News |access-date=8 February 2020 |archive-url=https://web.archive.org/web/20200206212717/https://www.buzzfeednews.com/article/janelytvynenko/coronavirus-disinformation-spread |archive-date=6 February 2020 |url-status=dead}}</ref> | |||
| In September 2020, the US Centers for Disease Control and Prevention (CDC) published preliminary estimates of the risk of death by age groups in the United States, but those estimates were widely misreported and misunderstood.<ref name=":1" /><ref>{{#invoke:Cite web||date=8 October 2020|title=Misleading claim circulates online about infection fatality ratio of Covid-19 in the US|url=https://factcheck.afp.com/misleading-claim-circulates-online-about-infection-fatality-ratio-covid-19-us|access-date=10 October 2020|website=Fact Check}}</ref> | |||
| ==Research== | |||
| == Other species == | |||
| {{Main|COVID-19 drug development}} | |||
| {{See also|Impact of the COVID-19 pandemic on animals}} | |||
| International ] programs on vaccines and therapeutic drug candidates having potential to reduce illnesses caused by COVID-19 are underway by government organisations, academic groups and industry researchers.<ref name="dhama">{{cite journal | vauthors=Dhama K, Sharun K, Tiwari R, Dadar M, Malik YS, Singh KP, Chaicumpa W | display-authors=6 | title=COVID-19, an emerging coronavirus infection: advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics | journal=Human Vaccines and Immunotherapeutics| date=March 2020 | issn=2164-5515 | pmid=32186952 | doi=10.1080/21645515.2020.1735227 | pages=1–7 | pmc=7103671 |doi-access=free}}</ref><ref name="zhang2020">{{cite journal | vauthors=Zhang L, Liu Y | title=Potential interventions for novel coronavirus in China: A systematic review | journal=Journal of Medical Virology | volume=92 | issue=5 | date=March 2020 | issn=0146-6615 | doi=10.1002/jmv.25707 | pages=479–90 | pmid=32052466 }}</ref> In March, the ] initiated the "]" in 10 countries, enrolling thousands of people infected with COVID-19 to assess treatment effects of four existing antiviral compounds with the most promise of efficacy.<ref name="kai">{{cite journal|last1=Kupferschmidt|first1=Kai|last2=Cohen|first2=Jon| title=WHO launches global megatrial of the four most promising coronavirus treatments |journal=Science Magazine | date=22 March 2020 | url=https://www.sciencemag.org/news/2020/03/who-launches-global-megatrial-four-most-promising-coronavirus-treatments | access-date=27 March 2020|doi=10.1126/science.abb8497}}</ref> | |||
| Humans appear to be capable of spreading the virus to some other animals,<ref name="Gryseels_2021_MammalReview">{{#invoke:cite journal||last1=Gryseels |first1=Sophie |last2=De Bruyn |first2=Luc |last3=Gyselings |first3=Ralf |last4=Calvignac-Spencer |first4=Sébastien |last5=Leendertz |first5=Fabian H. |last6=Leirs |first6=Herwig |title=Risk of human-to-wildlife transmission of SARS-CoV-2 |journal=Mammal Review |date=April 2021 |volume=51 |issue=2 |pages=272–292 |doi=10.1111/mam.12225 |pmid=33230363 |pmc=7675675 |hdl=10067/1726730151162165141 |issn=0305-1838}}</ref><ref name="TanLam2022_NatComm">{{#invoke:cite journal||last1=Tan |first1=Cedric C. S. |last2=Lam |first2=Su Datt |last3=Richard |first3=Damien |last4=Owen |first4=Christopher J. |last5=Berchtold |first5=Dorothea |last6=Orengo |first6=Christine |last7=Nair |first7=Meera Surendran |last8=Kuchipudi |first8=Suresh V. |last9=Kapur |first9=Vivek |last10=van Dorp |first10=Lucy |last11=Balloux |first11=François |title=Transmission of SARS-CoV-2 from humans to animals and potential host adaptation |journal=Nature Communications |date=27 May 2022 |volume=13 |issue=1 |pages=2988 |doi=10.1038/s41467-022-30698-6 |pmid=35624123 |pmc=9142586 |bibcode=2022NatCo..13.2988T |url=https://doi.org/10.1038/s41467-022-30698-6 |access-date=28 February 2023 |issn=2041-1723}}</ref> a type of disease transmission referred to as ].<ref name="Pappas_MDPI_2022">{{#invoke:cite journal ||last1=Pappas |first1=Georgios |last2=Vokou |first2=Despoina |last3=Sainis |first3=Ioannis |last4=Halley |first4=John M. |title=SARS-CoV-2 as a Zooanthroponotic Infection: Spillbacks, Secondary Spillovers, and Their Importance |journal=Microorganisms |date=November 2022 |volume=10 |issue=11 |pages=2166 |doi=10.3390/microorganisms10112166 |pmid=36363758 |pmc=9696655 |issn=2076-2607|doi-access=free }}</ref><ref name="MunirAshraf_2020">{{#invoke:cite journal ||last1=Munir |first1=Khalid |last2=Ashraf |first2=Shoaib |last3=Munir |first3=Isra |last4=Khalid |first4=Hamna |last5=Muneer |first5=Mohammad Akram |last6=Mukhtar |first6=Noreen |last7=Amin |first7=Shahid |last8=Ashraf |first8=Sohaib |last9=Imran |first9=Muhammad Ahmad |last10=Chaudhry |first10=Umer |last11=Zaheer |first11=Muhammad Usman |last12=Arshad |first12=Maria |last13=Munir |first13=Rukhsana |last14=Ahmad |first14=Ali |last15=Zhao |first15=Xin |title=Zoonotic and reverse zoonotic events of SARS-CoV-2 and their impact on global health |journal=Emerging Microbes & Infections |date=1 January 2020 |volume=9 |issue=1 |pages=2222–2235 |doi=10.1080/22221751.2020.1827984 |pmid=32967592 |pmc=7594747 }}</ref> | |||
| Some pets, especially cats and ]s, can catch this virus from infected humans.<ref name="Kampf-2020">{{#invoke:cite journal || vauthors = Kampf G, Brüggemann Y, Kaba HE, Steinmann J, Pfaender S, Scheithauer S, Steinmann E | title = Potential sources, modes of transmission and effectiveness of prevention measures against SARS-CoV-2 | journal = The Journal of Hospital Infection | volume = 106 | issue = 4 | pages = 678–697 | date = December 2020 | pmid = 32956786 | pmc = 7500278 | doi = 10.1016/j.jhin.2020.09.022 }}</ref><ref>{{#invoke:cite journal || vauthors = Shi J, Wen Z, Zhong G, Yang H, Wang C, Huang B, Liu R, He X, Shuai L, Sun Z, Zhao Y, Liu P, Liang L, Cui P, Wang J, Zhang X, Guan Y, Tan W, Wu G, Chen H, Bu Z | title = Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2 | journal = Science | volume = 368 | issue = 6494 | pages = 1016–1020 | date = May 2020 | pmid = 32269068 | pmc = 7164390 | doi = 10.1126/science.abb7015 }}</ref> Symptoms in cats include ] (such as a cough) and digestive symptoms.<ref name="Kampf-2020" /> Cats can spread the virus to other cats, and may be able to spread the virus to humans, but cat-to-human transmission of SARS-CoV-2 has not been proven.<ref name="Kampf-2020" /><ref name="Tazerji-2020">{{#invoke:cite journal || vauthors = Salajegheh Tazerji S, Magalhães Duarte P, Rahimi P, Shahabinejad F, Dhakal S, Singh Malik Y, Shehata AA, Lama J, Klein J, Safdar M, Rahman MT, Filipiak KJ, Rodríguez-Morales AJ, Sobur MA, Kabir F, Vazir B, Mboera L, Caporale M, Islam MS, Amuasi JH, Gharieb R, Roncada P, Musaad S, Tilocca B, Koohi MK, Taghipour A, Sait A, Subbaram K, Jahandideh A, Mortazavi P, Abedini MA, Hokey DA, Hogan U, Shaheen MN, Elaswad A, Elhaig MM, Fawzy M |title = Transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) to animals: an updated review | journal = Journal of Translational Medicine | volume = 18 | issue = 1 | pages = 358 | date = September 2020 | pmid = 32957995 | pmc = 7503431 | doi = 10.1186/s12967-020-02534-2 |doi-access=free}}</ref> Compared to cats, dogs are less susceptible to this infection.<ref name="Tazerji-2020" /> Behaviours which increase the risk of transmission include kissing, licking, and petting the animal.<ref name="Tazerji-2020" /> | |||
| Personal hygiene and a healthy lifestyle and ] have been recommended to improve immunity.<ref>{{cite journal |last1=Wang |first1=Li-sheng |last2=Wang |first2=Yi-ru |last3=Ye |first3=Da-wei |last4=Liu |first4=Qing-quan |title=A review of the 2019 novel coronavirus (COVID-19) based on current evidence |journal=International Journal of Antimicrobial Agents |date=19 March 2020 |page=105948 |doi=10.1016/j.ijantimicag.2020.105948 |pmid=32201353 |url=https://www.sciencedirect.com/science/article/pii/S0924857920300984 |accessdate=27 March 2020 |language=en |issn=0924-8579 |archive-url=https://web.archive.org/web/20200327232545/https://www.sciencedirect.com/science/article/pii/S0924857920300984 |archive-date=27 March 2020 |url-status=live }}</ref> | |||
| The virus does not appear to be able to infect ]s, ]s, or chickens at all.<ref name="Kampf-2020" /> ], rats, and rabbits, if they can be infected at all, are unlikely to be involved in spreading the virus.<ref name="Tazerji-2020" /> | |||
| ===Vaccine=== | |||
| Tigers and lions in zoos have become infected as a result of contact with infected humans.<ref name="Tazerji-2020" /> As expected, monkeys and ] species such as ]s can also be infected with the COVID‑19 virus.<ref name="Tazerji-2020" /> | |||
| {{main|COVID-19 vaccine}} | |||
| ]s, which are in the ] as ferrets, have been infected.<ref name="Tazerji-2020" /> Minks may be asymptomatic, and can also spread the virus to humans.<ref name="Tazerji-2020" /> Multiple countries have identified infected animals in ]s.<ref name="Gorman-2021">{{#invoke:cite news|| vauthors = Gorman J |date=22 January 2021|title=The Coronavirus Kills Mink, So They Too May Get a Vaccine|work=The New York Times |url=https://www.nytimes.com/2021/01/22/science/covid-mink-vaccine.html |archive-url=https://ghostarchive.org/archive/20211228/https://www.nytimes.com/2021/01/22/science/covid-mink-vaccine.html |archive-date=28 December 2021 |url-access=limited|access-date=24 February 2021|issn=0362-4331| url-status=live}}</ref> ], a major producer of mink pelts, ordered the slaughter of all minks over fears of viral mutations,<ref name="Gorman-2021" /> following an outbreak referred to as ]. A vaccine for mink and other animals is being researched.<ref name="Gorman-2021" /> | |||
| There is no available vaccine, but various agencies are actively developing vaccine candidates. Previous work on ] is being utilised because SARS-CoV-2 and SARS-CoV both use the ACE2 receptor to enter human cells.<ref>{{cite book | vauthors=Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R | chapter=Features, Evaluation and Treatment Coronavirus (COVID-19) | title=StatPearls | chapter-url=https://www.ncbi.nlm.nih.gov/books/NBK554776/ |date=March 2020 | publisher=StatPearls |pmid= 32150360 | id=Bookshelf ID: NBK554776 }}</ref> There are three vaccination strategies being investigated. First, researchers aim to build a whole virus vaccine. The use of such a virus, be it ] or dead, aims to elicit a prompt ] of the human body to a new infection with COVID-19. A second strategy, subunit vaccines, aims to create a vaccine that sensitises the immune system to certain subunits of the virus. In the case of SARS-CoV-2, such research focuses on the S-spike protein that helps the virus intrude the ] receptor. A third strategy is that of the nucleic acid vaccines (] or ], a novel technique for creating a vaccination). Experimental vaccines from any of these strategies would have to be tested for safety and efficacy.<ref name="Chen Strych Hotez Bottazzi p.">{{cite journal | vauthors = Chen WH, Strych U, Hotez PJ, Bottazzi ME | title=The SARS-CoV-2 Vaccine Pipeline: an Overview | journal=Current Tropical Medicine Reports | date=3 March 2020 | pages=1–4 |doi=10.1007/s40475-020-00201-6 |doi-access=free | pmid=32219057 | pmc=7094941 | name-list-format = vanc}}</ref> | |||
| == Research == | |||
| On 16 March 2020, the first clinical trial of a vaccine started with four volunteers in ]. The vaccine contains a harmless genetic code copied from the virus that causes the disease.<ref>{{Cite news |last=Roberts |first=Michelle |name-list-format=vanc |url=https://www.bbc.com/news/health-51906604 |title=Coronavirus: US volunteers test first vaccine |date=17 March 2020 |work=BBC News |access-date=17 March 2020 |language=en-GB |archive-url=https://web.archive.org/web/20200317034657/https://www.bbc.com/news/health-51906604 |archive-date=17 March 2020 |url-status=live }}</ref> | |||
| {{Further|COVID-19 drug development}} | |||
| International research on vaccines and medicines in COVID{{nbhyph}}19 is underway by government organisations, academic groups, and industry researchers.<ref name="dhama">{{#invoke:cite journal || vauthors = Dhama K, Sharun K, Tiwari R, Dadar M, Malik YS, Singh KP, Chaicumpa W | title = COVID-19, an emerging coronavirus infection: advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics | journal = Human Vaccines & Immunotherapeutics | volume = 16 | issue = 6 | pages = 1232–1238 | date = June 2020 | pmid = 32186952 | pmc = 7103671 | doi = 10.1080/21645515.2020.1735227 | title-link = doi | doi-access = free }}</ref><ref name="zhang2020">{{#invoke:cite journal || vauthors = Zhang L, Liu Y | title = Potential interventions for novel coronavirus in China: A systematic review | journal = Journal of Medical Virology | volume = 92 | issue = 5 | pages = 479–490 | date = May 2020 | pmid = 32052466 | pmc = 7166986 | doi = 10.1002/jmv.25707 }}</ref> The CDC has classified it to require a ] grade laboratory.<ref name=inlbg>{{#invoke:Cite web||title=Interim Laboratory Biosafety Guidelines for Handling and Processing Specimens Associated with Coronavirus Disease 2019 (COVID-19) |url=https://www.cdc.gov/coronavirus/2019-ncov/lab/lab-biosafety-guidelines.html |website=Coronavirus Disease 2019 (COVID-19) Lab Biosafety Guidelines |date=11 February 2020 |publisher=U.S. ] (CDC) |access-date=1 April 2020}}</ref> There has been a great deal of COVID‑19 research, involving accelerated research processes and publishing shortcuts to meet the global demand.<ref name="Aristovnik et al. 2020">{{#invoke:cite journal||vauthors=Aristovnik A, Ravšelj D, Umek L|date=November 2020|title=A Bibliometric Analysis of COVID-19 across Science and Social Science Research Landscape|journal=Sustainability|volume=12|issue=21|pages=9132|doi=10.3390/su12219132|doi-access=free | title-link = doi |bibcode=2020Sust...12.9132A }}</ref> | |||
| {{As of|2020|December}}, hundreds of ]s have been undertaken, with research happening on every continent except ].<ref>{{#invoke:cite journal || vauthors = Kupferschmidt K |title=First-of-its-kind African trial tests common drugs to prevent severe COVID-19 |journal=] |date=3 December 2020 |doi=10.1126/science.abf9987 |doi-access=free | title-link=doi |url=https://www.science.org/content/article/first-its-kind-african-trial-tests-common-drugs-prevent-severe-covid-19 |access-date=8 March 2022 }}</ref> {{As of|2020|November}}, more than 200 possible treatments have been studied in humans.<ref>{{#invoke:Cite magazine || vauthors = Reardon S |date= November 2020|title=For COVID Drugs, Months of Frantic Development Lead to Few Outright Successes|url=https://www.scientificamerican.com/article/for-covid-drugs-months-of-frantic-development-lead-to-few-outright-successes/|access-date=10 December 2020|magazine=] }}</ref> | |||
| ===Post-infection treatments=== | |||
| === Transmission and prevention research === | |||
| {{Further|COVID-19 vaccine}} | |||
| ] research has been conducted with several objectives, including predictions of the dynamics of transmission,<ref>{{#invoke:cite journal || vauthors = Kucharski AJ, Russell TW, Diamond C, Liu Y, Edmunds J, Funk S, Eggo RM | title = Early dynamics of transmission and control of COVID-19: a mathematical modelling study | journal = The Lancet. Infectious Diseases | volume = 20 | issue = 5 | pages = 553–558 | date = May 2020 | pmid = 32171059 | pmc = 7158569 | doi = 10.1016/S1473-3099(20)30144-4 | doi-access = free | title-link = doi }}</ref> diagnosis and prognosis of infection,<ref>{{#invoke:cite journal||date=3 February 2021|title=Update to living systematic review on prediction models for diagnosis and prognosis of covid-19|url=https://pubmed.ncbi.nlm.nih.gov/33536183|journal=BMJ (Clinical Research Ed.)|volume=372|pages=n236|doi=10.1136/bmj.n236|issn=1756-1833|pmid=33536183|s2cid=231775762}}</ref> estimation of the impact of interventions,<ref>{{#invoke:cite journal || vauthors = Giordano G, Blanchini F, Bruno R, Colaneri P, Di Filippo A, Di Matteo A, Colaneri M | title = Modelling the COVID-19 epidemic and implementation of population-wide interventions in Italy | journal = Nature Medicine | volume = 26 | issue = 6 | pages = 855–860 | date = June 2020 | pmid = 32322102 | pmc = 7175834 | doi = 10.1038/s41591-020-0883-7|arxiv=2003.09861 | doi-access = free | title-link = doi }}</ref><ref>{{#invoke:cite journal || vauthors = Prem K, Liu Y, Russell TW, Kucharski AJ, Eggo RM, Davies N, Jit M, Klepac P | title = The effect of control strategies to reduce social mixing on outcomes of the COVID-19 epidemic in Wuhan, China: a modelling study | journal = The Lancet. Public Health | volume = 5 | issue = 5 | pages = e261–e270 | date = May 2020 | pmid = 32220655 | pmc = 7158905 | doi = 10.1016/S2468-2667(20)30073-6 | doi-access = free | title-link = doi }}</ref> or allocation of resources.<ref>{{#invoke:cite journal || vauthors = Emanuel EJ, Persad G, Upshur R, Thome B, Parker M, Glickman A, Zhang C, Boyle C, Smith M, Phillips JP | title = Fair Allocation of Scarce Medical Resources in the Time of Covid-19 | journal = The New England Journal of Medicine | volume = 382 | issue = 21 | pages = 2049–2055 | date = May 2020 | pmid = 32202722 | doi = 10.1056/NEJMsb2005114 | doi-access = free | title-link = doi }}</ref> Modelling studies are mostly based on ],<ref>{{#invoke:cite journal ||doi=10.1098/rspa.1927.0118 |volume=115 |issue=772 |pages=700–721 |title=A contribution to the mathematical theory of epidemics |journal=Proceedings of the Royal Society of London. Series A, Containing Papers of a Mathematical and Physical Character |year=1927 |bibcode=1927RSPSA.115..700K |doi-access=free | title-link = doi | vauthors = Kermack WO, McKendrick AG }}</ref> estimating the number of infected people over time under given conditions. Several other types of models have been developed and used during the COVID{{nbhyph}}19 pandemic including ] models to study the flow physics of COVID{{nbhyph}}19,<ref>{{#invoke:cite journal ||doi=10.1017/jfm.2020.330 |volume=894 |pages=–2 | vauthors = Mittal R, Ni R, Seo JH |title=The flow physics of COVID-19 |journal=Journal of Fluid Mechanics |year=2020 |arxiv=2004.09354 |bibcode=2020JFM...894F...2M |doi-access=free | title-link = doi }}</ref> retrofits of crowd movement models to study occupant exposure,<ref>{{#invoke:cite journal || vauthors = Ronchi E, Lovreglio R | title = EXPOSED: An occupant exposure model for confined spaces to retrofit crowd models during a pandemic | journal = Safety Science | volume = 130 | pages = 104834 | date = October 2020 | pmid = 32834509 | pmc = 7373681 | doi = 10.1016/j.ssci.2020.104834 | arxiv = 2005.04007 | doi-access = free | title-link = doi }}</ref> mobility-data based models to investigate transmission,<ref>{{#invoke:cite journal || vauthors = Badr HS, Du H, Marshall M, Dong E, Squire MM, Gardner LM | title = Association between mobility patterns and COVID-19 transmission in the USA: a mathematical modelling study | journal = The Lancet Infectious Diseases | volume = 20 | issue = 11 | pages = 1247–1254 | date = November 2020 | pmid = 32621869 | pmc = 7329287 | doi = 10.1016/S1473-3099(20)30553-3 | doi-access = free | title-link = doi }}</ref> or the use of ] models to assess the economic impact of the pandemic.<ref>{{#invoke:cite journal || vauthors = McKibbin W, Roshen F | title = The global macroeconomic impacts of COVID-19: Seven scenarios |journal=CAMA Working Paper |year=2020 |doi=10.2139/ssrn.3547729 |s2cid=216307705 |url= https://cama.crawford.anu.edu.au/sites/default/files/publication/cama_crawford_anu_edu_au/2020-03/19_2020_mckibbin_fernando_0.pdf }}</ref> | |||
| === Treatment-related research === | |||
| {{Main|COVID-19 drug repurposing research}} | {{Main|COVID-19 drug repurposing research}} | ||
| ] | |||
| Repurposed ]s make up most of the research into COVID‑19 treatments.<ref name="milken">{{#invoke:Cite web||date=21 April 2020|title=COVID-19 treatment and vaccine tracker|url=https://milkeninstitute.org/sites/default/files/2020-04/Covid19%20Tracker%20NEW4-21-20-2.pdf|access-date=21 April 2020|publisher=Milken Institute }}</ref><ref name="koch">{{#invoke:Cite web|| vauthors = Koch S, Pong W |date=13 March 2020|title=First up for COVID-19: nearly 30 clinical readouts before end of April|url=https://www.biocentury.com/article/304658|access-date=1 April 2020|publisher=BioCentury Inc.}}</ref> Other candidates in trials include ]s, ]s, immune therapies, ], ], and ] angiotensin-converting enzyme 2.<ref name="koch" /> | |||
| In March 2020, the World Health Organization (WHO) initiated the ] to assess the treatment effects of some promising drugs:<ref name="kai">{{#invoke:cite journal||vauthors=Kupferschmidt K, Cohen J|date=March 2020|title=WHO launches global megatrial of the four most promising coronavirus treatments|journal=Science|doi=10.1126/science.abb8497|doi-access=free|title-link=doi}}</ref><ref>{{#invoke:Cite web||url=https://news.un.org/en/story/2020/03/1059722|title=UN health chief announces global 'solidarity trial' to jumpstart search for COVID-19 treatment|date=18 March 2020|website=UN News|access-date=23 March 2020|archive-url=https://web.archive.org/web/20200323101633/https://news.un.org/en/story/2020/03/1059722|archive-date=23 March 2020|url-status=live}}</ref> | |||
| According to two organisations tracking clinical trial progress on potential therapeutic drugs for COVID-19 infections, 29 Phase II-IV efficacy trials were concluded in March 2020 or scheduled to provide results in April from hospitals in China – which experienced the first outbreak of COVID-19 in late 2019.<ref name="milken">{{cite web |title=COVID-19 treatment and vaccine tracker |url=https://milkeninstitute.org/sites/default/files/2020-04/Covid19%20Tracker%20NEW4-2-20-final.pdf |publisher=Milken Institute |accessdate=2 April 2020 |date=2 April 2020 |lay-url=https://milkeninstitute.org/covid-19-tracker }}</ref><ref name="koch">{{cite web |author1=Selina Koch |author2=Winnie Pong |title=First up for COVID-19: nearly 30 clinical readouts before end of April |url=https://www.biocentury.com/article/304658 |publisher=BioCentury Inc. |accessdate=1 April 2020 |date=13 March 2020}}</ref> Seven trials were evaluating repurposed drugs already approved to treat ], including four studies on hydroxychloroquine or chloroquine phosphate.<ref name=koch/> Repurposed ]s make up most of the Chinese research, with nine Phase III trials on remdesivir across several countries due to report by the end of April.<ref name=milken/><ref name=koch/> Other potential therapeutic candidates under pivotal clinical trials concluding in March–April are ]s, ]s, ], ], ] and ] ], among others.<ref name=koch/> | |||
| * An ] called ] | |||
| * ] drugs ] and ] | |||
| * Two ], ] and ] | |||
| More than 300 active clinical trials are underway as of April 2020.<ref name="Sanders202022" /> | |||
| The COVID-19 Clinical Research Coalition has goals to 1) facilitate rapid reviews of clinical trial proposals by ]s and national regulatory agencies, 2) fast-track approvals for the candidate therapeutic compounds, 3) ensure standardised and rapid analysis of emerging efficacy and safety data and 4) facilitate sharing of clinical trial outcomes before publication.<ref name="coalition">{{cite journal | title=Global coalition to accelerate COVID-19 clinical research in resource-limited settings | journal=The Lancet |author=COVID-19 Clinical Research Coalition| year=2020 | issn=0140-6736 | doi=10.1016/s0140-6736(20)30798-4 |url=https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30798-4/fulltext#articleInformation}}</ref><ref name="maguire">{{cite journal | last=Maguire | first=Brittany J. | last2=Guérin | first2=Philippe J. | title=A living systematic review protocol for COVID-19 clinical trial registrations | journal=Wellcome Open Research | volume=5 | date=2 April 2020 | issn=2398-502X | doi=10.12688/wellcomeopenres.15821.1 | page=60|url=https://wellcomeopenresearch.org/articles/5-60/v1}}</ref> A dynamic review of clinical development for COVID-19 vaccine and drug candidates was in place, as of April 2020.<ref name=maguire/> | |||
| Research on the antimalarial drugs hydroxychloroquine and chloroquine showed that they were ineffective at best,<ref name="20200526nytimes">{{#invoke:Cite web||date=26 May 2020|title=Citing safety concerns, the W.H.O. paused tests of a drug Trump said he had taken|url=https://www.nytimes.com/2020/05/26/world/coronavirus-news.html |archive-url=https://web.archive.org/web/20200526041004/https://www.nytimes.com/2020/05/26/world/coronavirus-news.html |archive-date=26 May 2020 |url-access=subscription |url-status=live|work=The New York Times}}</ref><ref>{{citation-attribution|1={{#invoke:cite press release||title=Hydroxychloroquine does not benefit adults hospitalized with COVID-19|website=National Institutes of Health (NIH)|date=9 November 2020|url=https://www.nih.gov/news-events/news-releases/hydroxychloroquine-does-not-benefit-adults-hospitalized-covid-19|access-date=9 November 2020}} }}</ref> and that they may reduce the antiviral activity of remdesivir.<ref>{{citation-attribution|1={{#invoke:cite press release||title=Coronavirus (COVID-19) Update: FDA Warns of Newly Discovered Potential Drug Interaction That May Reduce Effectiveness of a COVID-19 Treatment Authorized for Emergency Use|website=U.S. ] (FDA)|date=15 June 2020|url=https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-warns-newly-discovered-potential-drug-interaction-may-reduce|access-date=15 June 2020}} }}</ref> {{As of|2020|May|alt=By May 2020}}, France, Italy, and Belgium had banned the use of hydroxychloroquine as a COVID‑19 treatment.<ref>{{#invoke:Cite web||date=27 May 2020|title=France bans use of hydroxychloroquine, drug touted by Trump, in coronavirus patients|url=https://www.cbsnews.com/news/france-bans-use-of-hydroxychloroquine-drug-touted-by-trump-to-treat-coronavirus/|publisher=CBS News}}</ref> | |||
| Several existing ] are being evaluated for treatment of COVID-19,<ref name="LiDeClerq" /> including ], ] and ], ] and lopinavir/ritonavir combined with ].<ref name=kai/><ref>{{cite web|url=https://news.un.org/en/story/2020/03/1059722|title=UN health chief announces global 'solidarity trial' to jumpstart search for COVID-19 treatment|date=18 March 2020|website=UN News|language=en|access-date=23 March 2020|archive-url=https://web.archive.org/web/20200323101633/https://news.un.org/en/story/2020/03/1059722|archive-date=23 March 2020|url-status=live}}</ref> There is tentative evidence for efficacy by remdesivir, as of March 2020.<ref>{{cite journal|vauthors=Ko WC, Rolain JM, Lee NY, Chen PL, Huang CT, Lee PI, Hsueh PR|date=March 2020|title=Arguments in favor of remdesivir for treating SARS-CoV-2 infections|journal=International Journal of Antimicrobial Agents|page=105933|doi=10.1016/j.ijantimicag.2020.105933|pmid=32147516|doi-access=free}}</ref> Remdesivir inhibits SARS-CoV-2 '']''.<ref name="pmid32020029">{{cite journal|display-authors=6|vauthors=Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, Hu Z, Zhong W, Xiao G|date=February 2020 |title= Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro|journal=Cell Research |volume= 30| issue= 3|pages=269–71|doi=10.1038/s41422-020-0282-0|pmc=7054408|pmid=32020029|doi-access=free|name-list-format=vanc}}</ref> ]s are being conducted in the U.S., China and Italy.<ref name="LiDeClerq" /><ref name=milken/><ref>{{cite journal |last1=Beeching |first1=Nicholas J. |last2=Fletcher |first2=Tom E. |last3=Fowler |first3=Robert |name-list-format=vanc |date=2020 |title=BMJ Best Practices: COVID-19 |url= https://bestpractice.bmj.com/topics/en-gb/3000168/pdf/3000168/COVID-19.pdf |url-status=live |journal=BMJ |access-date=11 March 2020 |archive-url=https://web.archive.org/web/20200222170544/https://bestpractice.bmj.com/topics/en-gb/3000168/pdf/3000168/COVID-19.pdf |archive-date=22 February 2020 }}</ref> | |||
| In June, initial results from the randomised ] in the United Kingdom showed that dexamethasone reduced mortality by one third for people who are critically ill on ventilators and one fifth for those receiving supplemental oxygen.<ref>{{#invoke:cite news || vauthors = Boseley S |title=Recovery trial for Covid-19 treatments: what we know so far |url= https://www.theguardian.com/world/2020/jun/16/recovery-trial-for-covid-19-treatments-what-we-know-so-far |access-date=21 June 2020 |work=] |date=16 June 202}}</ref> Because this is a well-tested and widely available treatment, it was welcomed by the WHO, which is in the process of updating treatment guidelines to include dexamethasone and other steroids.<ref>{{#invoke:cite press release ||title=WHO welcomes preliminary results about dexamethasone use in treating critically ill COVID-19 patients |url=https://www.who.int/news-room/detail/16-06-2020-who-welcomes-preliminary-results-about-dexamethasone-use-in-treating-critically-ill-covid-19-patients |website=] (WHO) |access-date=21 June 2020 |date=16 June 2020}}</ref><ref>{{#invoke:Cite press release||title=Q&A: Dexamethasone and COVID-19|url=https://www.who.int/news-room/q-a-detail/q-a-dexamethasone-and-covid-19|access-date=12 July 2020|website=] (WHO)}}</ref> Based on those preliminary results, dexamethasone treatment has been recommended by the NIH for peoples with COVID‑19 who are mechanically ventilated or who require supplemental oxygen but not in people with COVID‑19 who do not require supplemental oxygen.<ref>{{#invoke:Cite web||title=Corticosteroids|url=https://www.covid19treatmentguidelines.nih.gov/immune-based-therapy/immunomodulators/corticosteroids/|access-date=12 July 2020|website=COVID-19 Treatment Guidelines|publisher=National Institutes of Health}}</ref> | |||
| Chloroquine, previously used to treat ], was studied in China in February 2020, with preliminary results.<ref name="JianjunZhXu_chloroquine">{{cite journal | vauthors = Gao J, Tian Z, Yang X | title = Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies | journal = Bioscience Trends | volume = 14 | pages = 72–73 | date = February 2020 | issue = 1 | pmid = 32074550 |doi=10.5582/bst.2020.01047 |doi-access=free | name-list-format = vanc}}</ref> However, there are calls for peer review of the research.<ref>{{cite journal | vauthors = Touret F, de Lamballerie X | title = Of chloroquine and COVID-19 | journal = Antiviral Research | volume = 177 | page = 104762 | date = March 2020 | pmid = 32147496 | doi = 10.1016/j.antiviral.2020.104762 }}</ref> The Guangdong Provincial Department of Science and Technology and the Guangdong Provincial Health and Health Commission issued a report stating that chloroquine phosphate "improves the success rate of treatment and shortens the length of person's hospital stay" and recommended it for people diagnosed with mild, moderate and severe cases of novel coronavirus pneumonia.<ref>{{cite journal | title = | journal = Zhonghua Jie He He Hu Xi Za Zhi = Zhonghua Jiehe He Huxi Zazhi = Chinese Journal of Tuberculosis and Respiratory Diseases | volume = 43 | pages = E019 | date = February 2020 | pmid = 32075365 | doi = 10.3760/cma.j.issn.1001-0939.2020.0019 | author1 = multicenter collaboration group of Department of Science Technology of Guangdong Province Health Commission of Guangdong Province for chloroquine in the treatment of novel coronavirus pneumonia }}</ref> | |||
| In September 2020, the WHO released updated guidance on using corticosteroids for COVID‑19.<ref name="WHO guidance">{{#invoke:cite report || vauthors=((World Health Organization)) | year=2020 | title=Corticosteroids for COVID-19: living guidance, 2 September 2020 | author-link=World Health Organization | id=WHO/2019-nCoV/Corticosteroids/2020.1 | hdl=10665/334125 | hdl-access=free }}</ref><ref>{{#invoke:Cite web|| title=WHO updates clinical care guidance with corticosteroid recommendations | publisher=] (WHO) | url=https://www.who.int/news-room/feature-stories/detail/who-updates-clinical-care-guidance-with-corticosteroid-recommendations | access-date=25 January 2022}}</ref> The WHO recommends systemic corticosteroids rather than no systemic corticosteroids for the treatment of people with severe and critical COVID‑19 (strong recommendation, based on moderate certainty evidence).<ref name="WHO guidance" /> The WHO suggests not to use corticosteroids in the treatment of people with non-severe COVID‑19 (conditional recommendation, based on low certainty evidence).<ref name="WHO guidance" /> The updated guidance was based on a meta-analysis of clinical trials of people critically ill with COVID‑19.<ref>{{#invoke:cite journal || vauthors = Sterne JA, Murthy S, Diaz JV, Slutsky AS, Villar J, Angus DC, Annane D, Azevedo LC, Berwanger O, Cavalcanti AB, Dequin PF, Du B, Emberson J, Fisher D, Giraudeau B, Gordon AC, Granholm A, Green C, Haynes R, Heming N, Higgins JP, Horby P, Jüni P, Landray MJ, Le Gouge A, Leclerc M, Lim WS, Machado FR, McArthur C, Meziani F, Møller MH, Perner A, Petersen MW, Savovic J, Tomazini B, Veiga VC, Webb S, Marshall JC | title = Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis | journal = JAMA | volume = 324 | issue = 13 | pages = 1330–1341 | date = October 2020 | pmid = 32876694 | pmc = 7489434 | doi = 10.1001/jama.2020.17023 | collaboration = The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group | s2cid = 221467783 | doi-access = free | title-link = doi }}</ref><ref>{{#invoke:cite journal || vauthors = Prescott HC, Rice TW | title = Corticosteroids in COVID-19 ARDS: Evidence and Hope During the Pandemic | journal = JAMA | volume = 324 | issue = 13 | pages = 1292–1295 | date = October 2020 | pmid = 32876693 | doi = 10.1001/jama.2020.16747 | s2cid = 221468015 | doi-access = free | title-link = doi }}</ref> | |||
| On 17 March, the Italian Pharmaceutical Agency included chloroquine and hydroxychloroquine in the list of drugs with positive preliminary results for treatment of COVID-19.<ref name=":15">{{cite web|url=https://aifa.gov.it/-/azioni-intraprese-per-favorire-la-ricerca-e-l-accesso-ai-nuovi-farmaci-per-il-trattamento-del-covid-19|title=Azioni intraprese per favorire la ricerca e l'accesso ai nuovi farmaci per il trattamento del COVID-19 |website= aifa.gov.it|language=it-IT|access-date=18 March 2020}}</ref> Korean and Chinese Health Authorities recommend the use of ].<ref>{{cite web|url=http://m.koreabiomed.com/news/articleView.html?idxno=7428|title=Physicians work out treatment guidelines for coronavirus|date=13 February 2020|website=m.koreabiomed.com|language=Korean|name-list-format=vanc|access-date=10 March 2020|archive-url= https://web.archive.org/web/20200317061347/http://m.koreabiomed.com/news/articleView.html?idxno=7428|archive-date=17 March 2020|url-status=live}}</ref><ref name=":9">{{cite web|url=https://www.chinalawtranslate.com/en/coronavirus-treatment-plan-7/|title=Novel Coronavirus Pneumonia Diagnosis and Treatment Plan (Provisional 7th Edition)|date=4 March 2020|website=China Law Translate|name-list-format=vanc|access-date=10 March 2020|archive-url= https://web.archive.org/web/20200310082919/https://www.chinalawtranslate.com/en/coronavirus-treatment-plan-7/|archive-date=10 March 2020|url-status=live}}</ref> However, the Wuhan Institute of Virology, while recommending a daily dose of one gram, notes that twice that dose is highly dangerous and could be lethal. On 28 March 2020, the ] issued an ] for hydroxychloroquine and chloroquine at the discretion of physicians treating people with COVID-19.<ref name="hinton">{{cite web |author1=Denise M Hinton |title=Request for Emergency Use Authorization For Use of Chloroquine Phosphate or Hydroxychloroquine Sulfate Supplied From the Strategic National Stockpile for Treatment of 2019 Coronavirus Disease |url=https://www.fda.gov/media/136534/download |publisher=US Food and Drug Administration |accessdate=30 March 2020 |date=28 March 2020 |archive-url=https://web.archive.org/web/20200330074008/https://www.fda.gov/media/136534/download |archive-date=30 March 2020 |url-status=live }}</ref><ref>{{cite web |last1=Commissioner |first1=Office of the |title=Emergency Use Authorization |url=https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization |website=FDA |accessdate=30 March 2020 |language=en |date=29 March 2020 |archive-url=https://web.archive.org/web/20200305013118/https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization |archive-date=5 March 2020 |url-status=live }}</ref> | |||
| In September 2020, the ] (EMA) endorsed the use of dexamethasone in adults and adolescents from twelve years of age and weighing at least {{convert|40|kg}} who require supplemental oxygen therapy.<ref name="EMA PR">{{#invoke:cite press release || title=EMA endorses use of dexamethasone in COVID-19 patients on oxygen or mechanical ventilation | website=] (EMA) | date=18 September 2020 | url=https://www.ema.europa.eu/en/news/ema-endorses-use-dexamethasone-covid-19-patients-oxygen-mechanical-ventilation | access-date=21 September 2020}} Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.</ref><ref>{{#invoke:cite report ||url=https://www.ema.europa.eu/en/documents/other/dexamethasone-covid19-article-53-procedure-assessment-report_en.pdf |title=Dexamethasone in hospitalised patients with COVID-19 |publisher=European Medicines Agency |date=17 September 2020}}</ref> Dexamethasone can be taken ] or given as an injection or ].<ref name="EMA PR" /> | |||
| The Chinese 7th edition guidelines also include ], ] or ] for use against COVID-19.<ref name=":9" /> | |||
| In November 2020, the US ] (FDA) issued an emergency use authorisation for the investigational monoclonal antibody therapy ] for the treatment of mild-to-moderate COVID‑19.<ref name="FDA bamlanivimab EUA">{{citation-attribution|1={{#invoke:cite press release || title=Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibody for Treatment of COVID-19 | website=U.S. ] (FDA) | date=9 November 2020 | url=https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibody-treatment-covid-19 | access-date=9 November 2020}} }}</ref> Bamlanivimab is authorised for people with positive results of direct SARS-CoV-2 viral testing who are twelve years of age and older weighing at least {{convert|40|kg}}, and who are at high risk for progressing to severe COVID‑19 or hospitalisation.<ref name="FDA bamlanivimab EUA" /> This includes those who are 65 years of age or older, or who have chronic medical conditions.<ref name="FDA bamlanivimab EUA" /> | |||
| In 2020, a trial found that lopinavir/ritonavir was ineffective in the treatment of severe illness.<ref>{{cite journal | vauthors = Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, Li X, Xia J, Chen N, Xiang J, Yu T, Bai T, Xie X, Zhang L, Li C, Yuan Y, Chen H, Li H, Huang H, Tu S, Gong F, Liu Y, Wei Y, Dong C, Zhou F, Gu X, Xu J, Liu Z, Zhang Y, Li H, Shang L, Wang K, Li K, Zhou X, Dong X, Qu Z, Lu S, Hu X, Ruan S, Luo S, Wu J, Peng L, Cheng F, Pan L, Zou J, Jia C, Wang J, Liu X, Wang S, Wu X, Ge Q, He J, Zhan H, Qiu F, Guo L, Huang C, Jaki T, Hayden FG, Horby PW, Zhang D, Wang C | display-authors = 6 | title = A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19 | journal = The New England Journal of Medicine | date = March 2020 | pmid = 32187464 | doi = 10.1056/NEJMoa2001282 }}</ref> ] has been recommended for further '']'' study after demonstrating low concentration inhibition of SARS-CoV-2.<ref name="pmid32020029" /> | |||
| In February 2021, the FDA issued an emergency use authorisation (EUA) for bamlanivimab and ] administered together for the treatment of mild to moderate COVID‑19 in people twelve years of age or older weighing at least {{convert|40|kg|lb}} who test positive for SARS‑CoV‑2 and who are at high risk for progressing to severe COVID‑19. The authorised use includes treatment for those who are 65 years of age or older or who have certain chronic medical conditions.<ref name="FDA PR 20210209">{{citation-attribution|1={{#invoke:cite press release||title=FDA Authorizes Monoclonal Antibodies for Treatment of COVID-19|website=U.S. ] (FDA)|date=10 February 2021|url=https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibodies-treatment-covid-19-0|access-date=9 February 2021}} }}</ref> | |||
| Studies have demonstrated that initial spike protein priming by transmembrane protease serine{{nbsp}}2 (]) is essential for entry of SARS-CoV-2 via interaction with the ] receptor.<ref>{{cite journal | vauthors = Iwata-Yoshikawa N, Okamura T, Shimizu Y, Hasegawa H, Takeda M, Nagata N | title = TMPRSS2 Contributes to Virus Spread and Immunopathology in the Airways of Murine Models after Coronavirus Infection | journal = Journal of Virology | volume = 93 | issue = 6 | date = March 2019 | pmid = 30626688 | pmc = 6401451 | doi = 10.1128/JVI.01815-18 | doi-access = free }}</ref> These findings suggest that the TMPRSS2 inhibitor ] approved for use in Japan for inhibiting fibrosis in liver and kidney disease might constitute an effective off-label treatment. | |||
| In April 2021, the FDA revoked the emergency use authorisation (EUA) that allowed for the investigational monoclonal antibody therapy bamlanivimab, when administered alone, to be used for the treatment of mild-to-moderate COVID‑19 in adults and certain paediatric patients.<ref name="FDA PR 20210416">{{citation-attribution|1={{#invoke:cite press release || title=Coronavirus (COVID-19) Update: FDA Revokes Emergency Use Authorization for Monoclonal Antibody Bamlanivimab | website=U.S. ] (FDA) | date=16 April 2021 | url=https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-monoclonal-antibody-bamlanivimab | access-date=16 April 2021}} }}</ref> | |||
| In February 2020, ] was being studied in China for experimental treatment of the emergent COVID-19 disease.<ref>Li G, De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV). ''Nature Reviews Drug Discovery'' 2020 Feb {{doi|10.1038/d41573-020-00016-0}}</ref><ref> {{Webarchive|url=https://web.archive.org/web/20200318220901/https://www.reuters.com/article/brief-corrected-zhejiang-hisun-pharma-ge/brief-corrected-zhejiang-hisun-pharma-gets-approval-for-clinical-trial-to-test-flu-drug-favipiravir-for-pneumonia-caused-by-new-coronavirus-idUSL4N2AH0C8 |date=18 March 2020 }}. Reuters Healthcare, 16 February 2020.</ref> | |||
| ==== Cytokine storm ==== | |||
| In April 2020 ] is being studied in Australia for a possible treatment for COVID-19 and has been shown to stop viral growth within 48 hours in vitro.<ref>{{Cite journal|url=http://www.sciencedirect.com/science/article/pii/S0166354220302011|title=The FDA-approved Drug Ivermectin inhibits the replication of SARS-CoV-2 in vitro|first1=Leon|last1=Caly|first2=Julian D.|last2=Druce|first3=Mike G.|last3=Catton|first4=David A.|last4=Jans|first5=Kylie M.|last5=Wagstaff|date=3 April 2020|journal=Antiviral Research|page=104787|via=ScienceDirect|doi=10.1016/j.antiviral.2020.104787}}</ref><ref>{{cite web|website=Monash Biomedicine Discovery Institute|title= Possible coronavirus drug identified by Monash University scientists |url=https://www.monash.edu/discovery-institute/news-and-events/news/2020-articles/possible-coronavirus-drug-identified-by-monash-university-scientists |date=3 April 2020|access-date= 7 April 2020}}</ref> | |||
| ] | |||
| A ] can be a complication in the later stages of severe COVID‑19. A cytokine storm is a potentially deadly immune reaction where a large amount of pro-inflammatory cytokines and ]s are released too quickly. A cytokine storm can lead to ARDS and multiple organ failure.<ref>{{#invoke:cite journal || vauthors = Li X, Geng M, Peng Y, Meng L, Lu S | title = Molecular immune pathogenesis and diagnosis of COVID-19 | journal = Journal of Pharmaceutical Analysis | volume = 10 | issue = 2 | pages = 102–108 | date = April 2020 | pmid = 32282863 | pmc = 7104082 | doi = 10.1016/j.jpha.2020.03.001 }}</ref> Data collected from Jin Yin-tan Hospital in Wuhan, China indicates that people who had more severe responses to COVID‑19 had greater amounts of pro-inflammatory cytokines and chemokines in their system than people who had milder responses. These high levels of pro-inflammatory cytokines and chemokines indicate presence of a cytokine storm.<ref>{{#invoke:cite journal || vauthors = Zhao Z, Wei Y, Tao C | title = An enlightening role for cytokine storm in coronavirus infection | journal = Clinical Immunology | volume = 222 | pages = 108615 | date = January 2021 | pmid = 33203513 | pmc = 7583583 | doi = 10.1016/j.clim.2020.108615 }}</ref> | |||
| ] has been included in treatment guidelines by China's ] after a small study was completed.<ref name="tocil-1">{{#invoke:cite news ||vauthors = Liu R, Miller J |url = https://www.reuters.com/article/us-health-coronavirus-china-roche-hldg/china-approves-use-of-roche-arthritis-drug-for-coronavirus-patients-idUSKBN20R0LF |title=China approves use of Roche drug in battle against coronavirus complications |date=3 March 2020 |work=] |access-date=14 March 2020 |archive-url=https://web.archive.org/web/20200312204625/https://www.reuters.com/article/us-health-coronavirus-china-roche-hldg/china-approves-use-of-roche-arthritis-drug-for-coronavirus-patients-idUSKBN20R0LF |archive-date=12 March 2020 |url-status=live}}</ref><ref name="tocil-2">{{#invoke:cite journal ||vauthors = Xu X, Han M, Li T, Sun W, Wang D, Fu B, Zhou Y, Zheng X, Yang Y, Li X, Zhang X, Pan A, Wei H |title = Effective treatment of severe COVID-19 patients with tocilizumab |journal = Proceedings of the National Academy of Sciences of the United States of America |volume = 117 |issue = 20 |pages = 10970–10975 |date = May 2020 |pmid = 32350134 |pmc = 7245089 |doi = 10.1073/pnas.2005615117 |doi-access = free |title-link = doi |bibcode = 2020PNAS..11710970X }}</ref> It is undergoing a ] non-randomised trial at the national level in Italy after showing positive results in people with severe disease.<ref>{{#invoke:Cite web||vauthors = Ovadia D, Agenzia Z |title=COVID-19 – Italy launches an independent trial on tocilizumab |url=https://www.univadis.co.uk/viewarticle/covid-19-italy-launches-an-independent-trial-on-tocilizumab-715741 |website=Univadis from Medscape |publisher=Aptus Health |access-date=22 April 2020}}</ref><ref>{{#invoke:Cite web||title=Tocilizumab in COVID-19 Pneumonia (TOCIVID-19) (TOCIVID-19) |url=https://clinicaltrials.gov/ct2/show/NCT04317092 |website=clinicaltrials.gov |access-date=22 April 2020}}</ref> Combined with a serum ] blood test to identify a cytokine storm (also called cytokine storm syndrome, not to be confused with ]), it is meant to counter such developments, which are thought to be the cause of death in some affected people.<ref name="tocil-5,6,8">Various sources: | |||
| There are mixed results as of April 3 as to the effectiveness of ] as a treatment for COVID-19. With studies showing little to no improvement over the control groups.<ref>{{cite news |last1=Seley-Radtke |first1=Katherine |title=Professor of Chemistry and Biochemistry and President-Elect of the International Society for Antiviral Research, University of Maryland, Baltimore County |url=https://theconversation.com/a-small-trial-finds-that-hydroxychloroquine-is-not-effective-for-treating-coronavirus-135484 |accessdate=5 April 2020 |publisher=The Conversation |date=3 April 2020}}</ref> | |||
| * {{#invoke:Cite web||url=https://www.vox.com/2020/3/12/21176783/coronavirus-covid-19-deaths-china-treatment-cytokine-storm-syndrome|title=How doctors can potentially significantly reduce the number of deaths from Covid-19|work=]|access-date=14 March 2020|date=12 March 2020|archive-url=https://web.archive.org/web/20200319155218/https://www.vox.com/2020/3/12/21176783/coronavirus-covid-19-deaths-china-treatment-cytokine-storm-syndrome|archive-date=19 March 2020|url-status=live}} | |||
| * {{#invoke:cite journal || vauthors = Ruan Q, Yang K, Wang W, Jiang L, Song J | title = Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China | journal = Intensive Care Medicine | volume = 46 | issue = 5 | pages = 846–848 | date = May 2020 | pmid = 32125452 | pmc = 7080116 | doi = 10.1007/s00134-020-05991-x | ref = none }} | |||
| * {{#invoke:cite journal || vauthors = Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ | title = COVID-19: consider cytokine storm syndromes and immunosuppression | journal = Lancet | volume = 395 | issue = 10229 | pages = 1033–1034 | date = March 2020 | pmid = 32192578 | pmc = 7270045 | doi = 10.1016/S0140-6736(20)30628-0 | ref = none | title-link = doi | doi-access = free }}</ref> The ] (IL-6R) ] was approved by the FDA to undergo a Phase{{spaces}}III clinical trial assessing its effectiveness on COVID‑19 based on retrospective case studies for the treatment of steroid-refractory cytokine release syndrome induced by a different cause, ] ], in 2017.<ref name="CancerNetworkTocilizumabTrial">{{#invoke:Cite web|| vauthors = Slater H |title=FDA Approves Phase III Clinical Trial of Tocilizumab for COVID-19 Pneumonia |url=https://www.cancernetwork.com/news/fda-approves-phase-iii-clinical-trial-tocilizumab-covid-19-pneumonia |website=cancernetwork.com |date=26 March 2020 |publisher=Cancer Network |access-date=22 April 2020}}</ref> There is no randomised, controlled evidence that tocilizumab is an efficacious treatment for CRS. Prophylactic tocilizumab has been shown to increase serum IL-6 levels by saturating the IL-6R, driving IL-6 across the ], and exacerbating ] while having no effect on the incidence of CRS.<ref>{{#invoke:cite journal ||vauthors=Locke FL, Neelapu SS, Bartlett NL, Lekakis LJ, Jacobson CA, Braunschweig I, Oluwole OO, Siddiqi T, Lin Y, Timmerman JM, Reagan PM, Bot A, Rossi JM, Sherman M, Navale L, Jiang Y, Aycock JS, Elias M, Wiezorek JS, Go WY, Miklos DB |title=Preliminary Results of Prophylactic Tocilizumab after Axicabtageneciloleucel (axi-cel; KTE-C19) Treatment for Patients with Refractory, Aggressive Non-Hodgkin Lymphoma (NHL) |journal=Blood |year=2017 |volume=130 |issue=Supplement 1 |pages=1547 |doi=10.1182/blood.V130.Suppl_1.1547.1547 |s2cid=155698207 |url=https://ashpublications.org/blood/article/130/Supplement%201/1547/79746}}</ref> | |||
| ], an anti-GM-CSF ], is protective in murine models for CAR T cell-induced CRS and neurotoxicity and is a viable therapeutic option due to the observed increase of pathogenic GM-CSF secreting T{{spaces}}cells in hospitalised patients with COVID‑19.<ref>{{#invoke:cite journal || vauthors = Sterner RM, Sakemura R, Cox MJ, Yang N, Khadka RH, Forsman CL, Hansen MJ, Jin F, Ayasoufi K, Hefazi M, Schick KJ, Walters DK, Ahmed O, Chappell D, Sahmoud T, Durrant C, Nevala WK, Patnaik MM, Pease LR, Hedin KE, Kay NE, Johnson AJ, Kenderian SS | title = GM-CSF inhibition reduces cytokine release syndrome and neuroinflammation but enhances CAR T cell function in xenografts | journal = Blood | volume = 133 | issue = 7 | pages = 697–709 | date = February 2019 | pmid = 30463995 | pmc = 6376281 | doi = 10.1182/blood-2018-10-881722 }}</ref> | |||
| ===Anti-cytokine storm=== | |||
| ==== Passive antibodies ==== | |||
| ] can be a complication in the later stages of severe COVID-19. There is evidence that ] may have anti-cytokine storm properties.<ref name="pmid32150618">{{cite journal |vauthors=Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P, Liu X, Zhao L, Dong E, Song C, Zhan S, Lu R, Li H, Tan W, Liu D |title=In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) |journal=Clin. Infect. Dis. |volume= |issue= |pages= |date=March 2020 |pmid=32150618 |doi=10.1093/cid/ciaa237 |pmc=7108130 }}</ref> | |||
| ] therapy]] | |||
| Transferring purified and concentrated ] produced by the immune systems of those who have recovered from COVID‑19 to people who need them is being investigated as a non-vaccine method of ].<ref name="pmid-32167489">{{#invoke:cite journal || vauthors = Casadevall A, Pirofski LA | title = The convalescent sera option for containing COVID-19 | journal = The Journal of Clinical Investigation | volume = 130 | issue = 4 | pages = 1545–1548 | date = April 2020 | pmid = 32167489 | pmc = 7108922 | doi = 10.1172/JCI138003 }}</ref><ref name="Iannizzi-2023">{{#invoke:Cite journal ||last1=Iannizzi |first1=Claire |last2=Chai |first2=Khai Li |last3=Piechotta |first3=Vanessa |last4=Valk |first4=Sarah J. |last5=Kimber |first5=Catherine |last6=Monsef |first6=Ina |last7=Wood |first7=Erica M. |last8=Lamikanra |first8=Abigail A. |last9=Roberts |first9=David J. |last10=McQuilten |first10=Zoe |last11=So-Osman |first11=Cynthia |last12=Jindal |first12=Aikaj |last13=Cryns |first13=Nora |last14=Estcourt |first14=Lise J. |last15=Kreuzberger |first15=Nina |date=2023-05-10 |title=Convalescent plasma for people with COVID-19: a living systematic review |journal=The Cochrane Database of Systematic Reviews |volume=2023 |issue=5 |pages=CD013600 |doi=10.1002/14651858.CD013600.pub6 |issn=1469-493X |pmc=10171886 |pmid=37162745 }}</ref> ] is the anticipated ] by which passive antibody therapy can mediate defence against SARS-CoV-2. The spike protein of SARS-CoV-2 is the primary target for neutralising antibodies.<ref name="Ho-2020">{{#invoke:cite journal || vauthors = Ho M | title = Perspectives on the development of neutralizing antibodies against SARS-CoV-2 | journal = Antibody Therapeutics | volume = 3 | issue = 2 | pages = 109–114 | date = April 2020 | pmid = 32566896 | pmc = 7291920 | doi = 10.1093/abt/tbaa009 | title-link = doi | doi-access = free }}</ref> As of 8{{spaces}}August 2020, eight neutralising antibodies targeting the spike protein of SARS-CoV-2 have entered clinical studies.<ref>{{#invoke:cite journal || vauthors = Yang L, Liu W, Yu X, Wu M, Reichert JM, Ho M | title = COVID-19 antibody therapeutics tracker: a global online database of antibody therapeutics for the prevention and treatment of COVID-19 | journal = Antibody Therapeutics | volume = 3 | issue = 3 | pages = 205–212 | date = July 2020 | pmid = 33215063 | pmc = 7454247 | doi = 10.1093/abt/tbaa020 }}</ref> It has been proposed that selection of broad-neutralising antibodies against SARS-CoV-2 and SARS-CoV might be useful for treating not only COVID‑19 but also future SARS-related CoV infections.<ref name="Ho-2020" /> Other mechanisms, however, such as ] or ], may be possible.<ref name="pmid-32167489" /> Other forms of passive antibody therapy, for example, using manufactured monoclonal antibodies, are in development.<ref name="pmid-32167489" /> | |||
| The use of passive antibodies to treat people with active COVID{{nbhyph}}19 is also being studied. This involves the production of ], which consists of the liquid portion of the blood from people who recovered from the infection and contains antibodies specific to this virus, which is then administered to active patients.<ref name="pmid-32167489" /> This strategy was tried for SARS with inconclusive results.<ref name="pmid-32167489" /> An updated Cochrane review in May 2023 found high certainty evidence that, for the treatment of people with moderate to severe COVID‑19, convalescent plasma did not reduce mortality or bring about symptom improvement.<ref name="Iannizzi-2023" /> There continues to be uncertainty about the safety of convalescent plasma administration to people with COVID‑19 and differing outcomes measured in different studies limits their use in determining efficacy.<ref name="Iannizzi-2023" /> | |||
| ] has been included in treatment guidelines by China's ] after a small study was completed.<ref name="tocil-1">{{cite news|last1=Liu|first1=Roxanne|last2=Miller|first2=Josh|name-list-format=vanc|url=https://www.reuters.com/article/us-health-coronavirus-china-roche-hldg/china-approves-use-of-roche-arthritis-drug-for-coronavirus-patients-idUSKBN20R0LF|title=China approves use of Roche drug in battle against coronavirus complications|date=3 March 2020|work=]|access-date=14 March 2020|archive-url=https://web.archive.org/web/20200312204625/https://www.reuters.com/article/us-health-coronavirus-china-roche-hldg/china-approves-use-of-roche-arthritis-drug-for-coronavirus-patients-idUSKBN20R0LF|archive-date=12 March 2020|url-status=live}}</ref><ref name="tocil-2">{{cite document |date=5 March 2020 |title=Effective Treatment of Severe COVID-19 Patients with Tocilizumab |url=http://chinaxiv.org/abs/202003.00026 |publisher=ChinaXiv.org |doi=10.12074/202003.00026|doi-broken-date=2020-03-16 |accessdate=14 March 2020|journal=|archive-url=https://web.archive.org/web/20200319022047/http://chinaxiv.org/abs/202003.00026|archive-date=19 March 2020|url-status=live}}</ref> It is undergoing a ] non randomised test at the national level in Italy after showing positive results in people with severe disease.<ref name=":15" /><ref name="tocil-3">{{cite web|url=http://www.ansa.it/english/news/general_news/2020/03/13/3-patients-get-better-on-arthritis-drug_90d4764d-d93f-463e-ab07-168b34b084d0.html|title=3 patients get better on arthritis drug|date=5 March 2020|accessdate=14 March 2020|archive-url=https://web.archive.org/web/20200319022045/http://www.ansa.it/english/news/general_news/2020/03/13/3-patients-get-better-on-arthritis-drug_90d4764d-d93f-463e-ab07-168b34b084d0.html|archive-date=19 March 2020|url-status=live}}</ref><ref name="tocil-4">{{cite news|url=https://www.ilmessaggero.it/italia/coronavirus_farmaco_artrite_ultime_notizie_news_napoli-5109045.html|title=Coronavirus, via libera dell'Aifa al farmaco anti-artrite efficace su 3 pazienti e a un antivirale: test in 5 centri|trans-title=Coronavirus, Aifa gives go-ahead to effective anti-arthritis drug on 3 patients and an antiviral: test in 5 centers|newspaper=]|language=it|accessdate=14 March 2020|archive-url=https://web.archive.org/web/20200319022047/https://www.ilmessaggero.it/italia/coronavirus_farmaco_artrite_ultime_notizie_news_napoli-5109045.html|archive-date=19 March 2020|url-status=live}}</ref>{{MEDRS|date=March 2020}} Combined with a ] to identify ], it is meant to counter such developments, which are thought to be the cause of death in some affected people.<ref name="tocil-5">{{cite web|url=https://www.vox.com/2020/3/12/21176783/coronavirus-covid-19-deaths-china-treatment-cytokine-storm-syndrome|title=How doctors can potentially significantly reduce the number of deaths from Covid-19|publisher=]|accessdate=14 March 2020|date=12 March 2020|archive-url=https://web.archive.org/web/20200319155218/https://www.vox.com/2020/3/12/21176783/coronavirus-covid-19-deaths-china-treatment-cytokine-storm-syndrome|archive-date=19 March 2020|url-status=live}}</ref><ref name="tocil-6">{{cite journal | vauthors = Ruan Q, Yang K, Wang W, Jiang L, Song J | title = Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China | journal = Intensive Care Medicine | date = March 2020 | pmid = 32125452 | doi = 10.1007/s00134-020-05991-x | pmc = 7080116 }}</ref><ref name="tocil-8">{{cite journal |display-authors=3 |vauthors=Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ |title=COVID-19: consider cytokine storm syndromes and immunosuppression |journal=] |date=16 March 2020 |volume=395 |issue=10229 |pages=1033–1034 |doi=10.1016/S0140-6736(20)30628-0 |pmid=32192578 |url=https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30628-0/fulltext |access-date=19 March 2020 |archive-url=https://web.archive.org/web/20200322045751/https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30628-0/fulltext |archive-date=22 March 2020 |url-status=live }}</ref> The ] ] was approved by the ] for treatment against cytokine release syndrome induced by a different cause, ] ], in 2017.<ref name="tocil-7">{{cite web|url=https://www.fiercepharma.com/pharma-asia/china-turns-roche-arthritis-drug-actemra-against-covid-19-new-treatment-guidelines|title=China turns Roche arthritis drug Actemra against COVID-19 in new treatment guidelines|publisher=FiercePharma|accessdate=14 March 2020|archive-url=https://web.archive.org/web/20200319022042/https://www.fiercepharma.com/pharma-asia/china-turns-roche-arthritis-drug-actemra-against-covid-19-new-treatment-guidelines|archive-date=19 March 2020|url-status=live}}</ref>{{MEDRS|date=March 2020}} | |||
| === Bioethics === | |||
| The ] of ] announced in March a study on "a human antibody that may prevent the activity" of IL-6.<ref>{{cite news|url=https://www.longislandpress.com/2020/03/21/northwell-health-initiates-clinical-trials-of-2-covid-19-drugs/|title=Northwell Health Initiates Clinical Trials of 2 COVID-19 Drugs|date=21 March 2020|accessdate=23 March 2020|archive-url=https://web.archive.org/web/20200323081238/https://www.longislandpress.com/2020/03/21/northwell-health-initiates-clinical-trials-of-2-covid-19-drugs/|archive-date=23 March 2020|url-status=live}}</ref> | |||
| Since the outbreak of the COVID‑19 pandemic, scholars have explored the ], ], and ] of ] related to the public health crisis.<ref>{{#invoke:cite journal || vauthors = Maccaro A, Piaggio D, Pagliara S, Pecchia L | title = The role of ethics in science: a systematic literature review from the first wave of COVID-19 | journal = Health and Technology | volume = 11 | issue = 5 | pages = 1063–1071 | date = June 2021 | pmid = 34104626 | pmc = 8175060 | doi = 10.1007/s12553-021-00570-6|issn=2190-7188 }}</ref> Academics have pointed to the moral distress of healthcare workers, ethics of distributing scarce healthcare resources such as ventilators,<ref>{{#invoke:cite journal || vauthors = McGuire AL, Aulisio MP, Davis FD, Erwin C, Harter TD, Jagsi R, Klitzman R, Macauley R, Racine E, Wolf SM, Wynia M, Wolpe PR | title = Ethical Challenges Arising in the COVID-19 Pandemic: An Overview from the Association of Bioethics Program Directors (ABPD) Task Force | journal = The American Journal of Bioethics | volume = 20 | issue = 7 | pages = 15–27 | date = July 2020 | pmid = 32511078 | doi = 10.1080/15265161.2020.1764138 | s2cid = 219552665 }}</ref> and the global justice of vaccine diplomacies.<ref>{{Cite journal |last1=Hotez |first1=Peter J. |last2=Batista |first2=Carolina |last3=Amor |first3=Yanis Ben |last4=Ergonul |first4=Onder |last5=Figueroa |first5=J Peter |last6=Gilbert |first6=Sarah |last7=Gursel |first7=Mayda |last8=Hassanain |first8=Mazen |last9=Kang |first9=Gagandeep |last10=Kaslow |first10=David C. |last11=Kim |first11=Jerome H. |last12=Lall |first12=Bhavna |last13=Larson |first13=Heidi |last14=Naniche |first14=Denise |last15=Sheahan |first15=Timothy |date=2021 |title=Global public health security and justice for vaccines and therapeutics in the COVID-19 pandemic |journal=eClinicalMedicine |language=en |volume=39 |pages=101053 |doi=10.1016/j.eclinm.2021.101053 |pmc=8330385 |pmid=34368661}}</ref><ref>{{Cite journal |last1=Sparke |first1=Matthew |last2=Levy |first2=Orly |date=2022-08-15 |title=Competing Responses to Global Inequalities in Access to COVID Vaccines: Vaccine Diplomacy and Vaccine Charity Versus Vaccine Liberty |url=https://academic.oup.com/cid/article/75/Supplement_1/S86/6583150 |journal=Clinical Infectious Diseases |language=en |volume=75 |issue=Supplement_1 |pages=S86–S92 |doi=10.1093/cid/ciac361 |issn=1058-4838 |pmc=9376271 |pmid=35535787}}</ref> The socio-economic inequalities between genders,<ref>{{#invoke:cite journal || vauthors = Wenham C, Smith J, Morgan R | title = COVID-19: the gendered impacts of the outbreak | journal = Lancet | volume = 395 | issue = 10227 | pages = 846–848 | date = March 2020 | pmid = 32151325 | pmc = 7124625 | doi = 10.1016/S0140-6736(20)30526-2 }}</ref> races,<ref>{{#invoke:cite journal || vauthors = Tolchin B, Hull SC, Kraschel K | title = Triage and justice in an unjust pandemic: ethical allocation of scarce medical resources in the setting of racial and socioeconomic disparities | journal = Journal of Medical Ethics | volume = 47 | issue = 3 | pages = 200–202 | date = October 2020 | pmid = 33067315 | doi = 10.1136/medethics-2020-106457 | s2cid = 223558059 }}</ref> groups with disabilities,<ref>{{#invoke:cite journal || vauthors = Sabatello M, Burke TB, McDonald KE, Appelbaum PS | title = Disability, Ethics, and Health Care in the COVID-19 Pandemic | journal = American Journal of Public Health | volume = 110 | issue = 10 | pages = 1523–1527 | date = October 2020 | pmid = 32816541 | pmc = 7483109 | doi = 10.2105/AJPH.2020.305837 }}</ref> communities,<ref>{{#invoke:cite journal || vauthors = Chin T, Kahn R, Li R, Chen JT, Krieger N, Buckee CO, Balsari S, Kiang MV | title = US-county level variation in intersecting individual, household and community characteristics relevant to COVID-19 and planning an equitable response: a cross-sectional analysis | journal = BMJ Open | volume = 10 | issue = 9 | pages = e039886 | date = September 2020 | pmid = 32873684 | pmc = 7467554 | doi = 10.1136/bmjopen-2020-039886 }}</ref> regions, countries,<ref>{{#invoke:cite journal || vauthors = Elgar FJ, Stefaniak A, Wohl MJ | title = The trouble with trust: Time-series analysis of social capital, income inequality, and COVID-19 deaths in 84 countries | journal = Social Science & Medicine | volume = 263 | pages = 113365 | date = October 2020 | pmid = 32981770 | pmc = 7492158 | doi = 10.1016/j.socscimed.2020.113365 }}</ref> and continents have also drawn attention in academia and the general public.<ref>{{Cite journal |last1=Abu El Kheir-Mataria |first1=Wafa |last2=Khadr |first2=Zeinab |last3=El Fawal |first3=Hassan |last4=Chun |first4=Sungsoo |date=2024-03-21 |title=COVID-19 vaccine intercountry distribution inequality and its underlying factors: a combined concentration index analysis and multiple linear regression analysis |journal=Frontiers in Public Health |volume=12 |doi=10.3389/fpubh.2024.1348088 |doi-access=free |issn=2296-2565 |pmc=10993910 |pmid=38577285}}</ref><ref>{{Cite journal |last1=Mortiboy |first1=Marissa |last2=Zitta |first2=John-Paul |last3=Carrico |first3=Savannah |last4=Stevens |first4=Elizabeth |last5=Smith |first5=Alecia |last6=Morris |first6=Corey |last7=Jenkins |first7=Rodney |last8=Jenks |first8=Jeffrey D. |date=2024 |title=Combating COVID-19 Vaccine Inequity During the Early Stages of the COVID-19 Pandemic |journal=Journal of Racial and Ethnic Health Disparities |language=en |volume=11 |issue=2 |pages=621–630 |doi=10.1007/s40615-023-01546-0 |issn=2197-3792 |pmc=10019425 |pmid=36929491}}</ref> | |||
| == See also == | |||
| ===Passive antibody therapy=== | |||
| <!-- ************************************************************* | |||
| **** Please be very cautious when adding to this list, **** | |||
| **** especially if adding a page already mentioned in **** | |||
| **** the body. It should contain only the most **** | |||
| **** important links. If in doubt, check at talk first. **** | |||
| ************************************************************* --> | |||
| * ], a group of closely related syndromes | |||
| * ], a WHO term{{Clear}} | |||
| * {{annotated link|Law of declining virulence}} | |||
| * {{annotated link|Theory of virulence}} | |||
| == References == | |||
| Transferring purified and concentrated ] produced by the ]s of those who have recovered from COVID-19 to people who need them is being investigated as a non-vaccine method of ].<ref name="pmid-32167489">{{cite journal | vauthors = Casadevall A, Pirofski LA | title = The convalescent sera option for containing COVID-19 | journal = The Journal of Clinical Investigation | date = March 2020 | volume = 130 | issue = 4 | pages = 1545–1548 | pmid = 32167489 | doi = 10.1172/JCI138003 | pmc = 7108922 }}</ref> This strategy was tried for SARS with inconclusive results.<ref name="pmid-32167489" /> ] is the anticipated ] by which passive antibody therapy can mediate defence against SARS-CoV-2. Other mechanisms however, such as ] and/or ], may be possible.<ref name="pmid-32167489" /> Other forms of passive antibody therapy, for example, using manufactured ], are in development.<ref name="pmid-32167489" /> Production of ], which consists of the liquid portion of the blood from recovered patients and contains antibodies specific to this virus, could be increased for quicker deployment.<ref name="Pearce-2020-03-13">{{cite web |last=Pearce |first=Katie |name-list-format=vanc |date=13 March 2020 |title=Antibodies from COVID-19 survivors could be used to treat patients, protect those at risk: Infusions of antibody-laden blood have been used with reported success in prior outbreaks, including the SARS epidemic and the 1918 flu pandemic |work=The Hub at Johns Hopkins University |url=https://hub.jhu.edu/2020/03/13/covid-19-antibody-sera-arturo-casadevall/ |accessdate=14 March 2020 |archive-url=https://web.archive.org/web/20200314185825/https://hub.jhu.edu/2020/03/13/covid-19-antibody-sera-arturo-casadevall/ |archive-date=14 March 2020 |url-status=live }}</ref> | |||
| <references /> | |||
| <!-- | |||
| <ref name=cochrane>{{#invoke:cite journal || vauthors = Jefferson T, Dooley L, Ferroni E, Al-Ansary LA, van Driel ML, Bawazeer GA, Jones MA, Hoffmann TC, Clark J, Beller EM, Glasziou PP, Conly JM | title = Physical interventions to interrupt or reduce the spread of respiratory viruses | journal = The Cochrane Database of Systematic Reviews | volume = 1 | issue = 1 | pages = CD006207 | date = January 2023 | pmid = 36715243 | pmc = 9885521 | doi = 10.1002/14651858.CD006207.pub6 }}</ref> | |||
| <ref name=royalsoc>{{#invoke:cite journal || url=https://doi.org/10.1098/rsta.2023.0133 | doi=10.1098/rsta.2023.0133 | title=Effectiveness of face masks for reducing transmission of SARS-CoV-2: A rapid systematic review | date=2023 | last1=Boulos | first1=Leah | last2=Curran | first2=Janet A. | last3=Gallant | first3=Allyson | last4=Wong | first4=Helen | last5=Johnson | first5=Catherine | last6=Delahunty-Pike | first6=Alannah | last7=Saxinger | first7=Lynora | last8=Chu | first8=Derek | last9=Comeau | first9=Jeannette | last10=Flynn | first10=Trudy | last11=Clegg | first11=Julie | last12=Dye | first12=Christopher | journal=Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences | volume=381 | issue=2257 | pmid=37611625 | pmc=10446908 | bibcode=2023RSPTA.38130133B }}</ref> | |||
| --> | |||
| == |
== Further reading == | ||
| <templatestyles src="Refbegin/styles.css" /><div class="refbegin"> | |||
| * {{cite Q|Q98568681|doi-access = free | title-link = doi }} | |||
| * {{#invoke:cite report || title=COVID-19 infection prevention and control measures for primary care, including general practitioner practices, dental clinics and pharmacy settings: first update | website=] (ECDC) | url=https://www.ecdc.europa.eu/en/publications-data/covid-19-infection-prevention-and-control-primary-care | date=October 2020 }} | |||
| * {{cite Q|Q104287299|doi-access = free | title-link = doi | display-authors = all }} ] ]. | |||
| </div> | |||
| == External links == | |||
| * {{annotated link|Coronavirus Act 2020}} | |||
| {{Scholia|Q84263196}} | |||
| * ], a US law | |||
| * ]s, a group of closely related syndromes | |||
| * ] | |||
| * ], a ] term | |||
| * ], a doctor at ], who later contracted and died of COVID-19 after raising awareness of the spread of the virus. | |||
| * ] for conditions in various countries | |||
| === Health agencies === | |||
| ==Notes== | |||
| * by the World Health Organization (WHO) | |||
| * by the UK ] (NHS) | |||
| * by the US ] (CDC) | |||
| === Directories === | |||
| {{notelist}} | |||
| * at the ] | |||
| * {{Webarchive|url=https://web.archive.org/web/20220113160833/http://firemountain.net/covid19.html |date=13 January 2022 }} | |||
| * | |||
| === Medical journals === | |||
| ==References== | |||
| * by the ] | |||
| * by '']'' | |||
| * by ] | |||
| * by '']'' | |||
| * by '']'' | |||
| * {{Webarchive|url=https://web.archive.org/web/20200924195411/https://novel-coronavirus.onlinelibrary.wiley.com/ |date=24 September 2020 }} by ] | |||
| * by ] | |||
| === Treatment guidelines === | |||
| {{reflist|refs= | |||
| * {{#invoke:Cite web||title=Bouncing Back From COVID-19: Your Guide to Restoring Movement |website=Johns Hopkins Medicine|url=https://www.hopkinsmedicine.org/physical_medicine_rehabilitation/coronavirus-rehabilitation/_files/impact-of-covid-patient-recovery.pdf}} | |||
| <ref name="Reut_NIH_Moderna_3months">{{cite news | last1= Steenhuysen | first1= Julie | last2= Kelland | first2= Kate | name-list-format = vanc | title= With Wuhan virus genetic code in hand, scientists begin work on a vaccine | date= 24 January 2020 | agency= ] | url= https://www.reuters.com/article/us-china-health-vaccines-idUSKBN1ZN2J8 |accessdate=25 January 2020 |archive-url= https://web.archive.org/web/20200125203723/https://www.reuters.com/article/us-china-health-vaccines-idUSKBN1ZN2J8 |archive-date= 25 January 2020 |url-status=live }}</ref> | |||
| * {{#invoke:Cite web||title=Coronavirus Disease 2019 (COVID-19) Treatment Guidelines |website=National Institutes of Health |url=https://files.covid19treatmentguidelines.nih.gov/guidelines/covid19treatmentguidelines.pdf }} | |||
| * {{#invoke:Cite web||title=Guidelines on the Treatment and Management of Patients with COVID-19 |website=Infectious Diseases Society of America |url=https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/ }} | |||
| * {{#invoke:Cite web||title=JHMI Clinical Recommendations for Available Pharmacologic Therapies for COVID-19 |publisher=Johns Hopkins Medicine |url=https://www.hopkinsguides.com/hopkins/ub?cmd=repview&type=479-1279&name=40_538747_PDF |format=PDF}} | |||
| * {{#invoke:cite report || vauthors=((NHS England and NHS Improvement)) | title=National Guidance for post-COVID syndrome assessment clinics | url=https://www.england.nhs.uk/coronavirus/publication/national-guidance-for-post-covid-syndrome-assessment-clinics/ }} | |||
| * {{#invoke:cite report || vauthors=((World Health Organization)) | title=Therapeutics and COVID-19: living guideline, 14 January 2022 | author-link=World Health Organization | year=2022 | id=WHO/2019-nCoV/therapeutics/2022.1 | hdl=10665/351006 | hdl-access=free | url=https://www.who.int/publications/i/item/therapeutics-and-covid-19-living-guideline }} | |||
| {{Subject bar | |||
| |commons = yes | |||
| |d = yes | |||
| |n = yes | |||
| |q = yes | |||
| |v = yes | |||
| |portal1 = COVID-19 | |||
| |portal2 = Medicine | |||
| |portal3 = Viruses | |||
| }} | }} | ||
| ==External links== | |||
| {{Portalbar|border=n|Coronavirus disease 2019 | Medicine | Viruses}} | |||
| {{Sister project links|wikt=COVID-19|c=category:COVID-19|n=category:COVID-19|q=COVID-19|s=category:COVID-19|voy=2019–2020 coronavirus pandemic|v=COVID-19|b=no}} | |||
| {{Scholia|topic}} | |||
| '''Health agencies:''' | |||
| * by the ] | |||
| * by the ] | |||
| '''Directories:''' | |||
| * {{Curlie|Health/Conditions_and_Diseases/Respiratory_Disorders/COVID-19|COVID-19}} | |||
| * | |||
| '''Medical journals:''' | |||
| * by '']'' | |||
| * by the ] | |||
| * by ] | |||
| * by '']'' | |||
| * by '']'' | |||
| * by the '']'' | |||
| * by ] | |||
| '''Other:''' | |||
| * <!--referenced by https://medium.com/@tomaspueyo/coronavirus-the-hammer-and-the-dance-be9337092b56 , in turn endorsed by hundreds of experts https://medium.com/tomas-pueyo/coronavirus-articles-endorsements-fdc68614f8e3--> | |||
| {{medical resources| ICD10 = {{ICD10|U07.1}}, {{ICD10|U07.2}}}} | {{medical resources| ICD10 = {{ICD10|U07.1}}, {{ICD10|U07.2}}}} | ||
| {{SARS}} | |||
| {{#invoke:COVID-19 pandemic|}} | |||
| {{Respiratory pathology}} | {{Respiratory pathology}} | ||
| {{Viral diseases}} | {{Viral diseases}} | ||
| {{#invoke:Authority control|authorityControl}} | |||
| {{2019–20 coronavirus pandemic}} | |||
| {{Authority control}} | |||
| ] | ] | ||
| ] | ] | ||
| ] | |||
| ] | ] | ||
| ] | ] | ||
| ] | |||
| ] | |||
Latest revision as of 21:06, 16 January 2025
Contagious disease caused by SARS-CoV-2 This article is about the disease itself. For the global pandemic caused by the disease, see COVID-19 pandemic. For other diseases caused by coronaviruses, see Coronavirus diseases.Medical condition
| Coronavirus disease 2019 (COVID-19) | |
|---|---|
| Other names | COVID, (the) coronavirus |
 | |
| Transmission and life-cycle of SARS-CoV-2, which causes COVID-19 | |
| Pronunciation | |
| Specialty | Infectious disease |
| Symptoms | Fever, cough, fatigue, shortness of breath, vomiting, loss of taste or smell; some cases asymptomatic |
| Complications | Pneumonia, sepsis, ARDS, kidney failure, respiratory failure, pulmonary fibrosis, CKS, MIS-C, long COVID |
| Usual onset | 2–14 days (typically 5) after infection |
| Duration | 5 days to chronic |
| Causes | SARS-CoV-2 |
| Diagnostic method | RT‑PCR testing, CT scan, rapid antigen test |
| Prevention | Vaccination, face coverings, quarantine, social distancing, ventilation, hand washing |
| Treatment | Symptomatic and supportive |
| Frequency | 777,309,629 confirmed cases (true case count is expected to be much higher) |
| Deaths |
|
Coronavirus disease 2019 (COVID-19) is a contagious disease caused by the coronavirus SARS-CoV-2. In January 2020 the disease spread worldwide, resulting in the COVID-19 pandemic.
The symptoms of COVID‑19 can vary but often include fever, fatigue, cough, breathing difficulties, loss of smell, and loss of taste. Symptoms may begin one to fourteen days after exposure to the virus. At least a third of people who are infected do not develop noticeable symptoms. Of those who develop symptoms noticeable enough to be classified as patients, most (81%) develop mild to moderate symptoms (up to mild pneumonia), while 14% develop severe symptoms (dyspnea, hypoxia, or more than 50% lung involvement on imaging), and 5% develop critical symptoms (respiratory failure, shock, or multiorgan dysfunction). Older people have a higher risk of developing severe symptoms. Some complications result in death. Some people continue to experience a range of effects (long COVID) for months or years after infection, and damage to organs has been observed. Multi-year studies on the long-term effects are ongoing.
COVID‑19 transmission occurs when infectious particles are breathed in or come into contact with the eyes, nose, or mouth. The risk is highest when people are in close proximity, but small airborne particles containing the virus can remain suspended in the air and travel over longer distances, particularly indoors. Transmission can also occur when people touch their eyes, nose or mouth after touching surfaces or objects that have been contaminated by the virus. People remain contagious for up to 20 days and can spread the virus even if they do not develop symptoms.
Testing methods for COVID-19 to detect the virus's nucleic acid include real-time reverse transcription polymerase chain reaction (RT‑PCR), transcription-mediated amplification, and reverse transcription loop-mediated isothermal amplification (RT‑LAMP) from a nasopharyngeal swab.
Several COVID-19 vaccines have been approved and distributed in various countries, many of which have initiated mass vaccination campaigns. Other preventive measures include physical or social distancing, quarantining, ventilation of indoor spaces, use of face masks or coverings in public, covering coughs and sneezes, hand washing, and keeping unwashed hands away from the face. While drugs have been developed to inhibit the virus, the primary treatment is still symptomatic, managing the disease through supportive care, isolation, and experimental measures.
The first known case was identified in Wuhan, China, in December 2019. Most scientists believe the SARS-CoV-2 virus entered into human populations through natural zoonosis, similar to the SARS-CoV-1 and MERS-CoV outbreaks, and consistent with other pandemics in human history. Social and environmental factors including climate change, natural ecosystem destruction and wildlife trade increased the likelihood of such zoonotic spillover.
Nomenclature
Main article: COVID-19 namingDuring the initial outbreak in Wuhan, the virus and disease were commonly referred to as "coronavirus" and "Wuhan coronavirus", with the disease sometimes called "Wuhan pneumonia". In the past, many diseases have been named after geographical locations, such as the Spanish flu, Middle East respiratory syndrome, and Zika virus. In January 2020, the World Health Organization (WHO) recommended 2019-nCoV and 2019-nCoV acute respiratory disease as interim names for the virus and disease per 2015 guidance and international guidelines against using geographical locations or groups of people in disease and virus names to prevent social stigma. The official names COVID‑19 and SARS-CoV-2 were issued by the WHO on 11 February 2020 with COVID-19 being shorthand for "coronavirus disease 2019". The WHO additionally uses "the COVID‑19 virus" and "the virus responsible for COVID‑19" in public communications.
Symptoms and signs
Main article: Symptoms of COVID-19
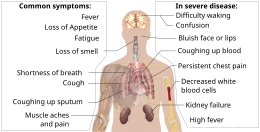
The symptoms of COVID-19 are variable depending on the type of variant contracted, ranging from mild symptoms to a potentially fatal illness. Common symptoms include coughing, fever, loss of smell (anosmia) and taste (ageusia), with less common ones including headaches, nasal congestion and runny nose, muscle pain, sore throat, diarrhea, eye irritation, and toes swelling or turning purple, and in moderate to severe cases, breathing difficulties. People with the COVID-19 infection may have different symptoms, and their symptoms may change over time.
Three common clusters of symptoms have been identified: a respiratory symptom cluster with cough, sputum, shortness of breath, and fever; a musculoskeletal symptom cluster with muscle and joint pain, headache, and fatigue; and a cluster of digestive symptoms with abdominal pain, vomiting, and diarrhea. In people without prior ear, nose, or throat disorders, loss of taste combined with loss of smell is associated with COVID-19 and is reported in as many as 88% of symptomatic cases.
Published data on the neuropathological changes related with COVID-19 have been limited and contentious, with neuropathological descriptions ranging from moderate to severe hemorrhagic and hypoxia phenotypes, thrombotic consequences, changes in acute disseminated encephalomyelitis (ADEM-type), encephalitis and meningitis. Many COVID-19 patients with co-morbidities have hypoxia and have been in intensive care for varying lengths of time, confounding interpretation of the data.
Of people who show symptoms, 81% develop only mild to moderate symptoms (up to mild pneumonia), while 14% develop severe symptoms (dyspnea, hypoxia, or more than 50% lung involvement on imaging) that require hospitalization, and 5% of patients develop critical symptoms (respiratory failure, septic shock, or multiorgan dysfunction) requiring ICU admission.
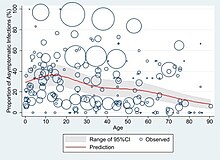
At least a third of the people who are infected with the virus do not develop noticeable symptoms at any point in time. These asymptomatic carriers tend not to get tested and can still spread the disease. Other infected people will develop symptoms later (called "pre-symptomatic") or have very mild symptoms and can also spread the virus.
As is common with infections, there is a delay, or incubation period, between the moment a person first becomes infected and the appearance of the first symptoms. The median delay for COVID-19 is four to five days possibly being infectious on 1–4 of those days. Most symptomatic people experience symptoms within two to seven days after exposure, and almost all will experience at least one symptom within 12 days.
Most people recover from the acute phase of the disease. However, some people continue to experience a range of effects, such as fatigue, for prolonged periods after an initial COVID-19 infection. This is the result of a condition called long COVID, which can be described as a range of persistent symptoms that continue for months or years. Long-term damage to organs has been observed after the onset of COVID-19. Multi-year studies are underway to further investigate the protracted effects of long COVID. Reducing the risk of long COVID includes staying up to date on the most recent COVID-19 vaccine, practicing good hygiene, maintaining clean indoor air, and physical distancing from people infected with a respiratory virus.
The Omicron variant became dominant in the U.S. in December 2021. Symptoms with the Omicron variant are less severe than they are with other variants.Complications
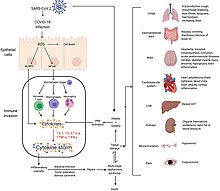
Complications may include pneumonia, acute respiratory distress syndrome (ARDS), multi-organ failure, septic shock, and death. Cardiovascular complications may include heart failure, arrhythmias (including atrial fibrillation), heart inflammation, thrombosis, particularly venous thromboembolism, and endothelial cell injury and dysfunction. Approximately 20–30% of people who present with COVID‑19 have elevated liver enzymes, reflecting liver injury.
Neurologic manifestations include seizure, stroke, encephalitis, and Guillain–Barré syndrome (which includes loss of motor functions). Following the infection, children may develop paediatric multisystem inflammatory syndrome, which has symptoms similar to Kawasaki disease, which can be fatal. In very rare cases, acute encephalopathy can occur, and it can be considered in those who have been diagnosed with COVID‑19 and have an altered mental status.
According to the US Centers for Disease Control and Prevention, pregnant women are at increased risk of becoming seriously ill from COVID‑19. This is because pregnant women with COVID‑19 appear to be more likely to develop respiratory and obstetric complications that can lead to miscarriage, premature delivery and intrauterine growth restriction.
Fungal infections such as aspergillosis, candidiasis, cryptococcosis and mucormycosis have been recorded in people recovering from COVID‑19.
Cause
COVID‑19 is caused by infection with a strain of coronavirus known as "severe acute respiratory syndrome coronavirus 2" (SARS-CoV-2).
Transmission
Main article: Transmission of COVID-19
COVID-19 is mainly transmitted when people breathe in air contaminated by droplets/aerosols and small airborne particles containing the virus. Infected people exhale those particles as they breathe, talk, cough, sneeze, or sing. Transmission is more likely the closer people are. However, infection can occur over longer distances, particularly indoors.
The transmission of the virus is carried out through virus-laden fluid particles, or droplets, which are created in the respiratory tract, and they are expelled by the mouth and the nose. There are three types of transmission: "droplet" and "contact", which are associated with large droplets, and "airborne", which is associated with small droplets. If the droplets are above a certain critical size, they settle faster than they evaporate, and therefore they contaminate surfaces surrounding them. Droplets that are below a certain critical size, generally thought to be <100μm diameter, evaporate faster than they settle; due to that fact, they form respiratory aerosol particles that remain airborne for a long period of time over extensive distances.
Infectivity can begin four to five days before the onset of symptoms. Infected people can spread the disease even if they are pre-symptomatic or asymptomatic. Most commonly, the peak viral load in upper respiratory tract samples occurs close to the time of symptom onset and declines after the first week after symptoms begin. Current evidence suggests a duration of viral shedding and the period of infectiousness of up to ten days following symptom onset for people with mild to moderate COVID-19, and up to 20 days for persons with severe COVID-19, including immunocompromised people.
Infectious particles range in size from aerosols that remain suspended in the air for long periods of time to larger droplets that remain airborne briefly or fall to the ground. Additionally, COVID-19 research has redefined the traditional understanding of how respiratory viruses are transmitted. The largest droplets of respiratory fluid do not travel far, but can be inhaled or land on mucous membranes on the eyes, nose, or mouth to infect. Aerosols are highest in concentration when people are in close proximity, which leads to easier viral transmission when people are physically close, but airborne transmission can occur at longer distances, mainly in locations that are poorly ventilated; in those conditions small particles can remain suspended in the air for minutes to hours.Virology
Main article: SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a novel severe acute respiratory syndrome coronavirus. It was first isolated from three people with pneumonia connected to the cluster of acute respiratory illness cases in Wuhan. All structural features of the novel SARS-CoV-2 virus particle occur in related coronaviruses in nature, particularly in Rhinolophus sinicus (Chinese horseshoe bats).
Outside the human body, the virus is destroyed by household soap which bursts its protective bubble. Hospital disinfectants, alcohols, heat, povidone-iodine, and ultraviolet-C (UV-C) irradiation are also effective disinfection methods for surfaces.
SARS-CoV-2 is closely related to the original SARS-CoV. It is thought to have an animal (zoonotic) origin. Genetic analysis has revealed that the coronavirus genetically clusters with the genus Betacoronavirus, in subgenus Sarbecovirus (lineage B) together with two bat-derived strains. It is 96% identical at the whole genome level to other bat coronavirus samples (BatCov RaTG13). The structural proteins of SARS-CoV-2 include membrane glycoprotein (M), envelope protein (E), nucleocapsid protein (N), and the spike protein (S). The M protein of SARS-CoV-2 is about 98% similar to the M protein of bat SARS-CoV, maintains around 98% homology with pangolin SARS-CoV, and has 90% homology with the M protein of SARS-CoV; whereas, the similarity is only around 38% with the M protein of MERS-CoV.
SARS-CoV-2 variants
Main article: Variants of SARS-CoV-2The many thousands of SARS-CoV-2 variants are grouped into either clades or lineages. The WHO, in collaboration with partners, expert networks, national authorities, institutions and researchers, have established nomenclature systems for naming and tracking SARS-CoV-2 genetic lineages by GISAID, Nextstrain and Pango. The expert group convened by the WHO recommended the labelling of variants using letters of the Greek alphabet, for example, Alpha, Beta, Delta, and Gamma, giving the justification that they "will be easier and more practical to discussed by non-scientific audiences". Nextstrain divides the variants into five clades (19A, 19B, 20A, 20B, and 20C), while GISAID divides them into seven (L, O, V, S, G, GH, and GR). The Pango tool groups variants into lineages, with many circulating lineages being classed under the B.1 lineage.
Several notable variants of SARS-CoV-2 emerged throughout 2020. Cluster 5 emerged among minks and mink farmers in Denmark. After strict quarantines and the slaughter of all the country's mink, the cluster was assessed to no longer be circulating among humans in Denmark as of 1 February 2021.
As of December 2021, there are five dominant variants of SARS-CoV-2 spreading among global populations: the Alpha variant (B.1.1.7, formerly called the UK variant), first found in London and Kent, the Beta variant (B.1.351, formerly called the South Africa variant), the Gamma variant (P.1, formerly called the Brazil variant), the Delta variant (B.1.617.2, formerly called the India variant), and the Omicron variant (B.1.1.529), which had spread to 57 countries as of 7 December.
On December 19, 2023, the WHO declared that another distinctive variant, JN.1, had emerged as a "variant of interest". Though the WHO expected an increase in cases globally, particularly for countries entering winter, the overall global health risk was considered low.
Pathophysiology

The SARS-CoV-2 virus can infect a wide range of cells and systems of the body. COVID‑19 is most known for affecting the upper respiratory tract (sinuses, nose, and throat) and the lower respiratory tract (windpipe and lungs). The lungs are the organs most affected by COVID‑19 because the virus accesses host cells via the receptor for the enzyme angiotensin-converting enzyme 2 (ACE2), which is most abundant on the surface of type II alveolar cells of the lungs. The virus uses a special surface glycoprotein called a "spike" to connect to the ACE2 receptor and enter the host cell.
Respiratory tract
Following viral entry, COVID‑19 infects the ciliated epithelium of the nasopharynx and upper airways. Autopsies of people who died of COVID‑19 have found diffuse alveolar damage, and lymphocyte-containing inflammatory infiltrates within the lung.
From the CT scans of COVID-19 infected lungs, white patches were observed containing fluid known as ground-glass opacity (GGO) or simply ground glass. This tended to correlate with the clear jelly liquid found in lung autopsies of people who died of COVID-19. One possibility addressed in medical research is that hyuralonic acid (HA) could be the leading factor for this observation of the clear jelly liquid found in the lungs, in what could be hyuralonic storm, in conjunction with cytokine storm.
Nervous system
One common symptom, loss of smell, results from infection of the support cells of the olfactory epithelium, with subsequent damage to the olfactory neurons. The involvement of both the central and peripheral nervous system in COVID‑19 has been reported in many medical publications. It is clear that many people with COVID-19 exhibit neurological or mental health issues. The virus is not detected in the central nervous system (CNS) of the majority of people with COVID-19 who also have neurological issues. However, SARS-CoV-2 has been detected at low levels in the brains of those who have died from COVID‑19, but these results need to be confirmed. While virus has been detected in cerebrospinal fluid of autopsies, the exact mechanism by which it invades the CNS remains unclear and may first involve invasion of peripheral nerves given the low levels of ACE2 in the brain. The virus may also enter the bloodstream from the lungs and cross the blood–brain barrier to gain access to the CNS, possibly within an infected white blood cell.

Research conducted when Alpha was the dominant variant has suggested COVID-19 may cause brain damage. Later research showed that all variants studied (including Omicron) killed brain cells, but the exact cells killed varied by variant. It is unknown if such damage is temporary or permanent. Observed individuals infected with COVID-19 (most with mild cases) experienced an additional 0.2% to 2% of brain tissue lost in regions of the brain connected to the sense of smell compared with uninfected individuals, and the overall effect on the brain was equivalent on average to at least one extra year of normal ageing; infected individuals also scored lower on several cognitive tests. All effects were more pronounced among older ages.
Gastrointestinal tract
The virus also affects gastrointestinal organs as ACE2 is abundantly expressed in the glandular cells of gastric, duodenal and rectal epithelium as well as endothelial cells and enterocytes of the small intestine.
Cardiovascular system
The virus can cause acute myocardial injury and chronic damage to the cardiovascular system. An acute cardiac injury was found in 12% of infected people admitted to the hospital in Wuhan, China, and is more frequent in severe disease. Rates of cardiovascular symptoms are high, owing to the systemic inflammatory response and immune system disorders during disease progression, but acute myocardial injuries may also be related to ACE2 receptors in the heart. ACE2 receptors are highly expressed in the heart and are involved in heart function.
A high incidence of thrombosis and venous thromboembolism occurs in people transferred to intensive care units with COVID‑19 infections, and may be related to poor prognosis. Blood vessel dysfunction and clot formation (as suggested by high D-dimer levels caused by blood clots) may have a significant role in mortality, incidents of clots leading to pulmonary embolisms, and ischaemic events (strokes) within the brain found as complications leading to death in people infected with COVID‑19. Infection may initiate a chain of vasoconstrictive responses within the body, including pulmonary vasoconstriction – a possible mechanism in which oxygenation decreases during pneumonia. Furthermore, damage of arterioles and capillaries was found in brain tissue samples of people who died from COVID‑19.
COVID‑19 may also cause substantial structural changes to blood cells, sometimes persisting for months after hospital discharge. A low level of blood lymphocytess may result from the virus acting through ACE2-related entry into lymphocytes.
Kidneys
Another common cause of death is complications related to the kidneys. Early reports show that up to 30% of people hospitalised with COVID-19 both in China and in New York have experienced some injury to their kidneys, including some persons with no previous kidney problems.
Immunopathology

Although SARS-CoV-2 has a tropism for ACE2-expressing epithelial cells of the respiratory tract, people with severe COVID‑19 have symptoms of systemic hyperinflammation. Clinical laboratory findings of elevated IL‑2, IL‑6, IL‑7, as well as the following suggest an underlying immunopathology:
- Granulocyte-macrophage colony-stimulating factor (GM‑CSF)
- Interferon gamma-induced protein 10 (IP‑10)
- Monocyte chemoattractant protein 1 (MCP1)
- Macrophage inflammatory protein 1‑alpha (MIP‑1‑alpha)
- Tumour necrosis factor (TNF‑α) indicative of cytokine release syndrome (CRS)
Interferon alpha plays a complex, Janus-faced role in the pathogenesis of COVID-19. Although it promotes the elimination of virus-infected cells, it also upregulates the expression of ACE-2, thereby facilitating the SARS-Cov2 virus to enter cells and to replicate. A competition of negative feedback loops (via protective effects of interferon alpha) and positive feedback loops (via upregulation of ACE-2) is assumed to determine the fate of people with COVID-19.
Additionally, people with COVID‑19 and acute respiratory distress syndrome (ARDS) have classical serum biomarkers of CRS, including elevated C-reactive protein (CRP), lactate dehydrogenase (LDH), D-dimer, and ferritin.
Systemic inflammation results in vasodilation, allowing inflammatory lymphocytic and monocytic infiltration of the lung and the heart. In particular, pathogenic GM-CSF-secreting T cells were shown to correlate with the recruitment of inflammatory IL-6-secreting monocytes and severe lung pathology in people with COVID‑19. Lymphocytic infiltrates have also been reported at autopsy.
Viral and host factors
Virus proteins

Multiple viral and host factors affect the pathogenesis of the virus. The S-protein, otherwise known as the spike protein, is the viral component that attaches to the host receptor via the ACE2 receptors. It includes two subunits: S1 and S2.
- S1 determines the virus-host range and cellular tropism via the receptor-binding domain.
- S2 mediates the membrane fusion of the virus to its potential cell host via the H1 and HR2, which are heptad repeat regions.
Studies have shown that S1 domain induced IgG and IgA antibody levels at a much higher capacity. It is the focus spike proteins expression that are involved in many effective COVID‑19 vaccines.
The M protein is the viral protein responsible for the transmembrane transport of nutrients. It is the cause of the bud release and the formation of the viral envelope. The N and E protein are accessory proteins that interfere with the host's immune response.
Host factors
Human angiotensin converting enzyme 2 (hACE2) is the host factor that SARS-CoV-2 virus targets causing COVID‑19. Theoretically, the usage of angiotensin receptor blockers (ARB) and ACE inhibitors upregulating ACE2 expression might increase morbidity with COVID‑19, though animal data suggest some potential protective effect of ARB; however no clinical studies have proven susceptibility or outcomes. Until further data is available, guidelines and recommendations for people with hypertension remain.
The effect of the virus on ACE2 cell surfaces leads to leukocytic infiltration, increased blood vessel permeability, alveolar wall permeability, as well as decreased secretion of lung surfactants. These effects cause the majority of the respiratory symptoms. However, the aggravation of local inflammation causes a cytokine storm eventually leading to a systemic inflammatory response syndrome.
Among healthy adults not exposed to SARS-CoV-2, about 35% have CD4 T cells that recognise the SARS-CoV-2 S protein (particularly the S2 subunit) and about 50% react to other proteins of the virus, suggesting cross-reactivity from previous common colds caused by other coronaviruses.
It is unknown whether different persons use similar antibody genes in response to COVID‑19.
Host cytokine response
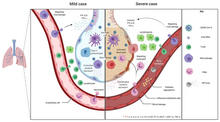
The severity of the inflammation can be attributed to the severity of what is known as the cytokine storm. Levels of interleukin 1B, interferon-gamma, interferon-inducible protein 10, and monocyte chemoattractant protein 1 were all associated with COVID‑19 disease severity. Treatment has been proposed to combat the cytokine storm as it remains to be one of the leading causes of morbidity and mortality in COVID‑19 disease.
A cytokine storm is due to an acute hyperinflammatory response that is responsible for clinical illness in an array of diseases but in COVID‑19, it is related to worse prognosis and increased fatality. The storm causes acute respiratory distress syndrome, blood clotting events such as strokes, myocardial infarction, encephalitis, acute kidney injury, and vasculitis. The production of IL-1, IL-2, IL-6, TNF-alpha, and interferon-gamma, all crucial components of normal immune responses, inadvertently become the causes of a cytokine storm. The cells of the central nervous system, the microglia, neurons, and astrocytes, are also involved in the release of pro-inflammatory cytokines affecting the nervous system, and effects of cytokine storms toward the CNS are not uncommon.
Pregnancy response
There are many unknowns for pregnant women during the COVID-19 pandemic. Given that they are prone to have complications and severe disease infection with other types of coronaviruses, they have been identified as a vulnerable group and advised to take supplementary preventive measures.
Physiological responses to pregnancy can include:
- Immunological: The immunological response to COVID-19, like other viruses, depends on a working immune system. It adapts during pregnancy to allow the development of the foetus whose genetic load is only partially shared with their mother, leading to a different immunological reaction to infections during the course of pregnancy.
- Respiratory: Many factors can make pregnant women more vulnerable to hard respiratory infections. One of them is the total reduction of the lungs' capacity and inability to clear secretions.
- Coagulation: During pregnancy, there are higher levels of circulating coagulation factors, and the pathogenesis of SARS-CoV-2 infection can be implicated. The thromboembolic events with associated mortality are a risk for pregnant women.
However, from the evidence base, it is difficult to conclude whether pregnant women are at increased risk of grave consequences of this virus.
In addition to the above, other clinical studies have proved that SARS-CoV-2 can affect the period of pregnancy in different ways. On the one hand, there is little evidence of its impact up to 12 weeks gestation. On the other hand, COVID-19 infection may cause increased rates of unfavourable outcomes in the course of the pregnancy. Some examples of these could be foetal growth restriction, preterm birth, and perinatal mortality, which refers to the foetal death past 22 or 28 completed weeks of pregnancy as well as the death among live-born children up to seven completed days of life. For preterm birth, a 2023 review indicates that there appears to be a correlation with COVID-19.
Unvaccinated women in later stages of pregnancy with COVID-19 are more likely than other people to need very intensive care. Babies born to mothers with COVID-19 are more likely to have breathing problems. Pregnant women are strongly encouraged to get vaccinated.
Diagnosis
Further information: COVID-19 testingCOVID‑19 can provisionally be diagnosed on the basis of symptoms and confirmed using reverse transcription polymerase chain reaction (RT-PCR) or other nucleic acid testing of infected secretions. Along with laboratory testing, chest CT scans may be helpful to diagnose COVID‑19 in individuals with a high clinical suspicion of infection. Detection of a past infection is possible with serological tests, which detect antibodies produced by the body in response to the infection.
Viral testing
Main article: COVID-19 testing
The standard methods of testing for presence of SARS-CoV-2 are nucleic acid tests, which detects the presence of viral RNA fragments. As these tests detect RNA but not infectious virus, its "ability to determine duration of infectivity of patients is limited". The test is typically done on respiratory samples obtained by a nasopharyngeal swab; however, a nasal swab or sputum sample may also be used. Results are generally available within hours. The WHO has published several testing protocols for the disease.
Several laboratories and companies have developed serological tests, which detect antibodies produced by the body in response to infection. Some have been evaluated by Public Health England and approved for use in the UK.
The University of Oxford's CEBM has pointed to mounting evidence that "a good proportion of 'new' mild cases and people re-testing positives after quarantine or discharge from hospital are not infectious, but are simply clearing harmless virus particles which their immune system has efficiently dealt with" and have called for "an international effort to standardize and periodically calibrate testing" In September 2020, the UK government issued "guidance for procedures to be implemented in laboratories to provide assurance of positive SARS-CoV-2 RNA results during periods of low prevalence, when there is a reduction in the predictive value of positive test results".
Imaging
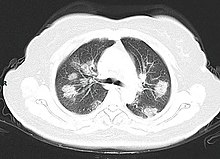


Chest CT scans may be helpful to diagnose COVID‑19 in individuals with a high clinical suspicion of infection but are not recommended for routine screening. Bilateral multilobar ground-glass opacities with a peripheral, asymmetric, and posterior distribution are common in early infection. Subpleural dominance, crazy paving (lobular septal thickening with variable alveolar filling), and consolidation may appear as the disease progresses. Characteristic imaging features on chest radiographs and computed tomography (CT) of people who are symptomatic include asymmetric peripheral ground-glass opacities without pleural effusions.
Many groups have created COVID‑19 datasets that include imagery such as the Italian Radiological Society which has compiled an international online database of imaging findings for confirmed cases. Due to overlap with other infections such as adenovirus, imaging without confirmation by rRT-PCR is of limited specificity in identifying COVID‑19. A large study in China compared chest CT results to PCR and demonstrated that though imaging is less specific for the infection, it is faster and more sensitive.
Coding
In late 2019, the WHO assigned emergency ICD-10 disease codes U07.1 for deaths from lab-confirmed SARS-CoV-2 infection and U07.2 for deaths from clinically or epidemiologically diagnosed COVID‑19 without lab-confirmed SARS-CoV-2 infection.
Pathology
The main pathological findings at autopsy are:
- Macroscopy: pericarditis, lung consolidation and pulmonary oedema
- Lung findings:
- Minor serous exudation, minor fibrin exudation
- Pulmonary oedema, pneumocyte hyperplasia, large atypical pneumocytes, interstitial inflammation with lymphocytic infiltration and multinucleated giant cell formation
- Diffuse alveolar damage (DAD) with diffuse alveolar exudates. DAD is the cause of acute respiratory distress syndrome (ARDS) and severe hypoxaemia.
- Organisation of exudates in alveolar cavities and pulmonary interstitial fibrosis
- Plasmocytosis in bronchoalveolar lavage (BAL)
- Blood and vessels: disseminated intravascular coagulation (DIC); leukoerythroblastic reaction, endotheliitis, hemophagocytosis
- Heart: cardiac muscle cell necrosis
- Liver: microvesicular steatosis
- Nose: shedding of olfactory epithelium
- Brain: infarction
- Kidneys: acute tubular damage.
- Spleen: white pulp depletion.
Prevention
Further information: COVID-19 vaccine, Workplace hazard controls for COVID-19, Pandemic prevention, Non-pharmaceutical intervention, Preparations prior to COVID-19, COVID-19 surveillance, and COVID-19 apps
Preventive measures to reduce the chances of infection include getting vaccinated, staying at home, wearing a mask in public, avoiding crowded places, keeping distance from others, ventilating indoor spaces, managing potential exposure durations, washing hands with soap and water often and for at least twenty seconds, practising good respiratory hygiene, and avoiding touching the eyes, nose, or mouth with unwashed hands.
Those diagnosed with COVID‑19 or who believe they may be infected are advised by the CDC to stay home except to get medical care, call ahead before visiting a healthcare provider, wear a face mask before entering the healthcare provider's office and when in any room or vehicle with another person, cover coughs and sneezes with a tissue, regularly wash hands with soap and water and avoid sharing personal household items.
The first COVID‑19 vaccine was granted regulatory approval on 2 December 2020 by the UK medicines regulator MHRA. It was evaluated for emergency use authorisation (EUA) status by the US FDA, and in several other countries. Initially, the US National Institutes of Health guidelines do not recommend any medication for prevention of COVID‑19, before or after exposure to the SARS-CoV-2 virus, outside the setting of a clinical trial. Without a vaccine, other prophylactic measures, or effective treatments, a key part of managing COVID‑19 is trying to decrease and delay the epidemic peak, known as "flattening the curve". This is done by slowing the infection rate to decrease the risk of health services being overwhelmed, allowing for better treatment of active cases, and delaying additional cases until effective treatments or a vaccine become available.
Vaccine
Main article: COVID-19 vaccine

Knowledge about the structure and function of previous coronaviruses causing diseases like severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) accelerated the development of various vaccine platforms in early 2020. In 2020, the first COVID‑19 vaccines were developed and made available to the public through emergency authorizations and conditional approvals. However, immunity from the vaccines was found to wane over time, requiring people to get booster doses of the vaccine to maintain protection against COVID‑19.
The COVID‑19 vaccines are widely credited for their role in reducing the spread of COVID‑19 and reducing the severity and death caused by COVID‑19. Many countries implemented phased distribution plans that prioritized those at highest risk of complications, such as the elderly, and those at high risk of exposure and transmission, such as healthcare workers.
Common side effects of COVID‑19 vaccines include soreness, redness, rash, inflammation at the injection site, fatigue, headache, myalgia (muscle pain), and arthralgia (joint pain), which resolve without medical treatment within a few days. COVID‑19 vaccination is safe for people who are pregnant or are breastfeeding.
As of 12 August 2024, 13.72 billion doses of COVID‑19 vaccines have been administered worldwide, based on official reports from national public health agencies. By December 2020, more than 10 billion vaccine doses had been preordered by countries, with about half of the doses purchased by high-income countries comprising 14% of the world's population.Face masks and respiratory hygiene
Main article: Face masks during the COVID-19 pandemic

In community and healthcare settings, the use of face masks is intended as source control to limit transmission of the virus and for personal protection to prevent infection. Properly worn masks both limit the respiratory droplets and aerosols spread by infected individuals and help protect healthy individuals from infection.
Reviews of various kinds of scientific studies have concluded that masking is effective in protecting the individual against COVID-19. Various case-control and population-based studies have also shown that increased levels of masking in a community reduces the spread of SARS-CoV-2, though there is a paucity of evidence from randomized controlled trials (RCTs). Masks vary in how well they work. Fitted N95s outperform surgical masks, while cloth masks provide marginal protection.
During the public health emergency, governments widely recommended and mandated mask-wearing, and prominent national and intergovernmental health agencies and their leaders recommended the use of masks to reduce transmission, including the WHO, American, European, and Chinese Centers for Disease Control and Prevention.Indoor ventilation and avoiding crowded indoor spaces
The CDC states that avoiding crowded indoor spaces reduces the risk of COVID-19 infection. When indoors, increasing the rate of air change, decreasing recirculation of air and increasing the use of outdoor air can reduce transmission. The WHO recommends ventilation and air filtration in public spaces to help clear out infectious aerosols.
Exhaled respiratory particles can build-up within enclosed spaces with inadequate ventilation. The risk of COVID‑19 infection increases especially in spaces where people engage in physical exertion or raise their voice (e.g., exercising, shouting, singing) as this increases exhalation of respiratory droplets. Prolonged exposure to these conditions, typically more than 15 minutes, leads to higher risk of infection.
Displacement ventilation with large natural inlets can move stale air directly to the exhaust in laminar flow while significantly reducing the concentration of droplets and particles. Passive ventilation reduces energy consumption and maintenance costs but may lack controllability and heat recovery. Displacement ventilation can also be achieved mechanically with higher energy and maintenance costs. The use of large ducts and openings helps to prevent mixing in closed environments. Recirculation and mixing should be avoided because recirculation prevents dilution of harmful particles and redistributes possibly contaminated air, and mixing increases the concentration and range of infectious particles and keeps larger particles in the air.
Hand-washing and hygiene
Main article: Hand washing
Thorough hand hygiene after any cough or sneeze is required. The WHO also recommends that individuals wash hands often with soap and water for at least twenty seconds, especially after going to the toilet or when hands are visibly dirty, before eating and after blowing one's nose. When soap and water are not available, the CDC recommends using an alcohol-based hand sanitiser with at least 60% alcohol. For areas where commercial hand sanitisers are not readily available, the WHO provides two formulations for local production. In these formulations, the antimicrobial activity arises from ethanol or isopropanol. Hydrogen peroxide is used to help eliminate bacterial spores in the alcohol; it is "not an active substance for hand antisepsis". Glycerol is added as a humectant.
Social distancing
Main article: Social distancing measures related to the COVID-19 pandemicSocial distancing (also known as physical distancing) includes infection control actions intended to slow the spread of the disease by minimising close contact between individuals. Methods include quarantines; travel restrictions; and the closing of schools, workplaces, stadiums, theatres, or shopping centres. Individuals may apply social distancing methods by staying at home, limiting travel, avoiding crowded areas, using no-contact greetings, and physically distancing themselves from others.
In 2020, outbreaks occurred in prisons due to crowding and an inability to enforce adequate social distancing. In the United States, the prisoner population is ageing and many of them are at high risk for poor outcomes from COVID‑19 due to high rates of coexisting heart and lung disease, and poor access to high-quality healthcare.
Surface cleaning
After being expelled from the body, coronaviruses can survive on surfaces for hours to days. If a person touches the dirty surface, they may deposit the virus at the eyes, nose, or mouth where it can enter the body and cause infection. Evidence indicates that contact with infected surfaces is not the main driver of COVID‑19, leading to recommendations for optimised disinfection procedures to avoid issues such as the increase of antimicrobial resistance through the use of inappropriate cleaning products and processes. Deep cleaning and other surface sanitation has been criticised as hygiene theatre, giving a false sense of security against something primarily spread through the air.
The amount of time that the virus can survive depends significantly on the type of surface, the temperature, and the humidity. Coronaviruses die very quickly when exposed to the UV light in sunlight. Like other enveloped viruses, SARS-CoV-2 survives longest when the temperature is at room temperature or lower, and when the relative humidity is low (<50%).
On many surfaces, including glass, some types of plastic, stainless steel, and skin, the virus can remain infective for several days indoors at room temperature, or even about a week under ideal conditions. On some surfaces, including cotton fabric and copper, the virus usually dies after a few hours. The virus dies faster on porous surfaces than on non-porous surfaces due to capillary action within pores and faster aerosol droplet evaporation. However, of the many surfaces tested, two with the longest survival times are N95 respirator masks and surgical masks, both of which are considered porous surfaces.
The CDC says that in most situations, cleaning surfaces with soap or detergent, not disinfecting, is enough to reduce risk of transmission. The CDC recommends that if a COVID‑19 case is suspected or confirmed at a facility such as an office or day care, all areas such as offices, bathrooms, common areas, shared electronic equipment like tablets, touch screens, keyboards, remote controls, and ATMs used by the ill persons should be disinfected. Surfaces may be decontaminated with the following:
- 62–71% ethanol
- 50–100% isopropanol
- 0.1% sodium hypochlorite
- 0.5% hydrogen peroxide
- 0.2–7.5% povidone-iodine
- 50–200 ppm hypochlorous acid
Other solutions, such as benzalkonium chloride and chlorhexidine gluconate, are less effective. Ultraviolet germicidal irradiation may also be used, although popular devices require 5–10 min exposure and may deteriorate some materials over time. A datasheet listing the authorised substances to disinfection in the food industry (including suspension or surface tested, kind of surface, use dilution, disinfectant and inoculum volumes) can be seen in the supplementary material of a 2021 Foods article.
Self-isolation
Self-isolation at home has been recommended for those diagnosed with COVID‑19 and those who suspect they have been infected. Health agencies have issued detailed instructions for proper self-isolation. Many governments have mandated or recommended self-quarantine for entire populations. The strongest self-quarantine instructions have been issued to those in high-risk groups. Those who may have been exposed to someone with COVID‑19 and those who have recently travelled to a country or region with the widespread transmission have been advised to self-quarantine for 14 days from the time of last possible exposure.
International travel-related control measures
A 2021 Cochrane rapid review found that based upon low-certainty evidence, international travel-related control measures such as restricting cross-border travel may help to contain the spread of COVID‑19. Additionally, symptom/exposure-based screening measures at borders may miss many positive cases. While test-based border screening measures may be more effective, it could also miss many positive cases if only conducted upon arrival without follow-up. The review concluded that a minimum 10-day quarantine may be beneficial in preventing the spread of COVID‑19 and may be more effective if combined with an additional control measure like border screening.
Treatment
Main article: Treatment and management of COVID-19The treatment and management of COVID-19 combines both supportive care, which includes treatment to relieve symptoms, fluid therapy, oxygen support as needed, and a growing list of approved medications. Highly effective vaccines have reduced mortality related to SARS-CoV-2; however, for those awaiting vaccination, as well as for the estimated millions of immunocompromised persons who are unlikely to respond robustly to vaccination, treatment remains important. Some people may experience persistent symptoms or disability after recovery from the infection, known as long COVID, but there is still limited information on the best management and rehabilitation for this condition.
Most cases of COVID-19 are mild. In these, supportive care includes medication such as paracetamol or NSAIDs to relieve symptoms (fever, body aches, cough), proper intake of fluids, rest, and nasal breathing. Good personal hygiene and a healthy diet are also recommended. As of April 2020 the U.S. Centers for Disease Control and Prevention (CDC) recommended that those who suspect they are carrying the virus isolate themselves at home and wear a face mask. As of November 2020 use of the glucocorticoid dexamethasone had been strongly recommended in those severe cases treated in hospital with low oxygen levels, to reduce the risk of death. Noninvasive ventilation and, ultimately, admission to an intensive care unit for mechanical ventilation may be required to support breathing. Extracorporeal membrane oxygenation (ECMO) has been used to address respiratory failure, but its benefits are still under consideration. Some of the cases of severe disease course are caused by systemic hyper-inflammation, the so-called cytokine storm.
Although several medications have been approved in different countries as of April 2022, not all countries have these medications. Patients with mild to moderate symptoms who are in the risk groups can take nirmatrelvir/ritonavir (marketed as Paxlovid) or remdesivir, either of which reduces the risk of serious illness or hospitalization. In the US, the Biden Administration COVID-19 action plan includes the Test to Treat initiative, where people can go to a pharmacy, take a COVID test, and immediately receive free Paxlovid if they test positive.
Several experimental treatments are being actively studied in clinical trials. These include the antivirals molnupiravir (developed by Merck), and nirmatrelvir/ritonavir (developed by Pfizer). Others were thought to be promising early in the pandemic, such as hydroxychloroquine and lopinavir/ritonavir, but later research found them to be ineffective or even harmful, like fluvoxamine, a cheap and widely available antidepressant; As of December 2020, there was not enough high-quality evidence to recommend so-called early treatment. In December 2020, two monoclonal antibody-based therapies were available in the United States, for early use in cases thought to be at high risk of progression to severe disease. The antiviral remdesivir has been available in the U.S., Canada, Australia, and several other countries, with varying restrictions; however, it is not recommended for people needing mechanical ventilation, and has been discouraged altogether by the World Health Organization (WHO), due to limited evidence of its efficacy. In November 2021, the UK approved the use of molnupiravir as a COVID treatment for vulnerable patients recently diagnosed with the disease.Prognosis and risk factors
See also: COVID-19 pandemic death rates by countryThe severity of COVID‑19 varies. The disease may take a mild course with few or no symptoms, resembling other common upper respiratory diseases such as the common cold. In 3–4% of cases (7.4% for those over age 65) symptoms are severe enough to cause hospitalisation. Mild cases typically recover within two weeks, while those with severe or critical diseases may take three to six weeks to recover. Among those who have died, the time from symptom onset to death has ranged from two to eight weeks. The Italian Istituto Superiore di Sanità reported that the median time between the onset of symptoms and death was twelve days, with seven being hospitalised. However, people transferred to an ICU had a median time of ten days between hospitalisation and death. Abnormal sodium levels during hospitalisation with COVID-19 are associated with poor prognoses: high sodium with a greater risk of death, and low sodium with an increased chance of needing ventilator support. Prolonged prothrombin time and elevated C-reactive protein levels on admission to the hospital are associated with severe course of COVID‑19 and with a transfer to ICU.
Some early studies suggest 10% to 20% of people with COVID‑19 will experience symptoms lasting longer than a month. A majority of those who were admitted to hospital with severe disease report long-term problems including fatigue and shortness of breath. On 30 October 2020, WHO chief Tedros Adhanom warned that "to a significant number of people, the COVID virus poses a range of serious long-term effects". He has described the vast spectrum of COVID‑19 symptoms that fluctuate over time as "really concerning". They range from fatigue, a cough and shortness of breath, to inflammation and injury of major organs – including the lungs and heart, and also neurological and psychologic effects. Symptoms often overlap and can affect any system in the body. Infected people have reported cyclical bouts of fatigue, headaches, months of complete exhaustion, mood swings, and other symptoms. Tedros therefore concluded that a strategy of achieving herd immunity by infection, rather than vaccination, is "morally unconscionable and unfeasible".
In terms of hospital readmissions about 9% of 106,000 individuals had to return for hospital treatment within two months of discharge. The average to readmit was eight days since first hospital visit. There are several risk factors that have been identified as being a cause of multiple admissions to a hospital facility. Among these are advanced age (above 65 years of age) and presence of a chronic condition such as diabetes, COPD, heart failure or chronic kidney disease.
According to scientific reviews smokers are more likely to require intensive care or die compared to non-smokers. Acting on the same ACE2 pulmonary receptors affected by smoking, air pollution has been correlated with the disease. Short-term and chronic exposure to air pollution seems to enhance morbidity and mortality from COVID‑19. Pre-existing heart and lung diseases and also obesity, especially in conjunction with fatty liver disease, contributes to an increased health risk of COVID‑19.
It is also assumed that those that are immunocompromised are at higher risk of getting severely sick from SARS-CoV-2. One research study that looked into the COVID‑19 infections in hospitalised kidney transplant recipients found a mortality rate of 11%.
Men with untreated hypogonadism were 2.4 times more likely than men with eugonadism to be hospitalised if they contracted COVID-19; Hypogonad men treated with testosterone were less likely to be hospitalised for COVID-19 than men who were not treated for hypogonadism.
Genetic risk factors
Genetics plays an important role in the ability to fight off Covid. For instance, those that do not produce detectable type I interferons or produce auto-antibodies against these may get much sicker from COVID‑19. Genetic screening is able to detect interferon effector genes. Some genetic variants are risk factors in specific populations. For instance, an allele of the DOCK2 gene (dedicator of cytokinesis 2 gene) is a common risk factor in Asian populations but much less common in Europe. The mutation leads to lower expression of DOCK2 especially in younger people with severe COVID-19 infections. In fact, many other genes and genetic variants have been found that determine the outcome of SARS-CoV-2 infections.
Children
See also: Impact of the COVID-19 pandemic on childrenWhile very young children have experienced lower rates of infection, older children have a rate of infection that is similar to the population as a whole. Children are likely to have milder symptoms and are at lower risk of severe disease than adults. The CDC reports that in the US roughly a third of hospitalised children were admitted to the ICU, while a European multinational study of hospitalised children from June 2020, found that about 8% of children admitted to a hospital needed intensive care. Four of the 582 children (0.7%) in the European study died, but the actual mortality rate may be "substantially lower" since milder cases that did not seek medical help were not included in the study.
Long-term effects
Further information: Long COVIDAround 10% to 30% of non-hospitalised people with COVID-19 go on to develop long COVID. For those that do need hospitalisation, the incidence of long-term effects is over 50%. Long COVID is an often severe multisystem disease with a large set of symptoms. There are likely various, possibly coinciding, causes. Organ damage from the acute infection can explain a part of the symptoms, but long COVID is also observed in people where organ damage seems to be absent.
By a variety of mechanisms, the lungs are the organs most affected in COVID‑19. In people requiring hospital admission, up to 98% of CT scans performed show lung abnormalities after 28 days of illness even if they had clinically improved. People with advanced age, severe disease, prolonged ICU stays, or who smoke are more likely to have long-lasting effects, including pulmonary fibrosis. Overall, approximately one-third of those investigated after four weeks will have findings of pulmonary fibrosis or reduced lung function as measured by DLCO, even in asymptomatic people, but with the suggestion of continuing improvement with the passing of more time. After severe disease, lung function can take anywhere from three months to a year or more to return to previous levels.
The risks of cognitive deficit, dementia, psychotic disorders, and epilepsy or seizures persists at an increased level two years after infection.
Immunity
See also: COVID-19 vaccine
The immune response by humans to SARS-CoV-2 virus occurs as a combination of the cell-mediated immunity and antibody production, just as with most other infections. B cells interact with T cells and begin dividing before selection into the plasma cell, partly on the basis of their affinity for antigen. Since SARS-CoV-2 has been in the human population only since December 2019, it remains unknown if the immunity is long-lasting in people who recover from the disease. The presence of neutralising antibodies in blood strongly correlates with protection from infection, but the level of neutralising antibody declines with time. Those with asymptomatic or mild disease had undetectable levels of neutralising antibody two months after infection. In another study, the level of neutralising antibodies fell four-fold one to four months after the onset of symptoms. However, the lack of antibodies in the blood does not mean antibodies will not be rapidly produced upon reexposure to SARS-CoV-2. Memory B cells specific for the spike and nucleocapsid proteins of SARS-CoV-2 last for at least six months after the appearance of symptoms.
As of August 2021, reinfection with COVID‑19 was possible but uncommon. The first case of reinfection was documented in August 2020. A systematic review found 17 cases of confirmed reinfection in medical literature as of May 2021. With the Omicron variant, as of 2022, reinfections have become common, albeit it is unclear how common. COVID-19 reinfections are thought to likely be less severe than primary infections, especially if one was previously infected by the same variant.
Mortality
Main articles: COVID-19 pandemic and COVID-19 pandemic death rates by countrySeveral measures are commonly used to quantify mortality. These numbers vary by region and over time and are influenced by the volume of testing, healthcare system quality, treatment options, time since the initial outbreak, and population characteristics such as age, sex, and overall health.
The mortality rate reflects the number of deaths within a specific demographic group divided by the population of that demographic group. Consequently, the mortality rate reflects the prevalence as well as the severity of the disease within a given population. Mortality rates are highly correlated to age, with relatively low rates for young people and relatively high rates among the elderly. In fact, one relevant factor of mortality rates is the age structure of the countries' populations. For example, the case fatality rate for COVID‑19 is lower in India than in the US since India's younger population represents a larger percentage than in the US.
Case fatality rate
The case fatality rate (CFR) reflects the number of deaths divided by the number of diagnosed cases within a given time interval. Based on Johns Hopkins University statistics, the global death-to-case ratio is 1.02% (6,881,955/676,609,955) as of 10 March 2023. The number varies by region.
-
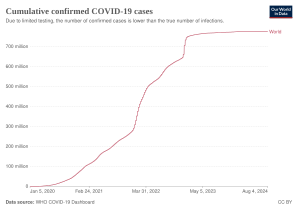 Total confirmed cases over time
Total confirmed cases over time
-
 Total confirmed cases of COVID‑19 per million people
Total confirmed cases of COVID‑19 per million people
-
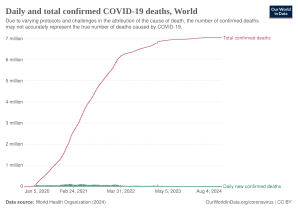 Total confirmed deaths over time
Total confirmed deaths over time
-
 Total confirmed deaths due to COVID‑19 per million people
Total confirmed deaths due to COVID‑19 per million people
Infection fatality rate
A key metric in gauging the severity of COVID‑19 is the infection fatality rate (IFR), also referred to as the infection fatality ratio or infection fatality risk. This metric is calculated by dividing the total number of deaths from the disease by the total number of infected individuals; hence, in contrast to the CFR, the IFR incorporates asymptomatic and undiagnosed infections as well as reported cases.
Estimates
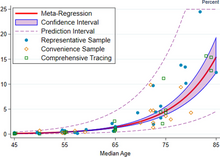

A December 2020 systematic review and meta-analysis estimated that population IFR during the first wave of the pandemic was about 0.5% to 1% in many locations (including France, Netherlands, New Zealand, and Portugal), 1% to 2% in other locations (Australia, England, Lithuania, and Spain), and exceeded 2% in Italy. That study also found that most of these differences in IFR reflected corresponding differences in the age composition of the population and age-specific infection rates; in particular, the metaregression estimate of IFR is very low for children and younger adults (e.g., 0.002% at age 10 and 0.01% at age 25) but increases progressively to 0.4% at age 55, 1.4% at age 65, 4.6% at age 75, and 15% at age 85. These results were also highlighted in a December 2020 report issued by the WHO.
| Age group | IFR |
|---|---|
| 0–34 | 0.004% |
| 35–44 | 0.068% |
| 45–54 | 0.23% |
| 55–64 | 0.75% |
| 65–74 | 2.5% |
| 75–84 | 8.5% |
| 85 + | 28.3% |
An analysis of those IFR rates indicates that COVID‑19 is hazardous not only for the elderly but also for middle-aged adults, for whom the infection fatality rate of COVID-19 is two orders of magnitude greater than the annualised risk of a fatal automobile accident and far more dangerous than seasonal influenza.
Earlier estimates of IFR
At an early stage of the pandemic, the World Health Organization reported estimates of IFR between 0.3% and 1%. On 2 July, The WHO's chief scientist reported that the average IFR estimate presented at a two-day WHO expert forum was about 0.6%. In August, the WHO found that studies incorporating data from broad serology testing in Europe showed IFR estimates converging at approximately 0.5–1%. Firm lower limits of IFRs have been established in a number of locations such as New York City and Bergamo in Italy since the IFR cannot be less than the population fatality rate. (After sufficient time however, people can get reinfected). As of 10 July, in New York City, with a population of 8.4 million, 23,377 individuals (18,758 confirmed and 4,619 probable) have died with COVID‑19 (0.3% of the population). Antibody testing in New York City suggested an IFR of ≈0.9%, and ≈1.4%. In Bergamo province, 0.6% of the population has died. In September 2020, the U.S. Centers for Disease Control and Prevention (CDC) reported preliminary estimates of age-specific IFRs for public health planning purposes.
Sex differences
Main article: Gendered impact of the COVID-19 pandemic| Percentage of infected people who are hospitalised | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0–19 | 20–29 | 30–39 | 40–49 | 50–59 | 60–69 | 70–79 | 80+ | Total | |
| Female | 0.1 (0.07–0.2) |
0.5 (0.3–0.8) |
0.9 (0.5–1.5) |
1.3 (0.7–2.1) |
2.6 (1.5–4.2) |
5.1 (2.9–8.3) |
7.8 (4.4–12.8) |
19.3 (10.9–31.6) |
2.6 (1.5–4.3) |
| Male | 0.2 (0.08–0.2) |
0.6 (0.3–0.9) |
1.2 (0.7–1.9) |
1.6 (0.9–2.6) |
3.2 (1.8–5.2) |
6.7 (3.7–10.9) |
11.0 (6.2–17.9) |
37.6 (21.1–61.3) |
3.3 (1.8–5.3) |
| Total | 0.1 (0.08–0.2) |
0.5 (0.3–0.8) |
1.1 (0.6–1.7) |
1.4 (0.8–2.3) |
2.9 (1.6–4.7) |
5.8 (3.3–9.5) |
9.3 (5.2–15.1) |
26.2 (14.8–42.7) |
2.9 (1.7–4.8) |
| Percentage of hospitalised people who go to Intensive Care Unit | |||||||||
| 0–19 | 20–29 | 30–39 | 40–49 | 50–59 | 60–69 | 70–79 | 80+ | Total | |
| Female | 16.7 (14.3–19.3) |
8.7 (7.5–9.9) |
11.9 (10.9–13.0) |
16.6 (15.6–17.7) |
20.7 (19.8–21.6) |
23.1 (22.2–24.0) |
18.7 (18.0–19.5) |
4.2 (4.0–4.5) |
14.3 (13.9–14.7) |
| Male | 26.9 (23.1–31.1) |
14.0 (12.2–16.0) |
19.2 (17.6–20.9) |
26.9 (25.4–28.4) |
33.4 (32.0–34.8) |
37.3 (36.0–38.6) |
30.2 (29.1–31.3) |
6.8 (6.5–7.2) |
23.1 (22.6–23.6) |
| Total | 22.2 (19.1–25.7) |
11.6 (10.1–13.2) |
15.9 (14.5–17.3) |
22.2 (21.0–23.5) |
27.6 (26.5–28.7) |
30.8 (29.8–31.8) |
24.9 (24.1–25.8) |
5.6 (5.3–5.9) |
19.0 (18.7–19.44) |
| Percent of hospitalised people who die | |||||||||
| 0–19 | 20–29 | 30–39 | 40–49 | 50–59 | 60–69 | 70–79 | 80+ | Total | |
| Female | 0.5 (0.2–1.0) |
0.9 (0.5–1.3) |
1.5 (1.2–1.9) |
2.6 (2.3–3.0) |
5.2 (4.8–5.6) |
10.1 (9.5–10.6) |
16.7 (16.0–17.4) |
25.2 (24.4–26.0) |
14.4 (14.0–14.8) |
| Male | 0.7 (0.3–1.5) |
1.3 (0.8–1.9) |
2.2 (1.7–2.7) |
3.8 (3.3–4.4) |
7.6 (7.0–8.2) |
14.8 (14.1–15.6) |
24.6 (23.7–25.6) |
37.1 (36.1–38.2) |
21.2 (20.8–21.7) |
| Total | 0.6 (0.2–1.3) |
1.1 (0.7–1.6) |
1.9 (1.5–2.3) |
3.3 (2.9–3.8) |
6.5 (6.0–7.0) |
12.6 (12.0–13.2) |
21.0 (20.3–21.7) |
31.6 (30.9–32.4) |
18.1 (17.8–18.4) |
| Percent of infected people who die – infection fatality rate (IFR) | |||||||||
| 0–19 | 20–29 | 30–39 | 40–49 | 50–59 | 60–69 | 70–79 | 80+ | Total | |
| Female | 0.001 (<0.001–0.002) |
0.004 (0.002–0.007) |
0.01 (0.007–0.02) |
0.03 (0.02–0.06) |
0.1 (0.08–0.2) |
0.5 (0.3–0.8) |
1.3 (0.7–2.1) |
4.9 (2.7–8.0) |
0.4 (0.2–0.6) |
| Male | 0.001 (<0.001–0.003) |
0.007 (0.003–0.01) |
0.03 (0.02–0.05) |
0.06 (0.03–0.1) |
0.2 (0.1–0.4) |
1.0 (0.6–1.6) |
2.7 (1.5–1.4) |
14.0 (7.9–22.7) |
0.7 (0.4–1.1) |
| Total | 0.001 (<0.001–0.002) |
0.005 (0.003–0.01) |
0.02 (0.01–0.03) |
0.05 (0.03–0.08) |
0.2 (0.1–0.3) |
0.7 (0.4–1.2) |
1.9 (1.1–3.2) |
8.3 (4.7–13.5) |
0.5 (0.3–0.9) |
| Numbers in parentheses are 95% credible intervals for the estimates. | |||||||||
COVID‑19 case fatality rates are higher among men than women in most countries. However, in a few countries like India, Nepal, Vietnam, and Slovenia the fatality cases are higher in women than men. Globally, men are more likely to be admitted to the ICU and more likely to die. One meta-analysis found that globally, men were more likely to get COVID‑19 than women; there were approximately 55 men and 45 women per 100 infections (CI: 51.43–56.58).
The Chinese Center for Disease Control and Prevention reported the death rate was 2.8% for men and 1.7% for women. Later reviews in June 2020 indicated that there is no significant difference in susceptibility or in CFR between genders. One review acknowledges the different mortality rates in Chinese men, suggesting that it may be attributable to lifestyle choices such as smoking and drinking alcohol rather than genetic factors. Smoking, which in some countries like China is mainly a male activity, is a habit that contributes to increasing significantly the case fatality rates among men. Sex-based immunological differences, lesser prevalence of smoking in women and men developing co-morbid conditions such as hypertension at a younger age than women could have contributed to the higher mortality in men. In Europe as of February 2020, 57% of the infected people were men and 72% of those died with COVID‑19 were men. As of April 2020, the US government is not tracking sex-related data of COVID‑19 infections. Research has shown that viral illnesses like Ebola, HIV, influenza and SARS affect men and women differently.
Ethnic differences
In the US, a greater proportion of deaths due to COVID‑19 have occurred among African Americans and other minority groups. Structural factors that prevent them from practising social distancing include their concentration in crowded substandard housing and in "essential" occupations such as retail grocery workers, public transit employees, health-care workers and custodial staff. Greater prevalence of lacking health insurance and care of underlying conditions such as diabetes, hypertension, and heart disease also increase their risk of death. Similar issues affect Native American and Latino communities. On the one hand, in the Dominican Republic there is a clear example of both gender and ethnic inequality. In this Latin American territory, there is great inequality and precariousness that especially affects Dominican women, with greater emphasis on those of Haitian descent. According to a US health policy non-profit, 34% of American Indian and Alaska Native People (AIAN) non-elderly adults are at risk of serious illness compared to 21% of white non-elderly adults. The source attributes it to disproportionately high rates of many health conditions that may put them at higher risk as well as living conditions like lack of access to clean water.
Leaders have called for efforts to research and address the disparities. In the UK, a greater proportion of deaths due to COVID‑19 have occurred in those of a Black, Asian, and other ethnic minority background. More severe impacts upon patients including the relative incidence of the necessity of hospitalisation requirements, and vulnerability to the disease has been associated via DNA analysis to be expressed in genetic variants at chromosomal region 3, features that are associated with European Neanderthal heritage. That structure imposes greater risks that those affected will develop a more severe form of the disease. The findings are from Professor Svante Pääbo and researchers he leads at the Max Planck Institute for Evolutionary Anthropology and the Karolinska Institutet. This admixture of modern human and Neanderthal genes is estimated to have occurred roughly between 50,000 and 60,000 years ago in Southern Europe.
Comorbidities
Biological factors (immune response) and the general behaviour (habits) can strongly determine the consequences of COVID‑19. Most of those who die of COVID‑19 have pre-existing (underlying) conditions, including hypertension, diabetes mellitus, and cardiovascular disease. According to March data from the United States, 89% of those hospitalised had preexisting conditions. The Italian Istituto Superiore di Sanità reported that out of 8.8% of deaths where medical charts were available, 96.1% of people had at least one comorbidity with the average person having 3.4 diseases. According to this report the most common comorbidities are hypertension (66% of deaths), type 2 diabetes (29.8% of deaths), ischaemic heart disease (27.6% of deaths), atrial fibrillation (23.1% of deaths) and chronic renal failure (20.2% of deaths).
Most critical respiratory comorbidities according to the US Centers for Disease Control and Prevention (CDC), are: moderate or severe asthma, pre-existing COPD, pulmonary fibrosis, cystic fibrosis. Evidence stemming from meta-analysis of several smaller research papers also suggests that smoking can be associated with worse outcomes. When someone with existing respiratory problems is infected with COVID‑19, they might be at greater risk for severe symptoms. COVID‑19 also poses a greater risk to people who misuse opioids and amphetamines, insofar as their drug use may have caused lung damage.
In August 2020, the CDC issued a caution that tuberculosis (TB) infections could increase the risk of severe illness or death. The WHO recommended that people with respiratory symptoms be screened for both diseases, as testing positive for COVID‑19 could not rule out co-infections. Some projections have estimated that reduced TB detection due to the pandemic could result in 6.3 million additional TB cases and 1.4 million TB-related deaths by 2025.
History
| This section needs to be updated. The reason given is: excessive detail about the very early pandemic while missing an overview of the later pandemic. Please help update this article to reflect recent events or newly available information. (July 2023) |
The virus is thought to be of natural animal origin, most likely through spillover infection. A joint-study conducted in early 2021 by the People's Republic of China and the World Health Organization indicated that the virus descended from a coronavirus that infects wild bats, and likely spread to humans through an intermediary wildlife host. There are several theories about where the index case originated and investigations into the origin of the pandemic are ongoing. According to articles published in July 2022 in Science, virus transmission into humans occurred through two spillover events in November 2019 and was likely due to live wildlife trade on the Huanan wet market in the city of Wuhan (Hubei, China). Doubts about the conclusions have mostly centered on the precise site of spillover. Earlier phylogenetics estimated that SARS-CoV-2 arose in October or November 2019. A phylogenetic algorithm analysis suggested that the virus may have been circulating in Guangdong before Wuhan.
Most scientists believe the virus spilled into human populations through natural zoonosis, similar to the SARS-CoV-1 and MERS-CoV outbreaks, and consistent with other pandemics in human history. According to the Intergovernmental Panel on Climate Change several social and environmental factors including climate change, natural ecosystem destruction and wildlife trade increased the likelihood of such zoonotic spillover. One study made with the support of the European Union found climate change increased the likelihood of the pandemic by influencing distribution of bat species.
Available evidence suggests that the SARS-CoV-2 virus was originally harboured by bats, and spread to humans multiple times from infected wild animals at the Huanan Seafood Market in Wuhan in December 2019. A minority of scientists and some members of the U.S intelligence community believe the virus may have been unintentionally leaked from a laboratory such as the Wuhan Institute of Virology. The US intelligence community has mixed views on the issue, but overall agrees with the scientific consensus that the virus was not developed as a biological weapon and is unlikely to have been genetically engineered. There is no evidence SARS-CoV-2 existed in any laboratory prior to the pandemic.
The first confirmed human infections were in Wuhan. A study of the first 41 cases of confirmed COVID‑19, published in January 2020 in The Lancet, reported the earliest date of onset of symptoms as 1 December 2019. Official publications from the WHO reported the earliest onset of symptoms as 8 December 2019. Human-to-human transmission was confirmed by the WHO and Chinese authorities by 20 January 2020. According to official Chinese sources, these were mostly linked to the Huanan Seafood Wholesale Market, which also sold live animals. In May 2020, George Gao, the director of the CDC, said animal samples collected from the seafood market had tested negative for the virus, indicating that the market was the site of an early superspreading event, but that it was not the site of the initial outbreak. Traces of the virus have been found in wastewater samples that were collected in Milan and Turin, Italy, on 18 December 2019.
By December 2019, the spread of infection was almost entirely driven by human-to-human transmission. The number of COVID-19 cases in Hubei gradually increased, reaching sixty by 20 December, and at least 266 by 31 December. On 24 December, Wuhan Central Hospital sent a bronchoalveolar lavage fluid (BAL) sample from an unresolved clinical case to sequencing company Vision Medicals. On 27 and 28 December, Vision Medicals informed the Wuhan Central Hospital and the Chinese CDC of the results of the test, showing a new coronavirus. A pneumonia cluster of unknown cause was observed on 26 December and treated by the doctor Zhang Jixian in Hubei Provincial Hospital, who informed the Wuhan Jianghan CDC on 27 December. On 30 December, a test report addressed to Wuhan Central Hospital, from company CapitalBio Medlab, stated an erroneous positive result for SARS, causing a group of doctors at Wuhan Central Hospital to alert their colleagues and relevant hospital authorities of the result. The Wuhan Municipal Health Commission issued a notice to various medical institutions on "the treatment of pneumonia of unknown cause" that same evening. Eight of these doctors, including Li Wenliang (punished on 3 January), were later admonished by the police for spreading false rumours and another, Ai Fen, was reprimanded by her superiors for raising the alarm.
The Wuhan Municipal Health Commission made the first public announcement of a pneumonia outbreak of unknown cause on 31 December, confirming 27 cases – enough to trigger an investigation.
During the early stages of the outbreak, the number of cases doubled approximately every seven and a half days. In early and mid-January 2020, the virus spread to other Chinese provinces, helped by the Chinese New Year migration and Wuhan being a transport hub and major rail interchange. On 20 January, China reported nearly 140 new cases in one day, including two people in Beijing and one in Shenzhen. Later official data shows 6,174 people had already developed symptoms by then, and more may have been infected. A report in The Lancet on 24 January indicated human transmission, strongly recommended personal protective equipment for health workers, and said testing for the virus was essential due to its "pandemic potential". On 30 January, the WHO declared COVID-19 a Public Health Emergency of International Concern. By this time, the outbreak spread by a factor of 100 to 200 times.
Italy had its first confirmed cases on 31 January 2020, two tourists from China. Italy overtook China as the country with the most deaths on 19 March 2020. By 26 March the United States had overtaken China and Italy with the highest number of confirmed cases in the world. Research on coronavirus genomes indicates the majority of COVID-19 cases in New York came from European travellers, rather than directly from China or any other Asian country. Retesting of prior samples found a person in France who had the virus on 27 December 2019, and a person in the United States who died from the disease on 6 February 2020.
RT-PCR testing of untreated wastewater samples from Brazil and Italy have suggested detection of SARS-CoV-2 as early as November and December 2019, respectively, but the methods of such sewage studies have not been optimised, many have not been peer-reviewed, details are often missing, and there is a risk of false positives due to contamination or if only one gene target is detected. A September 2020 review journal article said, "The possibility that the COVID‑19 infection had already spread to Europe at the end of last year is now indicated by abundant, even if partially circumstantial, evidence", including pneumonia case numbers and radiology in France and Italy in November and December.
As of 1 October 2021, Reuters reported that it had estimated the worldwide total number of deaths due to COVID‑19 to have exceeded five million.
The Public Health Emergency of International Concern for COVID-19 ended on May 5, 2023. By this time, everyday life in most countries had returned to how it was before the pandemic.
Misinformation
Main article: COVID-19 misinformationAfter the initial outbreak of COVID‑19, misinformation and disinformation regarding the origin, scale, prevention, treatment, and other aspects of the disease rapidly spread online.
In September 2020, the US Centers for Disease Control and Prevention (CDC) published preliminary estimates of the risk of death by age groups in the United States, but those estimates were widely misreported and misunderstood.
Other species
See also: Impact of the COVID-19 pandemic on animalsHumans appear to be capable of spreading the virus to some other animals, a type of disease transmission referred to as zooanthroponosis.
Some pets, especially cats and ferrets, can catch this virus from infected humans. Symptoms in cats include respiratory (such as a cough) and digestive symptoms. Cats can spread the virus to other cats, and may be able to spread the virus to humans, but cat-to-human transmission of SARS-CoV-2 has not been proven. Compared to cats, dogs are less susceptible to this infection. Behaviours which increase the risk of transmission include kissing, licking, and petting the animal.
The virus does not appear to be able to infect pigs, ducks, or chickens at all. Mice, rats, and rabbits, if they can be infected at all, are unlikely to be involved in spreading the virus.
Tigers and lions in zoos have become infected as a result of contact with infected humans. As expected, monkeys and great ape species such as orangutans can also be infected with the COVID‑19 virus.
Minks, which are in the same family as ferrets, have been infected. Minks may be asymptomatic, and can also spread the virus to humans. Multiple countries have identified infected animals in mink farms. Denmark, a major producer of mink pelts, ordered the slaughter of all minks over fears of viral mutations, following an outbreak referred to as Cluster 5. A vaccine for mink and other animals is being researched.
Research
Further information: COVID-19 drug developmentInternational research on vaccines and medicines in COVID‑19 is underway by government organisations, academic groups, and industry researchers. The CDC has classified it to require a BSL3 grade laboratory. There has been a great deal of COVID‑19 research, involving accelerated research processes and publishing shortcuts to meet the global demand.
As of December 2020, hundreds of clinical trials have been undertaken, with research happening on every continent except Antarctica. As of November 2020, more than 200 possible treatments have been studied in humans.
Transmission and prevention research
Further information: COVID-19 vaccineModelling research has been conducted with several objectives, including predictions of the dynamics of transmission, diagnosis and prognosis of infection, estimation of the impact of interventions, or allocation of resources. Modelling studies are mostly based on compartmental models in epidemiology, estimating the number of infected people over time under given conditions. Several other types of models have been developed and used during the COVID‑19 pandemic including computational fluid dynamics models to study the flow physics of COVID‑19, retrofits of crowd movement models to study occupant exposure, mobility-data based models to investigate transmission, or the use of macroeconomic models to assess the economic impact of the pandemic.
Treatment-related research
Main article: COVID-19 drug repurposing research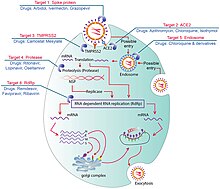
Repurposed antiviral drugs make up most of the research into COVID‑19 treatments. Other candidates in trials include vasodilators, corticosteroids, immune therapies, lipoic acid, bevacizumab, and recombinant angiotensin-converting enzyme 2.
In March 2020, the World Health Organization (WHO) initiated the Solidarity trial to assess the treatment effects of some promising drugs:
- An experimental drug called remdesivir
- Anti-malarial drugs chloroquine and hydroxychloroquine
- Two anti-HIV drugs, lopinavir/ritonavir and interferon-beta
More than 300 active clinical trials are underway as of April 2020.
Research on the antimalarial drugs hydroxychloroquine and chloroquine showed that they were ineffective at best, and that they may reduce the antiviral activity of remdesivir. By May 2020, France, Italy, and Belgium had banned the use of hydroxychloroquine as a COVID‑19 treatment.
In June, initial results from the randomised RECOVERY Trial in the United Kingdom showed that dexamethasone reduced mortality by one third for people who are critically ill on ventilators and one fifth for those receiving supplemental oxygen. Because this is a well-tested and widely available treatment, it was welcomed by the WHO, which is in the process of updating treatment guidelines to include dexamethasone and other steroids. Based on those preliminary results, dexamethasone treatment has been recommended by the NIH for peoples with COVID‑19 who are mechanically ventilated or who require supplemental oxygen but not in people with COVID‑19 who do not require supplemental oxygen.
In September 2020, the WHO released updated guidance on using corticosteroids for COVID‑19. The WHO recommends systemic corticosteroids rather than no systemic corticosteroids for the treatment of people with severe and critical COVID‑19 (strong recommendation, based on moderate certainty evidence). The WHO suggests not to use corticosteroids in the treatment of people with non-severe COVID‑19 (conditional recommendation, based on low certainty evidence). The updated guidance was based on a meta-analysis of clinical trials of people critically ill with COVID‑19.
In September 2020, the European Medicines Agency (EMA) endorsed the use of dexamethasone in adults and adolescents from twelve years of age and weighing at least 40 kilograms (88 lb) who require supplemental oxygen therapy. Dexamethasone can be taken by mouth or given as an injection or infusion (drip) into a vein.
In November 2020, the US Food and Drug Administration (FDA) issued an emergency use authorisation for the investigational monoclonal antibody therapy bamlanivimab for the treatment of mild-to-moderate COVID‑19. Bamlanivimab is authorised for people with positive results of direct SARS-CoV-2 viral testing who are twelve years of age and older weighing at least 40 kilograms (88 lb), and who are at high risk for progressing to severe COVID‑19 or hospitalisation. This includes those who are 65 years of age or older, or who have chronic medical conditions.
In February 2021, the FDA issued an emergency use authorisation (EUA) for bamlanivimab and etesevimab administered together for the treatment of mild to moderate COVID‑19 in people twelve years of age or older weighing at least 40 kilograms (88 lb) who test positive for SARS‑CoV‑2 and who are at high risk for progressing to severe COVID‑19. The authorised use includes treatment for those who are 65 years of age or older or who have certain chronic medical conditions.
In April 2021, the FDA revoked the emergency use authorisation (EUA) that allowed for the investigational monoclonal antibody therapy bamlanivimab, when administered alone, to be used for the treatment of mild-to-moderate COVID‑19 in adults and certain paediatric patients.
Cytokine storm

A cytokine storm can be a complication in the later stages of severe COVID‑19. A cytokine storm is a potentially deadly immune reaction where a large amount of pro-inflammatory cytokines and chemokines are released too quickly. A cytokine storm can lead to ARDS and multiple organ failure. Data collected from Jin Yin-tan Hospital in Wuhan, China indicates that people who had more severe responses to COVID‑19 had greater amounts of pro-inflammatory cytokines and chemokines in their system than people who had milder responses. These high levels of pro-inflammatory cytokines and chemokines indicate presence of a cytokine storm.
Tocilizumab has been included in treatment guidelines by China's National Health Commission after a small study was completed. It is undergoing a Phase II non-randomised trial at the national level in Italy after showing positive results in people with severe disease. Combined with a serum ferritin blood test to identify a cytokine storm (also called cytokine storm syndrome, not to be confused with cytokine release syndrome), it is meant to counter such developments, which are thought to be the cause of death in some affected people. The interleukin-6 receptor (IL-6R) antagonist was approved by the FDA to undergo a Phase III clinical trial assessing its effectiveness on COVID‑19 based on retrospective case studies for the treatment of steroid-refractory cytokine release syndrome induced by a different cause, CAR T cell therapy, in 2017. There is no randomised, controlled evidence that tocilizumab is an efficacious treatment for CRS. Prophylactic tocilizumab has been shown to increase serum IL-6 levels by saturating the IL-6R, driving IL-6 across the blood–brain barrier, and exacerbating neurotoxicity while having no effect on the incidence of CRS.
Lenzilumab, an anti-GM-CSF monoclonal antibody, is protective in murine models for CAR T cell-induced CRS and neurotoxicity and is a viable therapeutic option due to the observed increase of pathogenic GM-CSF secreting T cells in hospitalised patients with COVID‑19.
Passive antibodies

Transferring purified and concentrated antibodies produced by the immune systems of those who have recovered from COVID‑19 to people who need them is being investigated as a non-vaccine method of passive immunisation. Viral neutralisation is the anticipated mechanism of action by which passive antibody therapy can mediate defence against SARS-CoV-2. The spike protein of SARS-CoV-2 is the primary target for neutralising antibodies. As of 8 August 2020, eight neutralising antibodies targeting the spike protein of SARS-CoV-2 have entered clinical studies. It has been proposed that selection of broad-neutralising antibodies against SARS-CoV-2 and SARS-CoV might be useful for treating not only COVID‑19 but also future SARS-related CoV infections. Other mechanisms, however, such as antibody-dependant cellular cytotoxicity or phagocytosis, may be possible. Other forms of passive antibody therapy, for example, using manufactured monoclonal antibodies, are in development.
The use of passive antibodies to treat people with active COVID‑19 is also being studied. This involves the production of convalescent serum, which consists of the liquid portion of the blood from people who recovered from the infection and contains antibodies specific to this virus, which is then administered to active patients. This strategy was tried for SARS with inconclusive results. An updated Cochrane review in May 2023 found high certainty evidence that, for the treatment of people with moderate to severe COVID‑19, convalescent plasma did not reduce mortality or bring about symptom improvement. There continues to be uncertainty about the safety of convalescent plasma administration to people with COVID‑19 and differing outcomes measured in different studies limits their use in determining efficacy.
Bioethics
Since the outbreak of the COVID‑19 pandemic, scholars have explored the bioethics, normative economics, and political theories of healthcare policies related to the public health crisis. Academics have pointed to the moral distress of healthcare workers, ethics of distributing scarce healthcare resources such as ventilators, and the global justice of vaccine diplomacies. The socio-economic inequalities between genders, races, groups with disabilities, communities, regions, countries, and continents have also drawn attention in academia and the general public.
See also
- Coronavirus diseases, a group of closely related syndromes
- Disease X, a WHO term
- Law of declining virulence – Disproved hypothesis of epidemiologist Theobald Smith
- Theory of virulence – Theory by biologist Paul W. Ewald
References
- "Covid-19". Oxford English Dictionary (Online ed.). Oxford University Press. April 2020. Retrieved 15 April 2020. (Subscription or participating institution membership required.)
- "Symptoms of Coronavirus". U.S. Centers for Disease Control and Prevention (CDC). 13 May 2020. Archived from the original on 17 June 2020. Retrieved 18 June 2020.
- "Q&A on coronaviruses (COVID-19)". World Health Organization (WHO). 17 April 2020. Archived from the original on 14 May 2020. Retrieved 14 May 2020.
- ^ Mathieu E, Ritchie H, Rodés-Guirao L, Appel C, Giattino C, Hasell J, et al. (2020–2024). "Coronavirus Pandemic (COVID-19)". Our World in Data. Retrieved 21 January 2025.
- Mathieu E, Ritchie H, Rodés-Guirao L, Appel C, Giattino C, Hasell J, et al. (5 March 2020). "Coronavirus Pandemic (COVID-19)". Our World in Data. Archived from the original on 24 February 2024. Retrieved 24 February 2024.
- "The pandemic's true death toll". The Economist. 28 August 2023 . Retrieved 28 August 2023.
- Islam MA (April 2021). "Prevalence and characteristics of fever in adult and paediatric patients with coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis of 17515 patients". PLOS ONE. 16 (4): e0249788. Bibcode:2021PLoSO..1649788I. doi:10.1371/journal.pone.0249788. PMC 8023501. PMID 33822812.
- Saniasiaya J, Islam MA (April 2021). "Prevalence of Olfactory Dysfunction in Coronavirus Disease 2019 (COVID-19): A Meta-analysis of 27,492 Patients". The Laryngoscope. 131 (4): 865–878. doi:10.1002/lary.29286. ISSN 0023-852X. PMC 7753439. PMID 33219539.
- Saniasiaya J, Islam MA (November 2020). "Prevalence and Characteristics of Taste Disorders in Cases of COVID-19: A Meta-analysis of 29,349 Patients" (PDF). Otolaryngology–Head and Neck Surgery. 165 (1): 33–42. doi:10.1177/0194599820981018. PMID 33320033. S2CID 229174644.
- Agyeman AA, Chin KL, Landersdorfer CB, Liew D, Ofori-Asenso R (August 2020). "Smell and Taste Dysfunction in Patients With COVID-19: A Systematic Review and Meta-analysis". Mayo Clin. Proc. 95 (8): 1621–1631. doi:10.1016/j.mayocp.2020.05.030. PMC 7275152. PMID 32753137.
- Wang B, Andraweera P, Elliott S, Mohammed H, Lassi Z, Twigger A, et al. (March 2023). "Asymptomatic SARS-CoV-2 Infection by Age: A Global Systematic Review and Meta-analysis". The Pediatric Infectious Disease Journal. 42 (3): 232–239. doi:10.1097/INF.0000000000003791. PMC 9935239. PMID 36730054.
- Oran DP, Topol EJ (January 2021). "The Proportion of SARS-CoV-2 Infections That Are Asymptomatic: A Systematic Review". Annals of Internal Medicine. 174 (5): M20-6976. doi:10.7326/M20-6976. PMC 7839426. PMID 33481642.
- "Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID-19)". U.S. Centers for Disease Control and Prevention (CDC). 6 April 2020. Archived from the original on 2 March 2020. Retrieved 19 April 2020.
- ^ Davis HE, McCorkell L, Vogel JM, Topol EJ (March 2023). "Long COVID: major findings, mechanisms and recommendations". Nature Reviews. Microbiology. 21 (3): 133–146. doi:10.1038/s41579-022-00846-2. PMC 9839201. PMID 36639608.
- CDC (11 February 2020). "Post-COVID Conditions". U.S. Centers for Disease Control and Prevention (CDC). Retrieved 12 July 2021.
- "Coronavirus disease (COVID-19): How is it transmitted?". World Health Organization (WHO). Retrieved 13 April 2023.
- ^ "Overview of Testing for SARS-CoV-2, the virus that causes COVID-19". U.S. Centers for Disease Control and Prevention (CDC). 11 February 2020. Retrieved 31 July 2022.
- ^ "Nucleic Acid Amplification Tests (NAATs)". U.S. Centers for Disease Control and Prevention (CDC). 11 February 2020. Retrieved 31 July 2022.
- Gorzalski AJ, Tian H, Laverdure C, Morzunov S, Verma SC, VanHooser S, et al. (August 2020). "High-Throughput Transcription-mediated amplification on the Hologic Panther is a highly sensitive method of detection for SARS-CoV-2". Journal of Clinical Virology. 129: 104501. doi:10.1016/j.jcv.2020.104501. PMC 7286273. PMID 32619959.
- ^ Li C, Zhao C, Bao J, Tang B, Wang Y, Gu B (November 2020). "Laboratory diagnosis of coronavirus disease-2019 (COVID-19)". Clinica Chimica Acta; International Journal of Clinical Chemistry. 510: 35–46. doi:10.1016/j.cca.2020.06.045. PMC 7329657. PMID 32621814.
- Page J, Hinshaw D, McKay B (26 February 2021). "In Hunt for Covid-19 Origin, Patient Zero Points to Second Wuhan Market – The man with the first confirmed infection of the new coronavirus told the WHO team that his parents had shopped there". The Wall Street Journal. Retrieved 27 February 2021.
- ^ Pekar J (26 July 2022). "The molecular epidemiology of multiple zoonotic origins of SARS-CoV-2". Science. 377 (6609): 960–966. Bibcode:2022Sci...377..960P. doi:10.1126/science.abp8337. PMC 9348752. PMID 35881005.
- ^ Jiang X, Wang R (25 August 2022). "Wildlife trade is likely the source of SARS-CoV-2". Science. 377 (6609): 925–926. Bibcode:2022Sci...377..925J. doi:10.1126/science.add8384. PMID 36007033. S2CID 251843410. Retrieved 20 November 2022.
- ^ Terrestrial and Freshwater Ecosystems and Their Services. In: Climate Change 2022: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change (PDF). IPCC. 2022. pp. 233–235. Retrieved 14 March 2023.
- ^ Health, Wellbeing, and the Changing Structure of Communities. In: Climate Change 2022: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change (PDF). IPCC. 2022. pp. 1067–1070. Retrieved 14 March 2023.
- ^ "Climate change may have driven the emergence of SARS-CoV-2". University of Cambridge. Science of the Total Environment. 5 February 2021. Retrieved 14 March 2023.
- ^ "Climate change the culprit in the COVID-19 pandemic". European Commission. Retrieved 24 March 2023.
- "2nd U.S. Case Of Wuhan Coronavirus Confirmed". NPR. Retrieved 4 April 2020.
- McNeil Jr DG (2 February 2020). "Wuhan Coronavirus Looks Increasingly Like a Pandemic, Experts Say". The New York Times. ISSN 0362-4331. Archived from the original on 2 February 2020. Retrieved 4 April 2020.
- Griffiths J. "Wuhan coronavirus deaths spike again as outbreak shows no signs of slowing". CNN. Retrieved 4 April 2020.
- Jiang S, Xia S, Ying T, Lu L (May 2020). "A novel coronavirus (2019-nCoV) causing pneumonia-associated respiratory syndrome". Cellular & Molecular Immunology. 17 (5): 554. doi:10.1038/s41423-020-0372-4. PMC 7091741. PMID 32024976.
- Chan JF, Yuan S, Kok KH, To KK, Chu H, Yang J, et al. (February 2020). "A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster". Lancet. 395 (10223): 514–523. doi:10.1016/S0140-6736(20)30154-9. PMC 7159286. PMID 31986261.
- Shablovsky S (September 2017). "The legacy of the Spanish flu". Science. 357 (6357): 1245. Bibcode:2017Sci...357.1245S. doi:10.1126/science.aao4093. ISSN 0036-8075. S2CID 44116811.
- "Stop the coronavirus stigma now". Nature. 580 (7802): 165. 7 April 2020. Bibcode:2020Natur.580..165.. doi:10.1038/d41586-020-01009-0. PMID 32265571. S2CID 214809950. Retrieved 16 April 2020.
- "Novel Coronavirus (2019-nCoV) Situation Report – 1" (PDF). World Health Organization (WHO). 21 January 2020.
- "Novel Coronavirus(2019-nCoV) Situation Report – 10" (PDF). World Health Organization (WHO). 30 January 2020.
- "Novel coronavirus named 'Covid-19': WHO". Today. Singapore. Archived from the original on 21 March 2020. Retrieved 11 February 2020.
- "The coronavirus spreads racism against – and among – ethnic Chinese". The Economist. 17 February 2020. Archived from the original on 17 February 2020. Retrieved 17 February 2020.
- World Health Organization Best Practices for the Naming of New Human Infectious Diseases (PDF) (Report). World Health Organization (WHO). May 2015. hdl:10665/163636.
- ^ "Naming the coronavirus disease (COVID-19) and the virus that causes it". World Health Organization (WHO). Archived from the original on 28 February 2020. Retrieved 13 March 2020.
- "Novel Coronavirus(2019-nCoV) Situation Report – 22" (PDF). WHO. 11 February 2020.
- Gover AR, Harper SB, Langton L (July 2020). "Anti-Asian Hate Crime During the COVID-19 Pandemic: Exploring the Reproduction of Inequality". American Journal of Criminal Justice. 45 (4): 647–667. doi:10.1007/s12103-020-09545-1. PMC 7364747. PMID 32837171.
- "Symptoms of Coronavirus". U.S. Centers for Disease Control and Prevention (CDC). 22 February 2021. Archived from the original on 4 March 2021. Retrieved 4 March 2021.
- Grant MC, Geoghegan L, Arbyn M, Mohammed Z, McGuinness L, Clarke EL, et al. (23 June 2020). "The prevalence of symptoms in 24,410 adults infected by the novel coronavirus (SARS-CoV-2; COVID-19): A systematic review and meta-analysis of 148 studies from 9 countries". PLOS ONE. 15 (6): e0234765. Bibcode:2020PLoSO..1534765G. doi:10.1371/journal.pone.0234765. PMC 7310678. PMID 32574165. S2CID 220046286.
- Pardhan S, Vaughan M, Zhang J, Smith L, Chichger H (1 November 2020). "Sore eyes as the most significant ocular symptom experienced by people with COVID-19: a comparison between pre-COVID-19 and during COVID-19 states". BMJ Open Ophthalmology. 5 (1): e000632. doi:10.1136/bmjophth-2020-000632. PMC 7705420. PMID 34192153.
- "COVID toes, rashes: How the coronavirus can affect your skin". www.aad.org. Retrieved 20 March 2022.
- ^ "Clinical characteristics of COVID-19". European Centre for Disease Prevention and Control. 10 June 2020. Retrieved 29 December 2020.
- Paderno A, Mattavelli D, Rampinelli V, Grammatica A, Raffetti E, Tomasoni M, et al. (December 2020). "Olfactory and Gustatory Outcomes in COVID-19: A Prospective Evaluation in Nonhospitalized Subjects". Otolaryngology–Head and Neck Surgery. 163 (6): 1144–1149. doi:10.1177/0194599820939538. PMC 7331108. PMID 32600175.
- Chabot AB, Huntwork MP (September 2021). "Turmeric as a Possible Treatment for COVID-19-Induced Anosmia and Ageusia". Cureus. 13 (9): e17829. doi:10.7759/cureus.17829. PMC 8502749. PMID 34660038.
- Niazkar HR, Zibaee B, Nasimi A, Bahri N (July 2020). "The neurological manifestations of COVID-19: a review article". Neurological Sciences. 41 (7): 1667–1671. doi:10.1007/s10072-020-04486-3. PMC 7262683. PMID 32483687.
- Jafari E, Azizian R, Asareh A, Akrami S, Karimi N (2022). "Comparative study between bacterial meningitis vs. viral meningitis and COVID-19". Infectious Diseases Research. 3 (2): 9. doi:10.53388/IDR20220525009. ISSN 2703-4631.
- "Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID-19)". U.S. Centers for Disease Control and Prevention (CDC). 6 April 2020. Archived from the original on 2 March 2020. Retrieved 19 April 2020.
- ^ Wang B, Andraweera P, Elliott S, Mohammed H, Lassi Z, Twigger A, et al. (March 2023). "Asymptomatic SARS-CoV-2 Infection by Age: A Global Systematic Review and Meta-analysis". The Pediatric Infectious Disease Journal. 42 (3): 232–239. doi:10.1097/INF.0000000000003791. PMC 9935239. PMID 36730054.
- Multiple sources:
- Oran DP, Topol EJ (May 2021). "The Proportion of SARS-CoV-2 Infections That Are Asymptomatic : A Systematic Review". Annals of Internal Medicine. 174 (5): 655–662. doi:10.7326/M20-6976. PMC 7839426. PMID 33481642.
- "Transmission of COVID-19". European Centre for Disease Prevention and Control. Retrieved 6 December 2020.
- Nogrady B (November 2020). "What the data say about asymptomatic COVID infections". Nature. 587 (7835): 534–535. Bibcode:2020Natur.587..534N. doi:10.1038/d41586-020-03141-3. PMID 33214725. S2CID 227079692.
- ^ Gao Z, Xu Y, Sun C, Wang X, Guo Y, Qiu S, et al. (February 2021). "A systematic review of asymptomatic infections with COVID-19". Journal of Microbiology, Immunology, and Infection = Wei Mian Yu Gan Ran Za Zhi. 54 (1): 12–16. doi:10.1016/j.jmii.2020.05.001. PMC 7227597. PMID 32425996.
- Oran DP, Topol EJ (September 2020). "Prevalence of Asymptomatic SARS-CoV-2 Infection : A Narrative Review". Annals of Internal Medicine. 173 (5): 362–367. doi:10.7326/M20-3012. PMC 7281624. PMID 32491919.
- Lai CC, Liu YH, Wang CY, Wang YH, Hsueh SC, Yen MY, et al. (June 2020). "Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): Facts and myths". Journal of Microbiology, Immunology, and Infection = Wei Mian Yu Gan Ran Za Zhi. 53 (3): 404–412. doi:10.1016/j.jmii.2020.02.012. PMC 7128959. PMID 32173241.
- ^ Furukawa NW, Brooks JT, Sobel J (July 2020). "Evidence Supporting Transmission of Severe Acute Respiratory Syndrome Coronavirus 2 While Presymptomatic or Asymptomatic". Emerging Infectious Diseases. 26 (7). doi:10.3201/eid2607.201595. PMC 7323549. PMID 32364890.
- ^ Gandhi RT, Lynch JB, Del Rio C (October 2020). "Mild or Moderate Covid-19". The New England Journal of Medicine. 383 (18): 1757–1766. doi:10.1056/NEJMcp2009249. PMID 32329974.
- Byrne AW, McEvoy D, Collins AB, Hunt K, Casey M, Barber A, et al. (August 2020). "Inferred duration of infectious period of SARS-CoV-2: rapid scoping review and analysis of available evidence for asymptomatic and symptomatic COVID-19 cases". BMJ Open. 10 (8): e039856. doi:10.1136/bmjopen-2020-039856. PMC 7409948. PMID 32759252.
- Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC (August 2020). "Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review". JAMA. 324 (8): 782–793. doi:10.1001/jama.2020.12839. PMID 32648899. S2CID 220465311.
- ^ "Long COVID Basics". US Centers for Disease Control and Prevention. 11 July 2024. Retrieved 27 November 2024.
- CDC (29 March 2022). "Omicron Variant: What You Need to Know". Centers for Disease Control and Prevention. Retrieved 15 June 2022.
- Hui DS, I Azhar E, Madani TA, Ntoumi F, Kock R, Dar O, et al. (February 2020). "The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health – The latest 2019 novel coronavirus outbreak in Wuhan, China". International Journal of Infectious Diseases. 91: 264–266. doi:10.1016/j.ijid.2020.01.009. PMC 7128332. PMID 31953166.
- Murthy S, Gomersall CD, Fowler RA (April 2020). "Care for Critically Ill Patients With COVID-19". JAMA. 323 (15): 1499–1500. doi:10.1001/jama.2020.3633. PMID 32159735.
- Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R (2020). "Features, Evaluation and Treatment Coronavirus (COVID-19)". StatPearls. Treasure Island (FL): StatPearls Publishing. PMID 32150360. Retrieved 18 March 2020.
- Heymann DL, Shindo N, et al. (WHO Scientific and Technical Advisory Group for Infectious Hazards) (February 2020). "COVID-19: what is next for public health?". Lancet. 395 (10224): 542–545. doi:10.1016/s0140-6736(20)30374-3. PMC 7138015. PMID 32061313.
- Romiti GF, Corica B, Lip GY, Proietti M (June 2021). "Prevalence and Impact of Atrial Fibrillation in Hospitalized Patients with COVID-19: A Systematic Review and Meta-Analysis". Journal of Clinical Medicine. 10 (11): 2490. doi:10.3390/jcm10112490. PMC 8200114. PMID 34199857.
- Wen W, Zhang H, Zhou M, Cheng Y, Ye L, Chen J, et al. (November 2020). "Arrhythmia in patients with severe coronavirus disease (COVID-19): a meta-analysis". European Review for Medical and Pharmacological Sciences. 24 (21): 11395–11401. doi:10.26355/eurrev_202011_23632. PMID 33215461. S2CID 227077132.
- Long B, Brady WJ, Koyfman A, Gottlieb M (July 2020). "Cardiovascular complications in COVID-19". The American Journal of Emergency Medicine. 38 (7): 1504–1507. doi:10.1016/j.ajem.2020.04.048. PMC 7165109. PMID 32317203.
- Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, et al. (November 2020). "Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID-19)". JAMA Cardiology. 5 (11): 1265–1273. doi:10.1001/jamacardio.2020.3557. PMC 7385689. PMID 32730619.
- Lindner D, Fitzek A, Bräuninger H, Aleshcheva G, Edler C, Meissner K, et al. (November 2020). "Association of Cardiac Infection With SARS-CoV-2 in Confirmed COVID-19 Autopsy Cases". JAMA Cardiology. 5 (11): 1281–1285. doi:10.1001/jamacardio.2020.3551. PMC 7385672. PMID 32730555.
- Siripanthong B, Nazarian S, Muser D, Deo R, Santangeli P, Khanji MY, et al. (September 2020). "Recognizing COVID-19-related myocarditis: The possible pathophysiology and proposed guideline for diagnosis and management". Heart Rhythm. 17 (9): 1463–1471. doi:10.1016/j.hrthm.2020.05.001. PMC 7199677. PMID 32387246.
- Perico L, Benigni A, Remuzzi G (January 2024). "SARS-CoV-2 and the spike protein in endotheliopathy". Trends in Microbiology. 32 (1): 53–67. doi:10.1016/j.tim.2023.06.004. PMC 10258582. PMID 37393180.
- Xu L, Liu J, Lu M, Yang D, Zheng X (May 2020). "Liver injury during highly pathogenic human coronavirus infections". Liver International. 40 (5): 998–1004. doi:10.1111/liv.14435. PMC 7228361. PMID 32170806.
- ^ Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB (May 2020). "Pharmacologic Treatments for Coronavirus Disease 2019 (COVID-19): A Review". JAMA. 323 (18): 1824–1836. doi:10.1001/jama.2020.6019. PMID 32282022.
- Carod-Artal FJ (May 2020). "Neurological complications of coronavirus and COVID-19". Revista de Neurología. 70 (9): 311–322. doi:10.33588/rn.7009.2020179. PMID 32329044. S2CID 226200547.
- Toscano G, Palmerini F, Ravaglia S, Ruiz L, Invernizzi P, Cuzzoni MG, et al. (June 2020). "Guillain-Barré Syndrome Associated with SARS-CoV-2". The New England Journal of Medicine. 382 (26): 2574–2576. doi:10.1056/NEJMc2009191. PMC 7182017. PMID 32302082.
- "Multisystem inflammatory syndrome in children and adolescents temporally related to COVID-19". World Health Organization (WHO). 15 May 2020. Retrieved 20 May 2020.
- HAN Archive – 00432. U.S. Centers for Disease Control and Prevention (CDC) (Report). 15 May 2020. Retrieved 20 May 2020.
- Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B (August 2020). "COVID-19-associated Acute Hemorrhagic Necrotizing Encephalopathy: Imaging Features". Radiology. 296 (2): E119 – E120. doi:10.1148/radiol.2020201187. PMC 7233386. PMID 32228363.
- ^ Córdoba-Vives S, Peñaranda G (April 2020). "COVID-19 y Embarazo". Medical Journal of Costa Rica (in Spanish): 629. Archived from the original on 18 June 2021. Retrieved 14 February 2022.
- Das S, Dhar S (July 2021). "Mucormycosis Following COVID-19 Infections: an Insight". The Indian Journal of Surgery. 84 (3): 585–586. doi:10.1007/s12262-021-03028-1. PMC 8270771. PMID 34276145. S2CID 235782159.
- Baruah C, Devi P, Deka B, Sharma DK (June 2021). "Mucormycosis and Aspergillosis have been Linked to Covid-19-Related Fungal Infections in India". Advancements in Case Studies. 3 (1). doi:10.31031/AICS.2021.03.000555. ISSN 2639-0531. S2CID 244678882 – via ResearchGate.
- Hu B, Guo H, Zhou P, Shi ZL (March 2021). "Characteristics of SARS-CoV-2 and COVID-19". Nature Reviews. Microbiology. 19 (3): 141–154. doi:10.1038/s41579-020-00459-7. PMC 7537588. PMID 33024307.
- ^ Wang CC, Prather KA, Sznitman J, Jimenez JL, Lakdawala SS, Tufekci Z, et al. (August 2021). "Airborne transmission of respiratory viruses". Science. 373 (6558). doi:10.1126/science.abd9149. PMC 8721651. PMID 34446582.
- Greenhalgh T, Jimenez JL, Prather KA, Tufekci Z, Fisman D, Schooley R (May 2021). "Ten scientific reasons in support of airborne transmission of SARS-CoV-2". Lancet. 397 (10285): 1603–1605. doi:10.1016/s0140-6736(21)00869-2. PMC 8049599. PMID 33865497.
- Bourouiba L (13 July 2021). "Fluid Dynamics of Respiratory Infectious Diseases". Annual Review of Biomedical Engineering. 23 (1): 547–577. doi:10.1146/annurev-bioeng-111820-025044. hdl:1721.1/131115. PMID 34255991. S2CID 235823756. Retrieved 7 September 2021.
- Stadnytskyi V, Bax CE, Bax A, Anfinrud P (2 June 2020). "The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission". Proceedings of the National Academy of Sciences. 117 (22): 11875–11877. Bibcode:2020PNAS..11711875S. doi:10.1073/pnas.2006874117. PMC 7275719. PMID 32404416.
- Miller SL, Nazaroff WW, Jimenez JL, Boerstra A, Buonanno G, Dancer SJ, et al. (March 2021). "Transmission of SARS-CoV-2 by inhalation of respiratory aerosol in the Skagit Valley Chorale superspreading event". Indoor Air. 31 (2): 314–323. Bibcode:2021InAir..31..314M. doi:10.1111/ina.12751. PMC 7537089. PMID 32979298.
- ^ Mittal R (2020). "The flow physics of COVID-19". Journal of Fluid Mechanics. 894. arXiv:2004.09354. Bibcode:2020JFM...894F...2M. doi:10.1017/jfm.2020.330. S2CID 215827809.
- He X, Lau EH, Wu P, Deng X, Wang J, Hao X, et al. (September 2020). "Author Correction: Temporal dynamics in viral shedding and transmissibility of COVID-19". Nature Medicine. 26 (9): 1491–1493. doi:10.1038/s41591-020-1016-z. PMC 7413015. PMID 32770170. S2CID 221050261.
- ^ Communicable Diseases Network Australia. "Coronavirus Disease 2019 (COVID-19): CDNA National Guidelines for Public Health Units". 5.1. Communicable Diseases Network Australia/Australian Government Department of Health.
- "Clinical Questions about COVID-19: Questions and Answers". Centers for Disease Control and Prevention. 4 March 2021.
- "Scientific Brief: SARS-CoV-2 Transmission". Centers for Disease Control and Prevention. 7 May 2021. Retrieved 8 May 2021.
- "Coronavirus disease (COVID-19): How is it transmitted?". World Health Organization. 30 April 2021.
- ^ • "COVID-19: epidemiology, virology and clinical features". GOV.UK. Retrieved 18 October 2020.
• Communicable Diseases Network Australia. "Coronavirus Disease 2019 (COVID-19) - CDNA Guidelines for Public Health Units". Version 4.4. Australian Government Department of Health. Retrieved 17 May 2021.
• Public Health Agency of Canada (3 November 2020). "COVID-19: Main modes of transmission". aem. Retrieved 18 May 2021.
• "Transmission of COVID-19". European Centre for Disease Prevention and Control. 26 January 2021. Retrieved 18 May 2021.
• Meyerowitz EA, Richterman A, Gandhi RT, Sax PE (January 2021). "Transmission of SARS-CoV-2: A Review of Viral, Host, and Environmental Factors". Annals of Internal Medicine. 174 (1): 69–79. doi:10.7326/M20-5008. ISSN 0003-4819. PMC 7505025. PMID 32941052. - ^ Tang JW, Marr LC, Li Y, Dancer SJ (April 2021). "Covid-19 has redefined airborne transmission". BMJ. 373: n913. doi:10.1136/bmj.n913. PMID 33853842.
- ^ Morawska L, Allen J, Bahnfleth W, Bluyssen PM, Boerstra A, Buonanno G, et al. (May 2021). "A paradigm shift to combat indoor respiratory infection" (PDF). Science. 372 (6543): 689–691. Bibcode:2021Sci...372..689M. doi:10.1126/science.abg2025. PMID 33986171. S2CID 234487289. Archived from the original (PDF) on 6 December 2021. Retrieved 14 June 2021.
- Biswas Riddhideep, Pal Anish, Pal Ritam, Sarkar Sourav, Mukhopadhyay Achintya (2022). "Risk assessment of COVID infection by respiratory droplets from cough for various ventilation scenarios inside an elevator: An OpenFOAM-based computational fluid dynamics analysis". Physics of Fluids. 34 (1): 013318. arXiv:2109.12841. Bibcode:2022PhFl...34a3318B. doi:10.1063/5.0073694. PMC 8939552. PMID 35340680. S2CID 245828044.
- "Outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): increased transmission beyond China – fourth update" (PDF). European Centre for Disease Prevention and Control. 14 February 2020. Retrieved 8 March 2020.
- ^ Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF (April 2020). "The proximal origin of SARS-CoV-2". Nature Medicine. 26 (4): 450–452. doi:10.1038/s41591-020-0820-9. PMC 7095063. PMID 32284615.
- Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. (2020). "A pneumonia outbreak associated with a new coronavirus of probable bat origin". Nature. 579 (7798): 270–273. Bibcode:2020Natur.579..270Z. doi:10.1038/s41586-020-2012-7. PMC 7095418. PMID 32015507.
- Gibbens S (18 March 2020). "Why soap is preferable to bleach in the fight against coronavirus". National Geographic. Archived from the original on 2 April 2020. Retrieved 2 April 2020.
- Viana Martins CP, Xavier CS, Cobrado L (2022). "Disinfection methods against SARS-CoV-2: a systematic review". The Journal of Hospital Infection. 119: 84–117. doi:10.1016/j.jhin.2021.07.014. ISSN 1532-2939. PMC 8522489. PMID 34673114.
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. (February 2020). "A Novel Coronavirus from Patients with Pneumonia in China, 2019". The New England Journal of Medicine. 382 (8): 727–733. doi:10.1056/NEJMoa2001017. PMC 7092803. PMID 31978945.
- ^ Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19) (PDF) (Report). World Health Organization (WHO). February 2020. Archived (PDF) from the original on 29 February 2020. Retrieved 21 March 2020.
- "Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19)". World Health Organization (WHO). Retrieved 25 January 2022.
- Rathore JS, Ghosh C (August 2020). "Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), a newly emerged pathogen: an overview". Pathogens and Disease. 78 (6). doi:10.1093/femspd/ftaa042. OCLC 823140442. PMC 7499575. PMID 32840560.
- Thomas S (October 2020). "The Structure of the Membrane Protein of SARS-CoV-2 Resembles the Sugar Transporter SemiSWEET". Pathogens & Immunity. 5 (1): 342–363. doi:10.20411/pai.v5i1.377. PMC 7608487. PMID 33154981.
- Koyama T, Platt D, Parida L (July 2020). "Variant analysis of SARS-CoV-2 genomes". Bulletin of the World Health Organization. 98 (7): 495–504. doi:10.2471/BLT.20.253591 (inactive 5 December 2024). PMC 7375210. PMID 32742035.
We detected in total 65776 variants with 5775 distinct variants.
{{cite journal}}: CS1 maint: DOI inactive as of December 2024 (link) - ^ Rambaut A, Holmes EC, O'Toole Á, Hill V, McCrone JT, Ruis C, et al. (November 2020). "A dynamic nomenclature proposal for SARS-CoV-2 lineages to assist genomic epidemiology". Nature Microbiology. 5 (11): 1403–1407. doi:10.1038/s41564-020-0770-5. PMC 7610519. PMID 32669681.
- "Tracking SARS-CoV-2 variants". World Health Organization (WHO). 1 July 2021. Retrieved 5 July 2021.
- Alm E, Broberg EK, Connor T, Hodcroft EB, Komissarov AB, Maurer-Stroh S, et al. (August 2020). "Geographical and temporal distribution of SARS-CoV-2 clades in the WHO European Region, January to June 2020". Euro Surveillance. 25 (32). doi:10.2807/1560-7917.ES.2020.25.32.2001410. PMC 7427299. PMID 32794443.
- "PANGO lineages". cov-lineages.org. Archived from the original on 10 May 2021. Retrieved 9 May 2021.
- Lauring AS, Hodcroft EB (February 2021). "Genetic Variants of SARS-CoV-2-What Do They Mean?". JAMA. 325 (6): 529–531. doi:10.1001/jama.2020.27124. PMID 33404586. S2CID 230783233.
- Abdool Karim SS, de Oliveira T (May 2021). "New SARS-CoV-2 Variants – Clinical, Public Health, and Vaccine Implications". The New England Journal of Medicine. 384 (19). Massachusetts Medical Society: 1866–1868. doi:10.1056/nejmc2100362. ISSN 0028-4793. PMC 8008749. PMID 33761203.
- Mallapaty S (November 2020). "COVID mink analysis shows mutations are not dangerous – yet". Nature. 587 (7834): 340–341. Bibcode:2020Natur.587..340M. doi:10.1038/d41586-020-03218-z. PMID 33188367. S2CID 226947606.
- Larsen HD, Fonager J, Lomholt FK, Dalby T, Benedetti G, Kristensen B, et al. (February 2021). "Preliminary report of an outbreak of SARS-CoV-2 in mink and mink farmers associated with community spread, Denmark, June to November 2020". Euro Surveillance. 26 (5): 2100009. doi:10.2807/1560-7917.ES.2021.26.5.210009. PMC 7863232. PMID 33541485.
As at 1 February 2021, we assess that the cluster 5 variant is no longer circulating among humans in Denmark.
- "New COVID-19 Variants". U.S. Centers for Disease Control and Prevention (CDC). 28 June 2021 . Retrieved 15 July 2021.
- "COVID-19 Weekly Epidemiological Update Edition 69". World Health Organization (WHO). 7 December 2021.
- "Classification of Omicron (B.1.1.529): SARS-CoV-2 Variant of Concern". World Health Organization (WHO). Retrieved 9 December 2021.
- "JN.1" (PDF). 19 December 2023. Retrieved 21 December 2023.
- Benadjaoud Y (19 December 2023). "COVID variant JN.1 listed as 'variant of interest' by World Health Organization". ABC News. Retrieved 22 December 2023.
- Harrison AG, Lin T, Wang P (December 2020). "Mechanisms of SARS-CoV-2 Transmission and Pathogenesis". Trends in Immunology. 41 (12): 1100–1115. doi:10.1016/j.it.2020.10.004. PMC 7556779. PMID 33132005.
- Verdecchia P, Cavallini C, Spanevello A, Angeli F (June 2020). "The pivotal link between ACE2 deficiency and SARS-CoV-2 infection". European Journal of Internal Medicine. 76: 14–20. doi:10.1016/j.ejim.2020.04.037. PMC 7167588. PMID 32336612.
- Letko M, Marzi A, Munster V (April 2020). "Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses". Nature Microbiology. 5 (4): 562–569. doi:10.1038/s41564-020-0688-y. PMC 7095430. PMID 32094589.
- Marik PE, Iglesias J, Varon J, Kory P (January 2021). "A scoping review of the pathophysiology of COVID-19". International Journal of Immunopathology and Pharmacology. 35: 20587384211048026. doi:10.1177/20587384211048026. PMC 8477699. PMID 34569339.
- ^ Eketunde AO, Mellacheruvu SP, Oreoluwa P (July 2020). "A Review of Postmortem Findings in Patients With COVID-19". Cureus. 12 (7). Cureus, Inc.: e9438. doi:10.7759/cureus.9438. PMC 7451084. PMID 32864262. S2CID 221352704.
- Ground glass opacities of the lung before, during and post COVID-19 pandemic - PMC (nih.gov)
- Ontong P, Prachayasittikul V (15 January 2021). "Unraveled roles of hyaluronan in severe COVID-19". EXCLI Journal. 20: 117–125. doi:10.17179/excli2020-3215. ISSN 1611-2156. PMC 7868638. PMID 33564281.
- ^ Meunier N, Briand L, Jacquin-Piques A, Brondel L, Pénicaud L (June 2020). "COVID 19-Induced Smell and Taste Impairments: Putative Impact on Physiology". Frontiers in Physiology. 11: 625110. doi:10.3389/fphys.2020.625110. PMC 7870487. PMID 33574768.
- Guerrero JI, Barragán LA, Martínez JD, Montoya JP, Peña A, Sobrino FE, et al. (June 2021). "Central and peripheral nervous system involvement by COVID-19: a systematic review of the pathophysiology, clinical manifestations, neuropathology, neuroimaging, electrophysiology, and cerebrospinal fluid findings". BMC Infectious Diseases. 21 (1): 515. doi:10.1186/s12879-021-06185-6. PMC 8170436. PMID 34078305.
- ^ Pezzini A, Padovani A (November 2020). "Lifting the mask on neurological manifestations of COVID-19". Nature Reviews. Neurology. 16 (11): 636–644. doi:10.1038/s41582-020-0398-3. PMC 7444680. PMID 32839585.
- Li YC, Bai WZ, Hashikawa T (June 2020). "The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients". Journal of Medical Virology. 92 (6): 552–555. doi:10.1002/jmv.25728. PMC 7228394. PMID 32104915.
- Baig AM, Khaleeq A, Ali U, Syeda H (April 2020). "Evidence of the COVID-19 Virus Targeting the CNS: Tissue Distribution, Host-Virus Interaction, and Proposed Neurotropic Mechanisms". ACS Chemical Neuroscience. 11 (7): 995–998. doi:10.1021/acschemneuro.0c00122. PMC 7094171. PMID 32167747.
- Yavarpour-Bali H, Ghasemi-Kasman M (September 2020). "Update on neurological manifestations of COVID-19". Life Sciences. 257: 118063. doi:10.1016/j.lfs.2020.118063. PMC 7346808. PMID 32652139.
- Douaud G, Lee S, Alfaro-Almagro F, Arthofer C, Wang C, McCarthy P, et al. (March 2022). "SARS-CoV-2 is associated with changes in brain structure in UK Biobank". Nature. 604 (7907): 697–707. Bibcode:2022Natur.604..697D. doi:10.1038/s41586-022-04569-5. ISSN 1476-4687. LCCN 12037118. OCLC 01586310. PMC 9046077. PMID 35255491.
- Proust A, Queval CJ, Harvey R, Adams L, Bennett M, Wilkinson RJ (2023). "Differential effects of SARS-CoV-2 variants on central nervous system cells and blood–brain barrier functions". Journal of Neuroinflammation. 20 (184): 184. doi:10.1186/s12974-023-02861-3. PMC 10398935. PMID 37537664.
- Geddes L, Sample I (7 March 2022). "Covid can shrink brain and damage its tissue, finds research". The Guardian. Archived from the original on 7 March 2022. Retrieved 4 September 2023.
- Morelle R (7 March 2022). "Scans reveal how Covid may change the brain". BBC News. BBC. Retrieved 4 September 2023.
- "Even mild Covid is linked to brain damage months after illness, scans show". NBC News. 7 March 2022.
- Gu J, Han B, Wang J (May 2020). "COVID-19: Gastrointestinal Manifestations and Potential Fecal-Oral Transmission". Gastroenterology. 158 (6): 1518–1519. doi:10.1053/j.gastro.2020.02.054. PMC 7130192. PMID 32142785.
- Mönkemüller K, Fry L, Rickes S (May 2020). "COVID-19, coronavirus, SARS-CoV-2 and the small bowel". Revista Espanola de Enfermedades Digestivas. 112 (5): 383–388. doi:10.17235/reed.2020.7137/2020. PMID 32343593. S2CID 216645754.
- Almamlouk R, Kashour T, Obeidat S, Bois MC, Maleszewski JJ, Omrani OA, et al. (August 2022). "COVID-19-Associated cardiac pathology at the postmortem evaluation: a collaborative systematic review". Clinical Microbiology and Infection. 28 (8): 1066–1075. doi:10.1016/j.cmi.2022.03.021. PMC 8941843. PMID 35339672.
- ^ Zheng YY, Ma YT, Zhang JY, Xie X (May 2020). "COVID-19 and the cardiovascular system". Nature Reviews. Cardiology. 17 (5): 259–260. doi:10.1038/s41569-020-0360-5. PMC 7095524. PMID 32139904.
- ^ Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. (February 2020). "Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China". Lancet. 395 (10223): 497–506. doi:10.1016/S0140-6736(20)30183-5. PMC 7159299. PMID 31986264.
- "Coronavirus disease 2019 (COVID-19): Myocardial infarction and other coronary artery disease issues". UpToDate. Retrieved 28 September 2020.
- Turner AJ, Hiscox JA, Hooper NM (June 2004). "ACE2: from vasopeptidase to SARS virus receptor". Trends in Pharmacological Sciences. 25 (6): 291–4. doi:10.1016/j.tips.2004.04.001. PMC 7119032. PMID 15165741.
- Abou-Ismail MY, Diamond A, Kapoor S, Arafah Y, Nayak L (October 2020). "The hypercoagulable state in COVID-19: Incidence, pathophysiology, and management". Thrombosis Research. 194. Elsevier BV: 101–115. doi:10.1016/j.thromres.2020.06.029. PMC 7305763. PMID 32788101.
- ^ Wadman M (April 2020). "How does coronavirus kill? Clinicians trace a ferocious rampage through the body, from brain to toes". Science. doi:10.1126/science.abc3208.
- "NIH study uncovers blood vessel damage and inflammation in COVID-19 patients' brains but no infection". National Institutes of Health (NIH). 30 December 2020. Retrieved 17 January 2021.
- Lee MH, Perl DP, Nair G, Li W, Maric D, Murray H, et al. (February 2021). "Microvascular Injury in the Brains of Patients with Covid-19". The New England Journal of Medicine. 384 (5): 481–483. doi:10.1056/nejmc2033369. PMC 7787217. PMID 33378608.
- Kubánková M, Hohberger B, Hoffmanns J, Fürst J, Herrmann M, Guck J, et al. (July 2021). "Physical phenotype of blood cells is altered in COVID-19". Biophysical Journal. 120 (14): 2838–2847. Bibcode:2021BpJ...120.2838K. doi:10.1016/j.bpj.2021.05.025. PMC 8169220. PMID 34087216.
- Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, et al. (July 2020). "Extrapulmonary manifestations of COVID-19". Nature Medicine. 26 (7): 1017–1032. doi:10.1038/s41591-020-0968-3. PMID 32651579. S2CID 220462000.
- "Coronavirus: Kidney Damage Caused by COVID-19". Johns Hopkins Medicine. 14 May 2020. Retrieved 25 January 2022.
- Ziegler C, Allon SJ, Nyquist SK, Mbano IM, Miao VN, Tzouanas CN, et al. (28 May 2020). "SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues". Cell. HCA Lung Biological Network. 181 (5): 1016–1035.e19. doi:10.1016/j.cell.2020.04.035. PMC 7252096. PMID 32413319.
- Sajuthi SP, DeFord P, Li Y, Jackson ND, Montgomery MT, Everman JL, et al. (12 October 2020). "Type 2 and interferon inflammation regulate SARS-CoV-2 entry factor expression in the airway epithelium". Nature Communications. 11 (1): 5139. Bibcode:2020NatCo..11.5139S. doi:10.1038/s41467-020-18781-2. PMC 7550582. PMID 33046696.
- Tretter F, Peters E, Sturmberg J, Bennett J, Voit E, Dietrich JW, et al. (28 September 2022). "Perspectives of (/memorandum for) systems thinking on COVID-19 pandemic and pathology". Journal of Evaluation in Clinical Practice. 29 (3): 415–429. doi:10.1111/jep.13772. PMC 9538129. PMID 36168893. S2CID 252566067.
- Zhang C, Wu Z, Li JW, Zhao H, Wang GQ (May 2020). "Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality". International Journal of Antimicrobial Agents. 55 (5): 105954. doi:10.1016/j.ijantimicag.2020.105954. PMC 7118634. PMID 32234467.
- Gómez-Rial J, Rivero-Calle I, Salas A, Martinón-Torres F (2020). "Role of Monocytes/Macrophages in Covid-19 Pathogenesis: Implications for Therapy". Infection and Drug Resistance. 13: 2485–2493. doi:10.2147/IDR.S258639. PMC 7383015. PMID 32801787.
- Dai L, Gao GF (February 2021). "Viral targets for vaccines against COVID-19". Nature Reviews. Immunology. 21 (2): 73–82. doi:10.1038/s41577-020-00480-0. ISSN 1474-1733. PMC 7747004. PMID 33340022.
- ^ Boopathi S, Poma AB, Kolandaivel P (April 2020). "Novel 2019 coronavirus structure, mechanism of action, antiviral drug promises and rule out against its treatment". Journal of Biomolecular Structure & Dynamics. 39 (9): 3409–3418. doi:10.1080/07391102.2020.1758788. PMC 7196923. PMID 32306836.
- Kai H, Kai M (July 2020). "Interactions of coronaviruses with ACE2, angiotensin II, and RAS inhibitors-lessons from available evidence and insights into COVID-19". Hypertension Research. 43 (7): 648–654. doi:10.1038/s41440-020-0455-8. PMC 7184165. PMID 32341442.
- Chen HX, Chen ZH, Shen HH (October 2020). "". Sheng Li Xue Bao. 72 (5): 617–630. PMID 33106832.
- Jeyanathan M, Afkhami S, Smaill F, Miller MS, Lichty BD, Xing Z (4 September 2020). "Immunological considerations for COVID-19 vaccine strategies". Nature Reviews Immunology. 20 (10): 615–632. doi:10.1038/s41577-020-00434-6. ISSN 1474-1741. PMC 7472682. PMID 32887954.
- Zhang Q, Ju B, Ge J, Chan JF, Cheng L, Wang R, et al. (July 2021). "Potent and protective IGHV3-53/3-66 public antibodies and their shared escape mutant on the spike of SARS-CoV-2". Nature Communications. 12 (1): 4210. Bibcode:2021NatCo..12.4210Z. doi:10.1038/s41467-021-24514-w. PMC 8270942. PMID 34244522. S2CID 235786394.
- Soy M, Keser G, Atagündüz P, Tabak F, Atagündüz I, Kayhan S (July 2020). "Cytokine storm in COVID-19: pathogenesis and overview of anti-inflammatory agents used in treatment". Clinical Rheumatology. 39 (7): 2085–2094. doi:10.1007/s10067-020-05190-5. PMC 7260446. PMID 32474885.
- Quirch M, Lee J, Rehman S (August 2020). "Hazards of the Cytokine Storm and Cytokine-Targeted Therapy in Patients With COVID-19: Review". Journal of Medical Internet Research. 22 (8): e20193. doi:10.2196/20193. PMC 7428145. PMID 32707537.
- Bhaskar S, Sinha A, Banach M, Mittoo S, Weissert R, Kass JS, et al. (2020). "Cytokine Storm in COVID-19-Immunopathological Mechanisms, Clinical Considerations, and Therapeutic Approaches: The REPROGRAM Consortium Position Paper". Frontiers in Immunology. 11: 1648. doi:10.3389/fimmu.2020.01648. PMC 7365905. PMID 32754159.
- ^ Wastnedge EA, Reynolds RM, van Boeckel SR, Stock SJ, Denison FC, Maybin JA, et al. (January 2021). "Pregnancy and COVID-19". Physiological Reviews. 101 (1): 303–318. doi:10.1152/physrev.00024.2020. PMC 7686875. PMID 32969772.
- Digby AM, Dahan MH (12 January 2023). "Obstetrical and gynecologic implications of COVID-19: what have we learned over the first two years of the pandemic". Archives of Gynecology and Obstetrics. 308 (3): 813–819. doi:10.1007/s00404-022-06847-z. PMC 9838509. PMID 36633677.
- Campbell D (10 October 2021). "One in six most critically ill NHS Covid patients are unvaccinated pregnant women". The Guardian. Retrieved 25 January 2022.
- ^ Ai T, Yang Z, Hou H, Zhan C, Chen C, Lv W, et al. (August 2020). "Correlation of Chest CT and RT-PCR Testing for Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases". Radiology. 296 (2): E32 – E40. doi:10.1148/radiol.2020200642. PMC 7233399. PMID 32101510.
- ^ Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A (July 2020). "Coronavirus Disease 2019 (COVID-19): A Systematic Review of Imaging Findings in 919 Patients". AJR. American Journal of Roentgenology. 215 (1): 87–93. doi:10.2214/AJR.20.23034. PMID 32174129.
- "2019 Novel Coronavirus (2019-nCoV) Situation Summary". U.S. Centers for Disease Control and Prevention (CDC). 30 January 2020. Archived from the original on 26 January 2020. Retrieved 30 January 2020.
- "Coronavirus disease (COVID-19) technical guidance: Laboratory testing for 2019-nCoV in humans". World Health Organization (WHO). Archived from the original on 15 March 2020. Retrieved 14 March 2020.
- Bullard J, Dust K, Funk D, Strong JE, Alexander D, Garnett L, et al. (December 2020). "Predicting Infectious Severe Acute Respiratory Syndrome Coronavirus 2 From Diagnostic Samples". Clinical Infectious Diseases. 71 (10): 2663–2666. doi:10.1093/cid/ciaa638. PMC 7314198. PMID 32442256.
- "Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens from Persons for Coronavirus Disease 2019 (COVID-19)". U.S. Centers for Disease Control and Prevention (CDC). 11 February 2020. Archived from the original on 4 March 2020. Retrieved 26 March 2020.
- "Real-Time RT-PCR Panel for Detection 2019-nCoV". U.S. Centers for Disease Control and Prevention (CDC). 29 January 2020. Archived from the original on 30 January 2020. Retrieved 1 February 2020.
- "Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases". World Health Organization (WHO). Archived from the original on 17 March 2020. Retrieved 13 March 2020.
- "NHS staff will be first to get new coronavirus antibody test, medical chief promises". The Independent. 14 May 2020. Retrieved 14 May 2020.
- Heneghan C, Jefferson T (1 September 2020). "Virological characterization of COVID-19 patients that test re-positive for SARS-CoV-2 by RT-PCR". CEBM. Retrieved 19 September 2020.
- Lu J, Peng J, Xiong Q, Liu Z, Lin H, Tan X, et al. (September 2020). "Clinical, immunological and virological characterization of COVID-19 patients that test re-positive for SARS-CoV-2 by RT-PCR". eBioMedicine. 59: 102960. doi:10.1016/j.ebiom.2020.102960. PMC 7444471. PMID 32853988.
- Spencer E, Jefferson T, Brassey J, Heneghan C (11 September 2020). "When is Covid, Covid?". The Centre for Evidence-Based Medicine. Retrieved 19 September 2020.
- "SARS-CoV-2 RNA testing: assurance of positive results during periods of low prevalence". GOV.UK. Retrieved 19 September 2020.
- "ACR Recommendations for the use of Chest Radiography and Computed Tomography (CT) for Suspected COVID-19 Infection". American College of Radiology. 22 March 2020. Archived from the original on 28 March 2020.
- Pormohammad A, Ghorbani S, Khatami A, Razizadeh MH, Alborzi E, Zarei M, et al. (October 2020). "Comparison of influenza type A and B with COVID-19: A global systematic review and meta-analysis on clinical, laboratory and radiographic findings". Reviews in Medical Virology. 31 (3): e2179. doi:10.1002/rmv.2179. PMC 7646051. PMID 33035373. S2CID 222255245.
- Lee EY, Ng MY, Khong PL (April 2020). "COVID-19 pneumonia: what has CT taught us?". The Lancet. Infectious Diseases. 20 (4): 384–385. doi:10.1016/S1473-3099(20)30134-1. PMC 7128449. PMID 32105641.
- ^ Li Y, Xia L (June 2020). "Coronavirus Disease 2019 (COVID-19): Role of Chest CT in Diagnosis and Management". AJR. American Journal of Roentgenology. 214 (6): 1280–1286. doi:10.2214/AJR.20.22954. PMID 32130038. S2CID 212416282.
- "COVID-19 Database". Società Italiana di Radiologia Medica e Interventistica (in Italian). Retrieved 11 March 2020.
- "ICD-10 Version:2019". World Health Organization (WHO). 2019. Archived from the original on 31 March 2020. Retrieved 31 March 2020.
U07.2 – COVID-19, virus not identified – COVID-19 NOS – Use this code when COVID-19 is diagnosed clinically or epidemiologically but laboratory testing is inconclusive or not available. Use additional code, if desired, to identify pneumonia or other manifestations
- Giani M, Seminati D, Lucchini A, Foti G, Pagni F (May 2020). "Exuberant Plasmocytosis in Bronchoalveolar Lavage Specimen of the First Patient Requiring Extracorporeal Membrane Oxygenation for SARS-CoV-2 in Europe". Journal of Thoracic Oncology. 15 (5): e65 – e66. doi:10.1016/j.jtho.2020.03.008. PMC 7118681. PMID 32194247.
- Lillicrap D (April 2020). "Disseminated intravascular coagulation in patients with 2019-nCoV pneumonia". Journal of Thrombosis and Haemostasis. 18 (4): 786–787. doi:10.1111/jth.14781. PMC 7166410. PMID 32212240.
- Mitra A, Dwyre DM, Schivo M, Thompson GR, Cohen SH, Ku N, et al. (August 2020). "Leukoerythroblastic reaction in a patient with COVID-19 infection". American Journal of Hematology. 95 (8): 999–1000. doi:10.1002/ajh.25793. PMC 7228283. PMID 32212392.
- ^ Satturwar S, Fowkes M, Farver C, Wilson AM, Eccher A, Girolami I, et al. (May 2021). "Postmortem Findings Associated With SARS-CoV-2: Systematic Review and Meta-analysis". The American Journal of Surgical Pathology. 45 (5): 587–603. doi:10.1097/PAS.0000000000001650. PMC 8132567. PMID 33481385. S2CID 231679276.
- Maier BF, Brockmann D (May 2020). "Effective containment explains subexponential growth in recent confirmed COVID-19 cases in China". Science. 368 (6492): 742–746. arXiv:2002.07572. Bibcode:2020Sci...368..742M. doi:10.1126/science.abb4557. PMC 7164388. PMID 32269067. ("... initial exponential growth expected for an unconstrained outbreak".)
- "Viral Load Exposure Factors". ReallyCorrect.com.
- "Recommendation Regarding the Use of Cloth Face Coverings, Especially in Areas of Significant Community-Based Transmission". U.S. Centers for Disease Control and Prevention (CDC). 28 June 2020.
- "Scientific Brief: SARS-CoV-2 and Potential Airborne Transmission". COVID-19 Published Science and Research. U.S. Centers for Disease Control and Prevention (CDC). 11 February 2020. Retrieved 30 October 2020.
- Centers for Disease Control and Prevention (5 April 2020). "What to Do if You Are Sick". U.S. Centers for Disease Control and Prevention (CDC). Archived from the original on 14 February 2020. Retrieved 24 April 2020.
- "Coronavirus Disease 2019 (COVID-19) – Prevention & Treatment". U.S. Centers for Disease Control and Prevention (CDC). 10 March 2020. Archived from the original on 11 March 2020. Retrieved 11 March 2020.
- "UK medicines regulator gives approval for first UK COVID-19 vaccine". Medicines and Healthcare Products Regulatory Agency, Government of the UK. 2 December 2020. Retrieved 2 December 2020.
- Mueller B (2 December 2020). "U.K. Approves Pfizer Coronavirus Vaccine, a First in the West". The New York Times. Archived from the original on 2 December 2020. Retrieved 2 December 2020.
- "COVID-19 Treatment Guidelines". nih.gov. National Institutes of Health. Retrieved 21 April 2020.
- ^ Anderson RM, Heesterbeek H, Klinkenberg D, Hollingsworth TD (March 2020). "How will country-based mitigation measures influence the course of the COVID-19 epidemic?". Lancet. 395 (10228): 931–934. doi:10.1016/S0140-6736(20)30567-5. PMC 7158572. PMID 32164834.
A key issue for epidemiologists is helping policy makers decide the main objectives of mitigation – e.g. minimising morbidity and associated mortality, avoiding an epidemic peak that overwhelms health-care services, keeping the effects on the economy within manageable levels, and flattening the epidemic curve to wait for vaccine development and manufacture on scale and antiviral drug therapies.
- Wiles S (14 March 2020). "After 'Flatten the Curve', we must now 'Stop the Spread'. Here's what that means". The Spinoff. Archived from the original on 26 March 2020. Retrieved 13 March 2020.
- "Data on COVID-19 mortality by vaccination status". Our World in Data (CDC data). April 2023. Archived from the original on 16 October 2023.
Data source: Centers for Disease Control and Prevention, Vaccine Breakthrough/Surveillance and Analytics Team.
- Li YD, Chi WY, Su JH, Ferrall L, Hung CF, Wu TC (December 2020). "Coronavirus vaccine development: from SARS and MERS to COVID-19". Journal of Biomedical Science. 27 (1): 104. doi:10.1186/s12929-020-00695-2. PMC 7749790. PMID 33341119.
- ^ Rogers K (11 May 2022). "COVID-19 vaccine". Encyclopædia Britannica. Archived from the original on 12 June 2022. Retrieved 12 June 2022.
- "Swissmedic grants authorisation for the first COVID-19 vaccine in Switzerland" (Press release). Swiss Agency for Therapeutic Products (Swissmedic). 18 December 2020. Archived from the original on 2 May 2021. Retrieved 5 July 2022.
- "EMA recommends first COVID-19 vaccine for authorisation in the EU". European Medicines Agency (EMA) (Press release). 21 December 2020. Archived from the original on 30 January 2021. Retrieved 21 December 2020.
- Mallapaty S, Callaway E, Kozlov M, Ledford H, Pickrell J, Van Noorden R (December 2021). "How COVID vaccines shaped 2021 in eight powerful charts". Nature. 600 (7890): 580–583. Bibcode:2021Natur.600..580M. doi:10.1038/d41586-021-03686-x. PMID 34916666. S2CID 245262732.
- Beaumont P (18 November 2020). "Covid-19 vaccine: who are countries prioritising for first doses?". The Guardian. ISSN 0261-3077. Archived from the original on 18 January 2021. Retrieved 26 December 2020.
- Wang H, Xu R, Qu S, Schwartz M, Adams A, Chen X (October 2021). "Health inequities in COVID-19 vaccination among the elderly: Case of Connecticut". Journal of Infection and Public Health. 14 (10): 1563–1565. doi:10.1016/j.jiph.2021.07.013. PMC 8491089. PMID 34326008. S2CID 236515442.
- Background document on the mRNA-1273 vaccine (Moderna) against COVID-19 (Report). World Health Organization (WHO). February 2021. hdl:10665/339218. WHO/2019-nCoV/vaccines/SAGE_recommendation/mRNA-1273/background/2021.1. Archived from the original on 13 June 2021. Retrieved 24 July 2021.
- "Background document on the mRNA-1273 vaccine (Moderna) against COVID-19". World Health Organization (WHO). Archived from the original on 26 January 2022. Retrieved 23 January 2022.
- "Pregnancy, breastfeeding, fertility and coronavirus (COVID-19) vaccination". NHS. 5 October 2022. Archived from the original on 15 October 2022. Retrieved 15 October 2022.
- Richie H, Ortiz-Ospina E, Beltekian D, Methieu E, Hasell J, Macdonald B, et al. (March 2020). "Coronavirus (COVID-19) Vaccinations – Statistics and Research". Our World in Data. Archived from the original on 10 March 2021. Retrieved 7 February 2021.
- Mullard A (November 2020). "How COVID vaccines are being divvied up around the world". Nature. doi:10.1038/d41586-020-03370-6. PMID 33257891. S2CID 227246811.
- So AD, Woo J (December 2020). "Reserving coronavirus disease 2019 vaccines for global access: cross sectional analysis". BMJ. 371: m4750. doi:10.1136/bmj.m4750. PMC 7735431. PMID 33323376.
- Bourouiba L (July 2021). "Fluid Dynamics of Respiratory Infectious Diseases". Annual Review of Biomedical Engineering. 23 (1): 547–577. doi:10.1146/annurev-bioeng-111820-025044. hdl:1721.1/131115. PMID 34255991. S2CID 235823756.
- ^ Matuschek C, Moll F, Fangerau H, Fischer JC, Zänker K, van Griensven M, et al. (August 2020). "Face masks: benefits and risks during the COVID-19 crisis". European Journal of Medical Research. 25 (1): 32. doi:10.1186/s40001-020-00430-5. PMC 7422455. PMID 32787926.
- Catching A, Capponi S, Yeh MT, Bianco S, Andino R (August 2021). "Examining the interplay between face mask usage, asymptomatic transmission, and social distancing on the spread of COVID-19". Scientific Reports. 11 (1). Nature Portfolio: 15998. Bibcode:2021NatSR..1115998C. doi:10.1038/s41598-021-94960-5. PMC 8346500. PMID 34362936. S2CID 236947786.
Masks prevent the spread of droplets and aerosols generated by an infected individual, and when correctly worn surgical masks can reduce viral transmission by 95%. Uninfected individuals wearing a surgical mask are about 85% protected against infection.
- ^ Talic S, Shah S, Wild H, Gasevic D, Maharaj A, Ademi Z, et al. (November 2021). "Effectiveness of public health measures in reducing the incidence of covid-19, SARS-CoV-2 transmission, and covid-19 mortality: systematic review and meta-analysis". BMJ. 375: e068302. doi:10.1136/bmj-2021-068302. PMC 9423125. PMID 34789505. S2CID 244271780.
The results of additional studies that assessed mask wearing ... indicate a reduction in covid-19 incidence, SARS-CoV-2 transmission, and covid-19 mortality. Specifically, a natural experiment across 200 countries showed 45.7% fewer covid-19 related mortality in countries where mask-wearing was mandatory. Another natural experiment study in the US reported a 29% reduction in SARS-CoV-2 transmission (measured as the time-varying reproductive number Rt) (risk ratio 0.71, 95% confidence interval 0.58 to 0.75) in states where mask-wearing was mandatory. A comparative study in the Hong Kong Special Administrative Region reported a statistically significantly lower cumulative incidence of covid-19 associated with mask-wearing than in selected countries where mask-wearing was not mandatory.
- ^ "Science Brief: Community Use of Masks to Control the Spread of SARS-CoV-2". CDC. 11 February 2020.
Experimental and epidemiologic data support community masking to reduce the spread of SARS-CoV-2, including alpha and delta variants, among adults and children. Mask use has been found to be safe and is not associated with clinically significant impacts on respiration or gas exchange under most circumstances, except for intense exercise. The limited available data indicate no clear evidence that masking impairs emotional or language development in children. n combination with other contextual cues, masks are unlikely to produce serious impairments of children's social interactions. A study of 2-year-old children concluded that they were able to recognize familiar words presented without a mask and when hearing words through opaque masks. Among children with autism spectrum disorders (ASD), interventions including positive reinforcement and coaching caregivers to teach mask-wearing have improved participants' ability to wear a face mask. These findings suggest that even children who may have difficulty wearing a mask can do so effectively through targeted interventions.
- Jefferson T, Dooley L, Ferroni E, Al-Ansary LA, van Driel ML, Bawazeer GA, et al. (January 2023). "Physical interventions to interrupt or reduce the spread of respiratory viruses". The Cochrane Database of Systematic Reviews. 1 (1): CD006207. doi:10.1002/14651858.CD006207.pub6. PMC 9885521. PMID 36715243.
- Boulos L, Curran JA, Gallant A, Wong H, Johnson C, Delahunty-Pike A, et al. (2023). "Effectiveness of face masks for reducing transmission of SARS-CoV-2: A rapid systematic review". Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences. 381 (2257). Bibcode:2023RSPTA.38130133B. doi:10.1098/rsta.2023.0133. PMC 10446908. PMID 37611625.
- Ju JT, Boisvert LN, Zuo YY (June 2021). "Face masks against COVID-19: Standards, efficacy, testing and decontamination methods". Advances in Colloid and Interface Science. 292: 102435. doi:10.1016/j.cis.2021.102435. PMC 8084286. PMID 33971389.
- Zayas G, Chiang MC, Wong E, MacDonald F, Lange CF, Senthilselvan A, et al. (2013). "Effectiveness of cough etiquette maneuvers in disrupting the chain of transmission of infectious respiratory diseases". BMC Public Health. 13: 811. doi:10.1186/1471-2458-13-811. PMC 3846148. PMID 24010919.
- Ataei M, Shirazi FM, Nakhaee S, Abdollahi M, Mehrpour O (October 2021). "Assessment of cloth masks ability to limit Covid-19 particles spread: a systematic review". Environmental Science and Pollution Research International. 29 (2): 1645–1676. doi:10.1007/s11356-021-16847-2. PMC 8541808. PMID 34689269.
- Koh XQ, Sng A, Chee JY, Sadovoy A, Luo P, Daniel D (February 2022). "Outward and inward protection efficiencies of different mask designs for different respiratory activities". Journal of Aerosol Science. 160. Bibcode:2022JAerS.16005905K. doi:10.1016/j.jaerosci.2021.105905.
- ^ CDC (11 February 2020). "Scientific Brief: SARS-CoV-2 Transmission". U.S. Centers for Disease Control and Prevention (CDC). Retrieved 10 May 2021.
- "Transmission of COVID-19". European Centre for Disease Prevention and Control. 7 September 2020. Retrieved 14 October 2020.
- ^ National Center for Immunization and Respiratory Diseases (NCIRD) (9 July 2020). "COVID-19 Employer Information for Office Buildings". U.S. Centers for Disease Control and Prevention (CDC). Retrieved 9 July 2020.
- WHO's Science in 5 on COVID-19 – Ventilation – 30 October 2020. World Health Organization (WHO). 30 October 2020. Archived from the original on 25 October 2022. Retrieved 8 December 2022 – via YouTube.
- Somsen GA, van Rijn C, Kooij S, Bem RA, Bonn D (July 2020). "Small droplet aerosols in poorly ventilated spaces and SARS-CoV-2 transmission". The Lancet. Respiratory Medicine. 8 (7). Elsesier: 658–659. doi:10.1016/S2213-2600(20)30245-9. PMC 7255254. PMID 32473123.
- Lipinski T, Ahmad D, Serey N, Jouhara H (1 November 2020). "Review of ventilation strategies to reduce the risk of disease transmission in high occupancy buildings". International Journal of Thermofluids. 7–8: 100045. Bibcode:2020IJTf....700045L. doi:10.1016/j.ijft.2020.100045. ISSN 2666-2027. S2CID 221642242.
- "Social distancing: what you need to do – Coronavirus (COVID-19)". nhs.uk. 2 June 2020. Retrieved 18 August 2020.
- "Advice for the public on COVID-19 – World Health Organization". World Health Organization (WHO). Retrieved 18 August 2020.
- "COVID-19 and Your Health". U.S. Centers for Disease Control and Prevention (CDC). 11 February 2020. Retrieved 23 March 2021.
To prevent the spread of germs, including COVID-19, CDC recommends washing hands with soap and water whenever possible because it reduces the amount of many types of germs and chemicals on hands. But if soap and water are not readily available, using a hand sanitizer with at least 60% alcohol can help you avoid getting sick and spreading germs to others.
- "WHO-recommended handrub formulations". WHO Guidelines on Hand Hygiene in Health Care: First Global Patient Safety Challenge Clean Care Is Safer Care. World Health Organization (WHO). 19 March 2009. Retrieved 19 March 2020.
- Nussbaumer-Streit B, Mayr V, Dobrescu AI, Chapman A, Persad E, Klerings I, et al. (September 2020). "Quarantine alone or in combination with other public health measures to control COVID-19: a rapid review". The Cochrane Database of Systematic Reviews. 2020 (9): CD013574. doi:10.1002/14651858.CD013574.pub2. ISSN 1469-493X. PMC 8133397. PMID 33959956.
- ^ Hawks L, Woolhandler S, McCormick D (August 2020). "COVID-19 in Prisons and Jails in the United States". JAMA Internal Medicine. 180 (8): 1041–1042. doi:10.1001/jamainternmed.2020.1856. PMID 32343355.
- Waldstein D (6 May 2020). "To Fight Virus in Prisons, C.D.C. Suggests More Screenings". The New York Times. Archived from the original on 7 May 2020. Retrieved 14 May 2020.
- "How COVID-19 Spreads". U.S. Centers for Disease Control and Prevention (CDC). 18 September 2020. Archived from the original on 19 September 2020. Retrieved 20 September 2020.
- Goldman E (August 2020). "Exaggerated risk of transmission of COVID-19 by fomites". The Lancet. Infectious Diseases. 20 (8): 892–893. doi:10.1016/S1473-3099(20)30561-2. PMC 7333993. PMID 32628907.
- Weixel N (5 April 2021). "CDC says risk of COVID-19 transmission on surfaces 1 in 10,000". The Hill. Retrieved 19 December 2021.
- ^ "Science Brief: SARS-CoV-2 and Surface (Fomite) Transmission for Indoor Community Environments". U.S. Centers for Disease Control and Prevention (CDC). 5 April 2021. Archived from the original on 5 April 2021.
- ^ Pedreira A, Taşkın Y, García MR (January 2021). "A Critical Review of Disinfection Processes to Control SARS-CoV-2 Transmission in the Food Industry". Foods. 10 (2): 283. doi:10.3390/foods10020283. PMC 7911259. PMID 33572531. S2CID 231900820.
- Rezasoltani S, Yadegar A, Hatami B, Asadzadeh Aghdaei H, Zali MR (2020). "Antimicrobial Resistance as a Hidden Menace Lurking Behind the COVID-19 Outbreak: The Global Impacts of Too Much Hygiene on AMR". Frontiers in Microbiology. 11: 590683. doi:10.3389/fmicb.2020.590683. PMC 7769770. PMID 33384670.
- Thompson D (8 February 2021). "Hygiene Theater Is Still a Huge Waste of Time". The Atlantic. Retrieved 27 February 2021.
- Thompson D (27 July 2020). "Hygiene Theater Is a Huge Waste of Time". The Atlantic. Retrieved 27 February 2021.
- ^ Bueckert M, Gupta R, Gupta A, Garg M, Mazumder A (November 2020). "Infectivity of SARS-CoV-2 and Other Coronaviruses on Dry Surfaces: Potential for Indirect Transmission". Materials. 13 (22): 5211. Bibcode:2020Mate...13.5211B. doi:10.3390/ma13225211. PMC 7698891. PMID 33218120.
- Bhardwaj R, Agrawal A (November 2020). "How coronavirus survives for days on surfaces". Physics of Fluids. 32 (11): 111706. Bibcode:2020PhFl...32k1706B. doi:10.1063/5.0033306. PMC 7713872. PMID 33281435.
- Chatterjee S, Murallidharan JS, Agrawal A, Bhardwaj R (February 2021). "Why coronavirus survives longer on impermeable than porous surfaces". Physics of Fluids. 33 (2): 021701. Bibcode:2021PhFl...33b1701C. doi:10.1063/5.0037924. PMC 7978145. PMID 33746485.
- Anthes E (8 April 2021). "Has the Era of Overzealous Cleaning Finally Come to an End?". The New York Times. Archived from the original on 28 December 2021. Retrieved 12 April 2021.
- "Interim Recommendations for US Community Facilities with Suspected/Confirmed Coronavirus Disease 2019". U.S. Centers for Disease Control and Prevention (CDC). 11 February 2020. Retrieved 4 April 2020.
- "Yes, UV phone sanitizers work. That doesn't mean you need one". The Washington Post. 16 February 2021. Retrieved 29 April 2022.
- Patiño-Lugo DF, Vélez M, Velásquez Salazar P, Vera-Giraldo CY, Vélez V, Marín IC, et al. (June 2020). "Non-pharmaceutical interventions for containment, mitigation and suppression of COVID-19 infection". Colombia Medica. 51 (2): e4266. doi:10.25100/cm.v51i2.4266. PMC 7518730. PMID 33012884.
- "COVID-19 Informational Resources for High-Risk Groups | Keeping Education ACTIVE | Partnership to Fight Chronic Disease". fightchronicdisease.org. Retrieved 31 May 2020.
- "Quarantine and Isolation". U.S. Centers for Disease Control and Prevention (CDC). 29 July 2021. Retrieved 12 August 2021.
- ^ Burns J, Movsisyan A, Stratil JM, Biallas RL, Coenen M, Emmert-Fees KM, et al. (Cochrane Public Health Group) (March 2021). "International travel-related control measures to contain the COVID-19 pandemic: a rapid review". The Cochrane Database of Systematic Reviews. 2021 (3): CD013717. doi:10.1002/14651858.CD013717.pub2. PMC 8406796. PMID 33763851. S2CID 232356197.
- Fisher D, Heymann D (February 2020). "Q&A: The novel coronavirus outbreak causing COVID-19". BMC Medicine. 18 (1): 57. doi:10.1186/s12916-020-01533-w. PMC 7047369. PMID 32106852.
- Liu K, Fang YY, Deng Y, Liu W, Wang MF, Ma JP, et al. (May 2020). "Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province". Chinese Medical Journal. 133 (9): 1025–1031. doi:10.1097/CM9.0000000000000744. PMC 7147277. PMID 32044814.
- Wang T, Du Z, Zhu F, Cao Z, An Y, Gao Y, et al. (March 2020). "Comorbidities and multi-organ injuries in the treatment of COVID-19". Lancet. 395 (10228). Elsevier BV: e52. doi:10.1016/s0140-6736(20)30558-4. PMC 7270177. PMID 32171074.
- Tao K, Tzou PL, Nouhin J, Bonilla H, Jagannathan P, Shafer RW (July 2021). "SARS-CoV-2 Antiviral Therapy". Clinical Microbiology Reviews. 34 (4): e0010921. doi:10.1128/CMR.00109-21. PMC 8404831. PMID 34319150. S2CID 236472654.
- ^ Motseki TP (7 June 2022). "COVID-19 Vaccination Guidelines". www.nih.gov. National Institutes of Health. Archived from the original on 19 January 2021. Retrieved 18 January 2021.
- Wang Y, Wang Y, Chen Y, Qin Q (March 2020). "Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures". Journal of Medical Virology. 92 (6): 568–576. doi:10.1002/jmv.25748. PMC 7228347. PMID 32134116.
- "Coronavirus". WebMD. Archived from the original on 1 February 2020. Retrieved 1 February 2020.
- Martel J, Ko YF, Young JD, Ojcius DM (May 2020). "Could nasal breathing help to mitigate the severity of COVID-19". Microbes and Infection. 22 (4–5): 168–171. doi:10.1016/j.micinf.2020.05.002. PMC 7200356. PMID 32387333.
- "Coronavirus recovery: breathing exercises". www.hopkinsmedicine.org. Johns Hopkins Medicine. Archived from the original on 11 October 2020. Retrieved 30 July 2020.
- Wang L, Wang Y, Ye D, Liu Q (March 2020). "Review of the 2019 novel coronavirus (SARS-CoV-2) based on current evidence". International Journal of Antimicrobial Agents. 55 (6): 105948. doi:10.1016/j.ijantimicag.2020.105948. PMC 7156162. PMID 32201353.
- U.S. Centers for Disease Control and Prevention (5 April 2020). "What to Do if You Are Sick". U.S. Centers for Disease Control and Prevention (CDC). Archived from the original on 14 February 2020. Retrieved 24 April 2020.
- "Update to living WHO guideline on drugs for covid-19". BMJ (Clinical Research Ed.). 371: m4475. November 2020. doi:10.1136/bmj.m4475. ISSN 1756-1833. PMID 33214213. S2CID 227059995.
- "Q&A: Dexamethasone and COVID-19". World Health Organization (WHO). Archived from the original on 11 October 2020. Retrieved 11 July 2020.
- "Home". National COVID-19 Clinical Evidence Taskforce. Archived from the original on 11 October 2020. Retrieved 11 July 2020.
- Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. (April 2020). "Clinical Characteristics of Coronavirus Disease 2019 in China". The New England Journal of Medicine. 382 (18). Massachusetts Medical Society: 1708–1720. doi:10.1056/nejmoa2002032. PMC 7092819. PMID 32109013.
- Henry BM (April 2020). "COVID-19, ECMO, and lymphopenia: a word of caution". The Lancet. Respiratory Medicine. 8 (4). Elsevier BV: e24. doi:10.1016/s2213-2600(20)30119-3. PMC 7118650. PMID 32178774.
- Kim JS, Lee JY, Yang JW, Lee KH, Effenberger M, Szpirt W, et al. (2021). "Immunopathogenesis and treatment of cytokine storm in COVID-19". Theranostics. 11 (1): 316–329. doi:10.7150/thno.49713. PMC 7681075. PMID 33391477.
- "COVID Treatment Guidelines: Clinical Management Summary". NIH Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. 8 April 2022. Archived from the original on 5 November 2021. Retrieved 19 April 2022.
- Wise J (17 April 2022). "What Happened to Paxlovid, the COVID Wonder Drug?". Intelligencer. Archived from the original on 19 April 2022. Retrieved 19 April 2022.
- ^ "Drug treatments for covid-19: living systematic review and network meta-analysis". BMJ. 373: n967. April 2021. doi:10.1136/bmj.n967. hdl:11375/26524. PMID 33849936.
- Aripaka P (5 November 2021). "Britain approves Merck's COVID-19 pill in world first". Reuters. Archived from the original on 8 November 2021. Retrieved 8 November 2021.
- Beasley D (5 November 2021). "Pfizer says its antiviral pill slashes risk of severe COVID-19 by 89%". Reuters. Archived from the original on 7 November 2021. Retrieved 8 November 2021.
- Reis S, Metzendorf MI, Kuehn R, Popp M, Gagyor I, Kranke P, et al. (November 2023). "Nirmatrelvir combined with ritonavir for preventing and treating COVID-19". The Cochrane Database of Systematic Reviews. 2023 (11): CD015395. doi:10.1002/14651858.CD015395.pub3. PMC 10688265. PMID 38032024.
- ^ Kim PS, Read SW, Fauci AS (December 2020). "Therapy for Early COVID-19: A Critical Need". JAMA. 324 (21). American Medical Association (AMA): 2149–2150. doi:10.1001/jama.2020.22813. PMID 33175121.
- ^ "COVID-19 Treatment Guidelines". www.nih.gov. National Institutes of Health. Archived from the original on 19 January 2021. Retrieved 18 January 2021./
- Saima MS (2 November 2021). "Common Antidepressant Slashes Risk of COVID Death". Nature. Archived from the original on 8 November 2021. Retrieved 8 November 2021.
- Hsu J (November 2020). "Covid-19: What now for remdesivir?". BMJ. 371: m4457. doi:10.1136/bmj.m4457. PMID 33214186.
- Reed J (4 November 2021). "Molnupiravir: First pill to treat Covid gets approval in UK". www.bbc.co.uk. Archived from the original on 4 November 2021. Retrieved 23 November 2021.
- Doshi P (October 2020). "Will covid-19 vaccines save lives? Current trials aren't designed to tell us". BMJ. 371: m4037. doi:10.1136/bmj.m4037. PMID 33087398. S2CID 224817161.
- ^ Palmieri L, Andrianou X, Barbariol P, Bella A, Bellino S, Benelli E, et al. (22 July 2020). Characteristics of SARS-CoV-2 patients dying in Italy Report based on available data on July 22nd, 2020 (PDF) (Report). Istituto Superiore di Sanità. Retrieved 4 October 2020.
- Tzoulis P, Waung JA, Bagkeris E, Hussein Z, Biddanda A, Cousins J, et al. (May 2021). "Dysnatremia is a Predictor for Morbidity and Mortality in Hospitalized Patients with COVID-19". The Journal of Clinical Endocrinology and Metabolism. 106 (6): 1637–1648. doi:10.1210/clinem/dgab107. PMC 7928894. PMID 33624101.
- Tzoulis P, Grossman AB, Baldeweg SE, Bouloux P, Kaltsas G (September 2021). "MANAGEMENT OF ENDOCRINE DISEASE: Dysnatraemia in COVID-19: prevalence, prognostic impact, pathophysiology, and management". European Journal of Endocrinology. 185 (4): R103 – R111. doi:10.1530/EJE-21-0281. PMC 8428074. PMID 34370712.
- Baranovskii DS, Klabukov ID, Krasilnikova OA, Nikogosov DA, Polekhina NV, Baranovskaia DR, et al. (December 1975). "Prolonged prothrombin time as an early prognostic indicator of severe acute respiratory distress syndrome in patients with COVID-19 related pneumonia". Current Medical Research and Opinion. 229 (6): 21–25. doi:10.1080/03007995.2020.1853510. PMC 7738209. PMID 33210948. S2CID 227065216.
- Christensen B, Favaloro EJ, Lippi G, Van Cott EM (October 2020). "Hematology Laboratory Abnormalities in Patients with Coronavirus Disease 2019 (COVID-19)". Seminars in Thrombosis and Hemostasis. 46 (7): 845–849. doi:10.1055/s-0040-1715458. PMC 7645834. PMID 32877961.
- "Living with Covid19". NIHR Themed Reviews. National Institute for Health Research. 15 October 2020. doi:10.3310/themedreview_41169.
- "How long does COVID-19 last?". UK COVID Symptom Study. 6 June 2020. Retrieved 15 October 2020.
- "Summary of COVID-19 Long Term Health Effects: Emerging evidence and Ongoing Investigation" (PDF). University of Washington. 1 September 2020. Archived from the original (PDF) on 18 December 2020. Retrieved 15 October 2020.
- "Long-term symptoms of COVID-19 'really concerning', says WHO chief". UN News. 30 October 2020. Retrieved 7 March 2021.
- "Coronavirus disease 2019 (COVID-19) – Prognosis". BMJ. Retrieved 15 November 2020.
- Lavery AM, Preston LE, Ko JY, Chevinsky JR, DeSisto CL, Pennington AF, et al. (November 2020). "Characteristics of Hospitalized COVID-19 Patients Discharged and Experiencing Same-Hospital Readmission – United States, March–August 2020". MMWR. Morbidity and Mortality Weekly Report. 69 (45): 1695–1699. doi:10.15585/mmwr.mm6945e2. PMC 7660660. PMID 33180754.
- Vardavas CI, Nikitara K (March 2020). "COVID-19 and smoking: A systematic review of the evidence". Tobacco Induced Diseases. 18: 20. doi:10.18332/tid/119324. PMC 7083240. PMID 32206052.
- ^ Engin AB, Engin ED, Engin A (August 2020). "Two important controversial risk factors in SARS-CoV-2 infection: Obesity and smoking". Environmental Toxicology and Pharmacology. 78: 103411. Bibcode:2020EnvTP..7803411E. doi:10.1016/j.etap.2020.103411. PMC 7227557. PMID 32422280.
- Setti L, Passarini F, De Gennaro G, Barbieri P, Licen S, Perrone MG, et al. (September 2020). "Potential role of particulate matter in the spreading of COVID-19 in Northern Italy: first observational study based on initial epidemic diffusion". BMJ Open. 10 (9): e039338. doi:10.1136/bmjopen-2020-039338. PMC 7517216. PMID 32973066.
- Wu X, Nethery RC, Sabath MB, Braun D, Dominici F (November 2020). "Air pollution and COVID-19 mortality in the United States: Strengths and limitations of an ecological regression analysis". Science Advances. 6 (45): eabd4049. Bibcode:2020SciA....6.4049W. doi:10.1126/sciadv.abd4049. PMC 7673673. PMID 33148655.
- Pansini R, Fornacca D (June 2021). "Early Spread of COVID-19 in the Air-Polluted Regions of Eight Severely Affected Countries". Atmosphere. 12 (6): 795. Bibcode:2021Atmos..12..795P. doi:10.3390/atmos12060795.
- Comunian S, Dongo D, Milani C, Palestini P (June 2020). "Air Pollution and Covid-19: The Role of Particulate Matter in the Spread and Increase of Covid-19's Morbidity and Mortality". International Journal of Environmental Research and Public Health. 17 (12): 4487. doi:10.3390/ijerph17124487. PMC 7345938. PMID 32580440.
- Domingo JL, Marquès M, Rovira J (September 2020). "Influence of airborne transmission of SARS-CoV-2 on COVID-19 pandemic. A review". Environmental Research. 188: 109861. Bibcode:2020ER....18809861D. doi:10.1016/j.envres.2020.109861. PMC 7309850. PMID 32718835.
- "COVID-19: Who's at higher risk of serious symptoms?". Mayo Clinic.
- Tamara A, Tahapary DL (July 2020). "Obesity as a predictor for a poor prognosis of COVID-19: A systematic review". Diabetes & Metabolic Syndrome. 14 (4): 655–659. doi:10.1016/j.dsx.2020.05.020. PMC 7217103. PMID 32438328.
- Petrakis D, Margină D, Tsarouhas K, Tekos F, Stan M, Nikitovic D, et al. (July 2020). "Obesity – A risk factor for increased COVID-19, severity and lethality (Review)". Molecular Medicine Reports. 22 (1): 9–19. doi:10.3892/mmr.2020.11127. PMC 7248467. PMID 32377709.
- Roca-Fernández A, Dennis A, Nicholls R, McGonigle J, Kelly M, Banerjee R, et al. (29 March 2021). "Hepatic Steatosis, Rather Than Underlying Obesity, Increases the Risk of Infection and Hospitalization for COVID-19". Frontiers in Medicine. 8: 636637. doi:10.3389/fmed.2021.636637. ISSN 2296-858X. PMC 8039134. PMID 33855033.
- "Coronavirus Disease 2019 (COVID-19)". U.S. Centers for Disease Control and Prevention (CDC). 11 February 2020.
- Devresse A, Belkhir L, Vo B, Ghaye B, Scohy A, Kabamba B, et al. (November 2020). "COVID-19 Infection in Kidney Transplant Recipients: A Single-Center Case Series of 22 Cases From Belgium". Kidney Medicine. 2 (4): 459–466. doi:10.1016/j.xkme.2020.06.001. PMC 7295531. PMID 32775986.
- Dhindsa S, Champion C, Deol E, Lui M, Campbell R, Newman J, et al. (September 2022). "Association of Male Hypogonadism With Risk of Hospitalization for COVID-19". JAMA Network Open. 5 (9): e2229747. doi:10.1001/jamanetworkopen.2022.29747. PMC 9440397. PMID 36053534.
- Shelton JF, Shastri AJ, Ye C, Weldon CH, Filshtein-Sonmez T, Coker D, et al. (June 2021). "Trans-ancestry analysis reveals genetic and nongenetic associations with COVID-19 susceptibility and severity". Nature Genetics. 53 (6): 801–808. doi:10.1038/s41588-021-00854-7. PMID 33888907. S2CID 233372385.
- Wallis C. "One in Seven Dire COVID Cases May Result from a Faulty Immune Response". Scientific American.
- Bastard P, Rosen LB, Zhang Q, Michailidis E, Hoffmann HH, Zhang Y, et al. (October 2020). "Autoantibodies against type I IFNs in patients with life-threatening COVID-19". Science. 370 (6515): eabd4585. doi:10.1126/science.abd4585. PMC 7857397. PMID 32972996. S2CID 221914095.
- Fusco DN, Brisac C, John SP, Huang YW, Chin CR, Xie T, et al. (June 2013). "A genetic screen identifies interferon-α effector genes required to suppress hepatitis C virus replication". Gastroenterology. 144 (7): 1438–49, 1449.e1-9. doi:10.1053/j.gastro.2013.02.026. PMC 3665646. PMID 23462180.
- Namkoong H, Edahiro R, Takano T, Nishihara H, Shirai Y, Sonehara K, et al. (September 2022). "DOCK2 is involved in the host genetics and biology of severe COVID-19". Nature. 609 (7928): 754–760. Bibcode:2022Natur.609..754N. doi:10.1038/s41586-022-05163-5. PMC 9492544. PMID 35940203.
- Kousathanas A, Pairo-Castineira E, Rawlik K, Stuckey A, Odhams CA, Walker S, et al. (July 2022). "Whole-genome sequencing reveals host factors underlying critical COVID-19". Nature. 607 (7917): 97–103. Bibcode:2022Natur.607...97K. doi:10.1038/s41586-022-04576-6. PMC 9259496. PMID 35255492.
- "COVID-19 in children and the role of school settings in transmission – first update". European Centre for Disease Prevention and Control. 23 December 2020. Retrieved 6 April 2021.
- "Estimated Disease Burden of COVID-19". U.S. Centers for Disease Control and Prevention (CDC). 11 February 2020. Retrieved 6 April 2021.
- Reardon S (2 September 2021). "Why don't kids tend to get as sick from Covid-19?". Knowable Magazine. doi:10.1146/knowable-090121-1. S2CID 239653475. Retrieved 7 September 2021.
- "Information for Pediatric Healthcare Providers". U.S. Centers for Disease Control and Prevention (CDC). 11 February 2020. Retrieved 6 April 2021.
- Götzinger F, Santiago-García B, Noguera-Julián A, Lanaspa M, Lancella L, Calò Carducci FI, et al. (September 2020). "COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study". The Lancet. Child & Adolescent Health. 4 (9): 653–661. doi:10.1016/S2352-4642(20)30177-2. PMC 7316447. PMID 32593339.
- Fang L, Karakiulakis G, Roth M (April 2020). "Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection?". The Lancet. Respiratory Medicine. 8 (4): e21. doi:10.1016/S0140-6736(20)30311-1. PMC 7118626. PMID 32171062.
- "Coronavirus Disease 2019 (COVID-19)". U.S. Centers for Disease Control and Prevention (CDC). 11 February 2020. Archived from the original on 2 March 2020. Retrieved 2 March 2020.
- Castanares-Zapatero D, Chalon P, Kohn L, Dauvrin M, Detollenaere J, Maertens de Noordhout C, et al. (December 2022). "Pathophysiology and mechanism of long COVID: a comprehensive review". Annals of Medicine. 54 (1): 1473–1487. doi:10.1080/07853890.2022.2076901. PMC 9132392. PMID 35594336.
- ^ Torres-Castro R, Vasconcello-Castillo L, Alsina-Restoy X, Solis-Navarro L, Burgos F, Puppo H, et al. (November 2020). "Respiratory function in patients post-infection by COVID-19: a systematic review and meta-analysis". Pulmonology. 27 (4). Elsevier BV: 328–337. doi:10.1016/j.pulmoe.2020.10.013. PMC 7687368. PMID 33262076. S2CID 227162748.
- Shaw B, Daskareh M, Gholamrezanezhad A (January 2021). "The lingering manifestations of COVID-19 during and after convalescence: update on long-term pulmonary consequences of coronavirus disease 2019 (COVID-19)". La Radiologia Medica. 126 (1): 40–46. doi:10.1007/s11547-020-01295-8. PMC 7529085. PMID 33006087.
- Zhao YM, Shang YM, Song WB, Li QQ, Xie H, Xu QF, et al. (August 2020). "Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery". eClinicalMedicine. 25: 100463. doi:10.1016/j.ijtb.2020.11.003. PMC 7654356. PMID 32838236.
- "COVID-19 Lung Damage". Johns Hopkins Medicine. 28 February 2022. Retrieved 21 May 2022.
- Taquet M, Sillett R, Zhu L, Mendel J, Camplisson I, Dercon Q, et al. (August 2022). "Neurological and psychiatric risk trajectories after SARS-CoV-2 infection: an analysis of 2-year retrospective cohort studies including 1 284 437 patients". The Lancet Psychiatry. 9 (10): 815–827. doi:10.1016/S2215-0366(22)00260-7. ISSN 2215-0366. PMC 9385200. PMID 35987197. S2CID 251626731.
- "Immune responses and correlates of protective immunity against SARS-CoV-2". European Centre for Disease Prevention and Control. 18 May 2021. Retrieved 3 June 2021.
- Vabret N, Britton GJ, Gruber C, Hegde S, Kim J, Kuksin M, et al. (June 2020). "Immunology of COVID-19: Current State of the Science". Immunity. 52 (6): 910–941. doi:10.1016/j.immuni.2020.05.002. PMC 7200337. PMID 32505227.
- Wang Z, Muecksch F, Schaefer-Babajew D, Finkin S, Viant C, Gaebler C, et al. (July 2021). "Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection". Nature. 595 (7867): 426–431. Bibcode:2021Natur.595..426W. doi:10.1038/s41586-021-03696-9. PMC 8277577. PMID 34126625.
- ^ Cohen JI, Burbelo PD (December 2020). "Reinfection with SARS-CoV-2: Implications for Vaccines". Clinical Infectious Diseases. 73 (11): e4223 – e4228. doi:10.1093/cid/ciaa1866. PMC 7799323. PMID 33338197. S2CID 229323810.
- ^ Wang J, Kaperak C, Sato T, Sakuraba A (August 2021). "COVID-19 reinfection: a rapid systematic review of case reports and case series". Journal of Investigative Medicine. 69 (6): 1253–1255. doi:10.1136/jim-2021-001853. ISSN 1081-5589. PMID 34006572. S2CID 234773697.
- ^ "How soon after catching COVID-19 can you get it again?". ABC News. 2 May 2022. Retrieved 24 June 2022.
- Centers for Disease Control and Prevention (May 2012). "Lesson 3: Measures of Risk Section 3: Mortality Frequency Measures". Principles of Epidemiology in Public Health Practice (Third ed.). U.S. Centers for Disease Control and Prevention (CDC). No. SS1978. Archived from the original on 28 February 2020. Retrieved 28 March 2020.
- ^ Ritchie H, Roser M (25 March 2020). Chivers T (ed.). "What do we know about the risk of dying from COVID-19?". Our World in Data. Archived from the original on 28 March 2020. Retrieved 28 March 2020.
- Castagnoli R, Votto M, Licari A, Brambilla I, Bruno R, Perlini S, et al. (September 2020). "Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection in Children and Adolescents: A Systematic Review". JAMA Pediatrics. 174 (9): 882–889. doi:10.1001/jamapediatrics.2020.1467. PMID 32320004.
- Lu X, Zhang L, Du H, Zhang J, Li YY, Qu J, et al. (April 2020). "SARS-CoV-2 Infection in Children". The New England Journal of Medicine. 382 (17). Massachusetts Medical Society: 1663–1665. doi:10.1056/nejmc2005073. PMC 7121177. PMID 32187458.
- Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z, et al. (June 2020). "Epidemiology of COVID-19 Among Children in China". Pediatrics. 145 (6): e20200702. doi:10.1542/peds.2020-0702. PMID 32179660. S2CID 219118986.
- ^ Dehingia N (2021). "Sex differences in COVID-19 case fatality: do we know enough?". The Lancet. Global Health. 9 (1): e14 – e15. doi:10.1016/S2214-109X(20)30464-2. PMC 7834645. PMID 33160453.
- "COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU)". ArcGIS. Johns Hopkins University. Retrieved 10 March 2023.
- Lazzerini M, Putoto G (May 2020). "COVID-19 in Italy: momentous decisions and many uncertainties". The Lancet. Global Health. 8 (5): e641 – e642. doi:10.1016/S2214-109X(20)30110-8. PMC 7104294. PMID 32199072.
- "Total confirmed cases of COVID-19 per million people". Our World in Data. Archived from the original on 19 March 2020. Retrieved 21 June 2022.
- "Cumulative confirmed COVID-19 deaths per million people". Our World in Data.
- Mallapaty S (June 2020). "How deadly is the coronavirus? Scientists are close to an answer". Nature. 582 (7813): 467–468. Bibcode:2020Natur.582..467M. doi:10.1038/d41586-020-01738-2. PMID 32546810. S2CID 219726496.
- Alwan NA, Burgess RA, Ashworth S, Beale R, Bhadelia N, Bogaert D, et al. (October 2020). "Scientific consensus on the COVID-19 pandemic: we need to act now". Lancet. 396 (10260): e71 – e72. doi:10.1016/S0140-6736(20)32153-X. PMC 7557300. PMID 33069277.
- Meyerowitz-Katz G, Merone L (December 2020). "A systematic review and meta-analysis of published research data on COVID-19 infection fatality rates". International Journal of Infectious Diseases. 101: 138–148. doi:10.1016/j.ijid.2020.09.1464. PMC 7524446. PMID 33007452.
- Zhang D, Hu M, Ji Q (October 2020). "Financial markets under the global pandemic of COVID-19". Finance Research Letters. 36: 101528. Bibcode:2020CSFX....500043D. doi:10.1016/j.csfx.2020.100043. PMC 7402242. PMID 32837360.
- ^ Levin AT, Hanage WP, Owusu-Boaitey N, Cochran KB, Walsh SP, Meyerowitz-Katz G (December 2020). "Assessing the age specificity of infection fatality rates for COVID-19: systematic review, meta-analysis, and public policy implications". European Journal of Epidemiology. 35 (12): 1123–1138. doi:10.1007/s10654-020-00698-1. PMC 7721859. PMID 33289900.
 Text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License Archived 16 October 2017 at the Wayback Machine.
Text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License Archived 16 October 2017 at the Wayback Machine.
- World Health Organization (22 December 2020). "Background paper on Covid-19 disease and vaccines: prepared by the Strategic Advisory Group of Experts (SAGE) on immunization working group on COVID-19 vaccines". World Health Organization (WHO). hdl:10665/338095.
- "Coronavirus disease 2019 (COVID-19) Situation Report – 30" (PDF). 19 February 2020. Retrieved 3 June 2020.
- "Coronavirus disease 2019 (COVID-19) Situation Report – 31" (PDF). 20 February 2020. Retrieved 23 April 2020.
- McNeil Jr DG (4 July 2020). "The Pandemic's Big Mystery: How Deadly Is the Coronavirus? – Even with more than 500,000 dead worldwide, scientists are struggling to learn how often the virus kills. Here's why". The New York Times. Archived from the original on 4 July 2020. Retrieved 6 July 2020.
- "Global Research and Innovation Forum on COVID-19: Virtual Press Conference" (PDF). World Health Organization (WHO). 2 July 2020.
- "Estimating mortality from COVID-19". World Health Organization (WHO). Retrieved 21 September 2020.
- Shaffer C (23 October 2021). "Covid-19 still rife in Iran". New Scientist. 252 (3357): 10–11. Bibcode:2021NewSc.252...10S. doi:10.1016/S0262-4079(21)01865-0. ISSN 0262-4079. PMC 8536311. PMID 34720322.
- "COVID-19: Data". City of New York.
- Wilson L (May 2020). "SARS-CoV-2, COVID-19, Infection Fatality Rate (IFR) Implied by the Serology, Antibody, Testing in New York City". SSRN 3590771.
- Yang W, Kandula S, Huynh M, Greene SK, Van Wye G, Li W, et al. (February 2021). "Estimating the infection-fatality risk of SARS-CoV-2 in New York City during the spring 2020 pandemic wave: a model-based analysis". The Lancet. Infectious Diseases. 21 (2): 203–212. doi:10.1016/s1473-3099(20)30769-6. PMC 7572090. PMID 33091374.
- Modi C (21 April 2020). "How deadly is COVID-19? Data Science offers answers from Italy mortality data". Medium. Retrieved 23 April 2020.
- ^ "Coronavirus Disease 2019 (COVID-19)". U.S. Centers for Disease Control and Prevention (CDC). 10 September 2020. Retrieved 9 December 2020.
- Salje H, Tran Kiem C, Lefrancq N, Courtejoie N, Bosetti P, Paireau J, et al. (July 2020). "Estimating the burden of SARS-CoV-2 in France". Science. 369 (6500): 208–211. Bibcode:2020Sci...369..208S. doi:10.1126/science.abc3517. PMC 7223792. PMID 32404476.
- McIntosh K (April 2021). "Covid 19 Clinical Features". UpToDate. Retrieved 12 May 2021.
- Peckham H, de Gruijter NM, Raine C, Radziszewska A, Ciurtin C, Wedderburn LR, et al. (December 2020). "Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission". Nature Communications. 11 (1): 6317. Bibcode:2020NatCo..11.6317P. doi:10.1038/s41467-020-19741-6. PMC 7726563. PMID 33298944.
- Abate BB, Kassie AM, Kassaw MW, Aragie TG, Masresha SA (October 2020). "Sex difference in coronavirus disease (COVID-19): a systematic review and meta-analysis". BMJ Open. 10 (10): e040129. doi:10.1136/bmjopen-2020-040129. PMC 7539579. PMID 33028563.
- ^ The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team (February 2020). "The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID-19) – China, 2020". China CDC Weekly. 2 (8): 113–122. doi:10.46234/ccdcw2020.032. PMC 839292. PMID 34594836.
- Hu Y, Sun J, Dai Z, Deng H, Li X, Huang Q, et al. (June 2020). "Prevalence and severity of corona virus disease 2019 (COVID-19): A systematic review and meta-analysis". Journal of Clinical Virology. 127: 104371. doi:10.1016/j.jcv.2020.104371. PMC 7195434. PMID 32315817.
- Fu L, Wang B, Yuan T, Chen X, Ao Y, Fitzpatrick T, et al. (June 2020). "Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: A systematic review and meta-analysis". The Journal of Infection. 80 (6): 656–665. doi:10.1016/j.jinf.2020.03.041. PMC 7151416. PMID 32283155.
- Yuki K, Fujiogi M, Koutsogiannaki S (June 2020). "COVID-19 pathophysiology: A review". Clinical Immunology. 215: 108427. doi:10.1016/j.clim.2020.108427. PMC 7169933. PMID 32325252. S2CID 216028003.
- Rabin RC (20 March 2020). "In Italy, Coronavirus Takes a Higher Toll on Men". The New York Times. Archived from the original on 20 March 2020. Retrieved 7 April 2020.
- "COVID-19 weekly surveillance report". World Health Organization (WHO). Archived from the original on 15 March 2020. Retrieved 7 April 2020.
- ^ Gupta AH (3 April 2020). "Does Covid-19 Hit Women and Men Differently? U.S. Isn't Keeping Track". The New York Times. Archived from the original on 3 April 2020. Retrieved 7 April 2020.
- ^ Dorn AV, Cooney RE, Sabin ML (April 2020). "COVID-19 exacerbating inequalities in the US". Lancet. 395 (10232): 1243–1244. doi:10.1016/S0140-6736(20)30893-X. PMC 7162639. PMID 32305087.
- ^ Shauly-Aharonov M, Shafrir A, Paltiel O, Calderon-Margalit R, Safadi R, Bicher R, et al. (22 July 2021). "Both high and low pre-infection glucose levels associated with increased risk for severe COVID-19: New insights from a population-based study". PLOS ONE. 16 (7): e0254847. Bibcode:2021PLoSO..1654847S. doi:10.1371/journal.pone.0254847. ISSN 1932-6203. PMC 8297851. PMID 34293038.
- Adams ML, Katz DL, Grandpre J (August 2020). "Population-Based Estimates of Chronic Conditions Affecting Risk for Complications from Coronavirus Disease, United States". Emerging Infectious Diseases. 26 (8): 1831–1833. doi:10.3201/eid2608.200679. PMC 7392427. PMID 32324118.
- Batthyány K (13 October 2020). "Coronavirus y Desigualdades preexistentes: Género y Cuidados". CLACSO (Consejo Latinoamericano de Ciencias Sociales). Retrieved 22 April 2021.
- ^ "COVID-19 Presents Significant Risks for American Indian and Alaska Native People". 14 May 2020.
- Laurencin CT, McClinton A (June 2020). "The COVID-19 Pandemic: a Call to Action to Identify and Address Racial and Ethnic Disparities". Journal of Racial and Ethnic Health Disparities. 7 (3): 398–402. doi:10.1007/s40615-020-00756-0. PMC 7166096. PMID 32306369.
- "How coronavirus deaths in the UK compare by race and ethnicity". The Independent. 9 June 2020. Retrieved 10 June 2020.
- "Emerging findings on the impact of COVID-19 on black and minority ethnic people". The Health Foundation. Retrieved 10 June 2020.
- Butcher B, Massey J (9 June 2020). "Why are more BAME people dying from coronavirus?". BBC News. Retrieved 10 June 2020.
- ^ "The ancient Neanderthal hand in severe COVID-19". ScienceDaily. 30 September 2020. Retrieved 13 December 2020.
- "WHO Director-General's statement on the advice of the IHR Emergency Committee on Novel Coronavirus". World Health Organization (WHO).
- Garg S, Kim L, Whitaker M, O'Halloran A, Cummings C, Holstein R, et al. (April 2020). "Hospitalization Rates and Characteristics of Patients Hospitalized with Laboratory-Confirmed Coronavirus Disease 2019 – COVID-NET, 14 States, March 1–30, 2020". MMWR. Morbidity and Mortality Weekly Report. 69 (15): 458–464. doi:10.15585/mmwr.mm6915e3. PMC 7755063. PMID 32298251.
- ^ "Coronavirus Disease 2019 (COVID-19)". U.S. Centers for Disease Control and Prevention (CDC). 11 February 2020. Retrieved 19 June 2020.
- Zhao Q, Meng M, Kumar R, Wu Y, Huang J, Lian N, et al. (October 2020). "The impact of COPD and smoking history on the severity of COVID-19: A systemic review and meta-analysis". Journal of Medical Virology. 92 (10): 1915–1921. doi:10.1002/jmv.25889. PMC 7262275. PMID 32293753.
- "Smoking and COVID-19". World Health Organization (WHO). Retrieved 19 June 2020.
- DeRobertis J (3 May 2020). "People who use drugs are more vulnerable to coronavirus. Here's what clinics are doing to help". The Advocate (Louisiana). Retrieved 4 May 2020.
- "Coronavirus Disease 2019 (COVID-19)". U.S. Centers for Disease Control and Prevention (CDC). 11 February 2020.
- Frutos R, Gavotte L, Devaux CA (November 2021). "Understanding the origin of COVID-19 requires to change the paradigm on zoonotic emergence from the spillover to the circulation model". Infection, Genetics and Evolution. 95: 104812. Bibcode:2021InfGE..9504812F. doi:10.1016/j.meegid.2021.104812. PMC 7969828. PMID 33744401.
- Holmes EC, Goldstein SA, Rasmussen AL, Robertson DL, Crits-Christoph A, Wertheim JO, et al. (September 2021). "The origins of SARS-CoV-2: A critical review". Cell. 184 (19): 4848–4856. doi:10.1016/j.cell.2021.08.017. PMC 8373617. PMID 34480864.
- "WHO-convened Global Study of Origins of SARS-CoV-2: China Part". World Health Organization (WHO). 30 March 2021. Retrieved 29 July 2022.
- Duarte F (24 February 2020). "As the cases of coronavirus increase in China and around the world, the hunt is on to identify "patient zero"". BBC News. Retrieved 22 March 2020.
- Pekar JE, Magee P, Parker E, Moshiri N, Izhikevich K, Havens JL, et al. (26 July 2022). "The molecular epidemiology of multiple zoonotic origins of SARS-CoV-2". Science. 377 (6609): 960–966. Bibcode:2022Sci...377..960P. doi:10.1126/science.abp8337. PMC 9348752. PMID 35881005.
- Gill V (26 July 2022). "Covid origin studies say evidence points to Wuhan market". BBC News Online. BBC. Archived from the original on 26 July 2022. Retrieved 31 August 2023.
- Worobey M, Levy JI, Serrano LM, Crits-Christoph A, Pekar JE, Goldstein SA, et al. (July 2022). "The Huanan Seafood Wholesale Market in Wuhan was the early epicenter of the COVID-19 pandemic". Science. 377 (6609): 951–959. Bibcode:2022Sci...377..951W. doi:10.1126/science.abp8715. PMC 9348750. PMID 35881010. S2CID 251067542.
- "Debate deepens over Wuhan wet market's role in kickstarting the pandemic". National Geographic. 27 July 2022.
- Li X, Zai J, Zhao Q, Nie Q, Li Y, Foley BT, et al. (June 2020). "Evolutionary history, potential intermediate animal host, and cross-species analyses of SARS-CoV-2". Journal of Medical Virology. 92 (6): 602–611. doi:10.1002/jmv.25731. PMC 7228310. PMID 32104911.
- Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF (April 2020). "The proximal origin of SARS-CoV-2". Nature Medicine. 26 (4): 450–452. doi:10.1038/s41591-020-0820-9. PMC 7095063. PMID 32284615.
- van Dorp L, Acman M, Richard D, Shaw LP, Ford CE, Ormond L, et al. (September 2020). "Emergence of genomic diversity and recurrent mutations in SARS-CoV-2". Infection, Genetics and Evolution. 83: 104351. Bibcode:2020InfGE..8304351V. doi:10.1016/j.meegid.2020.104351. PMC 7199730. PMID 32387564.
- Grose TK (13 May 2020). "Did the Coronavirus Originate Outside of Wuhan?". U.S. News & World Report.
- Barnes JE (26 February 2023). "Lab Leak Most Likely Caused Pandemic, Energy Dept. Says". The New York Times. Retrieved 27 February 2023.
- Mueller J (26 February 2023). "Energy Department's COVID lab leak conclusion: What we know". The Hill. Retrieved 26 March 2023.
- LeBlanc P (27 February 2023). "New assessment on the origins of Covid-19 adds to the confusion | CNN Politics". CNN. Retrieved 27 February 2023.
- Davis N, Hawkins A (27 February 2023). "How seriously should we take the US DoE's Covid lab leak theory?". The Guardian. Retrieved 27 February 2023.
- Wolf ZB (25 May 2021). "Analysis: Why scientists are suddenly more interested in the lab-leak theory of Covid's origin". CNN. Retrieved 26 May 2021.
- Maxmen A (September 2021). "US COVID origins report: researchers pleased with scientific approach". Nature. 597 (7875): 159–160. Bibcode:2021Natur.597..159M. doi:10.1038/d41586-021-02366-0. PMID 34465917. S2CID 237373547.
- Paun C, Zeller S, Reader R, Leonard B, Scullion G (4 November 2022). "Cross-examining the lab-leak theorists". Politico. Retrieved 21 November 2022.
- Hosenball M, Zengerle P (30 October 2021). "U.S. spy agencies say origins of COVID-19 may never be known". Reuters. Retrieved 21 November 2022.
- Holmes EC, Goldstein SA, Rasmussen AL, Robertson DL, Crits-Christoph A, Wertheim JO, et al. (September 2021). "The origins of SARS-CoV-2: A critical review". Cell (Review). 184 (19): 4848–4856. doi:10.1016/j.cell.2021.08.017. PMC 8373617. PMID 34480864.
Under any laboratory escape scenario, SARS-CoV-2 would have to have been present in a laboratory prior to the pandemic, yet no evidence exists to support such a notion and no sequence has been identified that could have served as a precursor.
- Gorski D (31 May 2021). "The origin of SARS-CoV-2, revisited". Science-Based Medicine. Archived from the original on 1 June 2021. Retrieved 19 July 2021.
The second is the version that "reasonable" people consider plausible, but there is no good evidence for either version.
- Holmes EC (14 August 2022). "The COVID lab leak theory is dead. Here's how we know the virus came from a Wuhan market". The Conversation. Retrieved 4 September 2022.
For the lab leak theory to be true, SARS-CoV-2 must have been present in the Wuhan Institute of Virology before the pandemic started. This would convince me. But the inconvenient truth is there's not a single piece of data suggesting this. There's no evidence for a genome sequence or isolate of a precursor virus at the Wuhan Institute of Virology. Not from gene sequence databases, scientific publications, annual reports, student theses, social media, or emails. Even the intelligence community has found nothing. Nothing. And there was no reason to keep any work on a SARS-CoV-2 ancestor secret before the pandemic.
- Wu YC, Chen CS, Chan YJ (March 2020). "The outbreak of COVID-19: An overview". Journal of the Chinese Medical Association. 83 (3): 217–220. doi:10.1097/JCMA.0000000000000270. PMC 7153464. PMID 32134861.
- Wang C, Horby PW, Hayden FG, Gao GF (February 2020). "A novel coronavirus outbreak of global health concern". Lancet. 395 (10223): 470–473. doi:10.1016/S0140-6736(20)30185-9. PMC 7135038. PMID 31986257.
- Cohen J (January 2020). "Wuhan seafood market may not be source of novel virus spreading globally". Science. doi:10.1126/science.abb0611.
- "Novel Coronavirus – China". World Health Organization (WHO). 12 January 2020. Archived from the original on 14 January 2020.
- Kessler G (17 April 2020). "Trump's false claim that the WHO said the coronavirus was 'not communicable'". The Washington Post. Archived from the original on 17 April 2020. Retrieved 17 April 2020.
- Kuo L (21 January 2020). "China confirms human-to-human transmission of coronavirus". The Guardian. Retrieved 18 April 2020.
- Epidemiology Working Group For Ncip Epidemic Response, Chinese Center for Disease Control Prevention (February 2020). "". Zhonghua Liu Xing Bing Xue Za Zhi = Zhonghua Liuxingbingxue Zazhi (in Chinese). 41 (2): 145–151. doi:10.3760/cma.j.issn.0254-6450.2020.02.003. PMID 32064853. S2CID 211133882.
- Areddy JT (26 May 2020). "China Rules Out Animal Market and Lab as Coronavirus Origin". The Wall Street Journal. Retrieved 29 May 2020.
- Kelland K (19 June 2020). "Italy sewage study suggests COVID-19 was there in December 2019". Reuters. Retrieved 23 June 2020.
- Heymann DL, Shindo N (February 2020). "COVID-19: what is next for public health?". Lancet. 395 (10224): 542–545. doi:10.1016/S0140-6736(20)30374-3. PMC 7138015. PMID 32061313.
- Bryner J (14 March 2020). "1st known case of coronavirus traced back to November in China". livescience.com. Retrieved 31 May 2020.
- Canadian Politics (8 April 2020). "The birth of a pandemic: How COVID-19 went from Wuhan to Toronto". National Post. Retrieved 31 May 2020.
- 高昱 (26 February 2020). "独家 | 新冠病毒基因测序溯源: 警报是何时拉响的" [Exclusive | Tracing the New Coronavirus gene sequencing: when did the alarm sound]. Caixin (in Chinese). Archived from the original on 27 February 2020. Retrieved 1 March 2020.
- 路子康. "最早上报疫情的她, 怎样发现这种不一样的肺炎". 中国网新闻 (in Chinese (China)). 北京. Archived from the original on 2 March 2020. Retrieved 11 February 2020.
- "Undiagnosed pneumonia – China (HU): RFI". ProMED Mail. ProMED. Retrieved 7 May 2020.
- "'Hero who told the truth': Chinese rage over coronavirus death of whistleblower doctor". The Guardian. 7 February 2020.
- Kuo L (11 March 2020). "Coronavirus: Wuhan doctor speaks out against authorities". The Guardian. London.
- "Novel Coronavirus". World Health Organization (WHO). Archived from the original on 2 February 2020. Retrieved 6 February 2020.
- "武汉现不明原因肺炎 官方确认属实: 已经做好隔离". Xinhua Net 新華網. 31 December 2019. Retrieved 31 March 2020.
- 武汉市卫健委关于当前我市肺炎疫情的情况通报. WJW.Wuhan.gov.cn (in Chinese). Wuhan Municipal Health Commission. 31 December 2019. Archived from the original on 9 January 2020. Retrieved 8 February 2020.
- "Mystery pneumonia virus probed in China". BBC News. 3 January 2020. Archived from the original on 5 January 2020. Retrieved 29 January 2020.
- Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. (March 2020). "Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia". The New England Journal of Medicine. 382 (13): 1199–1207. doi:10.1056/NEJMoa2001316. PMC 7121484. PMID 31995857.
- "China confirms sharp rise in cases of SARS-like virus across the country". 20 January 2020. Archived from the original on 20 January 2020. Retrieved 20 January 2020.
- ^ "Flattery and foot dragging: China's influence over the WHO under scrutiny". The Globe and Mail. 25 April 2020.
- Horton R (18 March 2020). "Scientists have been sounding the alarm on coronavirus for months. Why did Britain fail to act?". The Guardian. Retrieved 23 April 2020.
- "China delayed releasing coronavirus info, frustrating WHO". Associated Press. 2 June 2020. Retrieved 3 June 2020.
- "Coronavirus: Primi due casi in Italia" [Coronavirus: First two cases in Italy]. Corriere della sera (in Italian). 31 January 2020. Retrieved 31 January 2020.
- "Coronavirus: Number of COVID-19 deaths in Italy surpasses China as total reaches 3,405". Sky News. Retrieved 7 May 2020.
- McNeil Jr DG (26 March 2020). "The U.S. Now Leads the World in Confirmed Coronavirus Cases". The New York Times. Archived from the original on 26 March 2020. Retrieved 27 March 2020.
- "Studies Show N.Y. Outbreak Originated in Europe". The New York Times. 8 April 2020. Archived from the original on 8 April 2020.
- Irish J (4 May 2020). Lough RM, Graff P (eds.). "After retesting samples, French hospital discovers COVID-19 case from December". Reuters. Retrieved 4 May 2020.
- Deslandes A, Berti V, Tandjaoui-Lambotte Y, Alloui C, Carbonnelle E, Zahar JR, et al. (June 2020). "SARS-CoV-2 was already spreading in France in late December 2019". International Journal of Antimicrobial Agents. 55 (6): 106006. doi:10.1016/j.ijantimicag.2020.106006. PMC 7196402. PMID 32371096.
- "2 died with coronavirus weeks before 1st U.S. virus death". PBS NewsHour. 22 April 2020. Retrieved 23 April 2020.
- Michael-Kordatou I, Karaolia P, Fatta-Kassinos D (October 2020). "Sewage analysis as a tool for the COVID-19 pandemic response and management: the urgent need for optimised protocols for SARS-CoV-2 detection and quantification". Journal of Environmental Chemical Engineering. 8 (5): 104306. doi:10.1016/j.jece.2020.104306. PMC 7384408. PMID 32834990.
- Platto S, Xue T, Carafoli E (September 2020). "COVID19: an announced pandemic". Cell Death & Disease. 11 (9): 799. doi:10.1038/s41419-020-02995-9. PMC 7513903. PMID 32973152.
- Kavya B, Abraham R (3 October 2021). Shumaker L, Wardell J (eds.). "Global COVID-19 deaths hit 5 million as Delta variant sweeps the world". Reuters.com. Reuters.
- "From emergency response to long-term COVID-19 disease management: sustaining gains made during the COVID-19 pandemic". World Health Organization (WHO). Retrieved 9 May 2023.
- Heyward G, Silver M (5 May 2023). "WHO ends global health emergency declaration for COVID-19". NPR. Retrieved 9 May 2023.
- "China coronavirus: Misinformation spreads online about origin and scale". BBC News. 30 January 2020. Archived from the original on 4 February 2020. Retrieved 10 February 2020.
- Taylor J (31 January 2020). "Bat soup, dodgy cures and 'diseasology': the spread of coronavirus misinformation". The Guardian. Archived from the original on 2 February 2020. Retrieved 3 February 2020.
- "Here's A Running List Of Disinformation Spreading About The Coronavirus". Buzzfeed News. Archived from the original on 6 February 2020. Retrieved 8 February 2020.
- "Misleading claim circulates online about infection fatality ratio of Covid-19 in the US". Fact Check. 8 October 2020. Retrieved 10 October 2020.
- Gryseels S, De Bruyn L, Gyselings R, Calvignac-Spencer S, Leendertz FH, Leirs H (April 2021). "Risk of human-to-wildlife transmission of SARS-CoV-2". Mammal Review. 51 (2): 272–292. doi:10.1111/mam.12225. hdl:10067/1726730151162165141. ISSN 0305-1838. PMC 7675675. PMID 33230363.
- Tan CC, Lam SD, Richard D, Owen CJ, Berchtold D, Orengo C, et al. (27 May 2022). "Transmission of SARS-CoV-2 from humans to animals and potential host adaptation". Nature Communications. 13 (1): 2988. Bibcode:2022NatCo..13.2988T. doi:10.1038/s41467-022-30698-6. ISSN 2041-1723. PMC 9142586. PMID 35624123. Retrieved 28 February 2023.
- Pappas G, Vokou D, Sainis I, Halley JM (November 2022). "SARS-CoV-2 as a Zooanthroponotic Infection: Spillbacks, Secondary Spillovers, and Their Importance". Microorganisms. 10 (11): 2166. doi:10.3390/microorganisms10112166. ISSN 2076-2607. PMC 9696655. PMID 36363758.
- Munir K, Ashraf S, Munir I, Khalid H, Muneer MA, Mukhtar N, et al. (1 January 2020). "Zoonotic and reverse zoonotic events of SARS-CoV-2 and their impact on global health". Emerging Microbes & Infections. 9 (1): 2222–2235. doi:10.1080/22221751.2020.1827984. PMC 7594747. PMID 32967592.
- ^ Kampf G, Brüggemann Y, Kaba HE, Steinmann J, Pfaender S, Scheithauer S, et al. (December 2020). "Potential sources, modes of transmission and effectiveness of prevention measures against SARS-CoV-2". The Journal of Hospital Infection. 106 (4): 678–697. doi:10.1016/j.jhin.2020.09.022. PMC 7500278. PMID 32956786.
- Shi J, Wen Z, Zhong G, Yang H, Wang C, Huang B, et al. (May 2020). "Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2". Science. 368 (6494): 1016–1020. doi:10.1126/science.abb7015. PMC 7164390. PMID 32269068.
- ^ Salajegheh Tazerji S, Magalhães Duarte P, Rahimi P, Shahabinejad F, Dhakal S, Singh Malik Y, et al. (September 2020). "Transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) to animals: an updated review". Journal of Translational Medicine. 18 (1): 358. doi:10.1186/s12967-020-02534-2. PMC 7503431. PMID 32957995.
- ^ Gorman J (22 January 2021). "The Coronavirus Kills Mink, So They Too May Get a Vaccine". The New York Times. ISSN 0362-4331. Archived from the original on 28 December 2021. Retrieved 24 February 2021.
- Dhama K, Sharun K, Tiwari R, Dadar M, Malik YS, Singh KP, et al. (June 2020). "COVID-19, an emerging coronavirus infection: advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics". Human Vaccines & Immunotherapeutics. 16 (6): 1232–1238. doi:10.1080/21645515.2020.1735227. PMC 7103671. PMID 32186952.
- Zhang L, Liu Y (May 2020). "Potential interventions for novel coronavirus in China: A systematic review". Journal of Medical Virology. 92 (5): 479–490. doi:10.1002/jmv.25707. PMC 7166986. PMID 32052466.
- "Interim Laboratory Biosafety Guidelines for Handling and Processing Specimens Associated with Coronavirus Disease 2019 (COVID-19)". Coronavirus Disease 2019 (COVID-19) Lab Biosafety Guidelines. U.S. Centers for Disease Control and Prevention (CDC). 11 February 2020. Retrieved 1 April 2020.
- Aristovnik A, Ravšelj D, Umek L (November 2020). "A Bibliometric Analysis of COVID-19 across Science and Social Science Research Landscape". Sustainability. 12 (21): 9132. Bibcode:2020Sust...12.9132A. doi:10.3390/su12219132.
- Kupferschmidt K (3 December 2020). "First-of-its-kind African trial tests common drugs to prevent severe COVID-19". Science. doi:10.1126/science.abf9987. Retrieved 8 March 2022.
- Reardon S (November 2020). "For COVID Drugs, Months of Frantic Development Lead to Few Outright Successes". Scientific American. Retrieved 10 December 2020.
- Kucharski AJ, Russell TW, Diamond C, Liu Y, Edmunds J, Funk S, et al. (May 2020). "Early dynamics of transmission and control of COVID-19: a mathematical modelling study". The Lancet. Infectious Diseases. 20 (5): 553–558. doi:10.1016/S1473-3099(20)30144-4. PMC 7158569. PMID 32171059.
- "Update to living systematic review on prediction models for diagnosis and prognosis of covid-19". BMJ (Clinical Research Ed.). 372: n236. 3 February 2021. doi:10.1136/bmj.n236. ISSN 1756-1833. PMID 33536183. S2CID 231775762.
- Giordano G, Blanchini F, Bruno R, Colaneri P, Di Filippo A, Di Matteo A, et al. (June 2020). "Modelling the COVID-19 epidemic and implementation of population-wide interventions in Italy". Nature Medicine. 26 (6): 855–860. arXiv:2003.09861. doi:10.1038/s41591-020-0883-7. PMC 7175834. PMID 32322102.
- Prem K, Liu Y, Russell TW, Kucharski AJ, Eggo RM, Davies N, et al. (May 2020). "The effect of control strategies to reduce social mixing on outcomes of the COVID-19 epidemic in Wuhan, China: a modelling study". The Lancet. Public Health. 5 (5): e261 – e270. doi:10.1016/S2468-2667(20)30073-6. PMC 7158905. PMID 32220655.
- Emanuel EJ, Persad G, Upshur R, Thome B, Parker M, Glickman A, et al. (May 2020). "Fair Allocation of Scarce Medical Resources in the Time of Covid-19". The New England Journal of Medicine. 382 (21): 2049–2055. doi:10.1056/NEJMsb2005114. PMID 32202722.
- Kermack WO, McKendrick AG (1927). "A contribution to the mathematical theory of epidemics". Proceedings of the Royal Society of London. Series A, Containing Papers of a Mathematical and Physical Character. 115 (772): 700–721. Bibcode:1927RSPSA.115..700K. doi:10.1098/rspa.1927.0118.
- Mittal R, Ni R, Seo JH (2020). "The flow physics of COVID-19". Journal of Fluid Mechanics. 894: –2. arXiv:2004.09354. Bibcode:2020JFM...894F...2M. doi:10.1017/jfm.2020.330.
- Ronchi E, Lovreglio R (October 2020). "EXPOSED: An occupant exposure model for confined spaces to retrofit crowd models during a pandemic". Safety Science. 130: 104834. arXiv:2005.04007. doi:10.1016/j.ssci.2020.104834. PMC 7373681. PMID 32834509.
- Badr HS, Du H, Marshall M, Dong E, Squire MM, Gardner LM (November 2020). "Association between mobility patterns and COVID-19 transmission in the USA: a mathematical modelling study". The Lancet Infectious Diseases. 20 (11): 1247–1254. doi:10.1016/S1473-3099(20)30553-3. PMC 7329287. PMID 32621869.
- McKibbin W, Roshen F (2020). "The global macroeconomic impacts of COVID-19: Seven scenarios" (PDF). CAMA Working Paper. doi:10.2139/ssrn.3547729. S2CID 216307705.
- "COVID-19 treatment and vaccine tracker" (PDF). Milken Institute. 21 April 2020. Retrieved 21 April 2020.
- ^ Koch S, Pong W (13 March 2020). "First up for COVID-19: nearly 30 clinical readouts before end of April". BioCentury Inc. Retrieved 1 April 2020.
- Kupferschmidt K, Cohen J (March 2020). "WHO launches global megatrial of the four most promising coronavirus treatments". Science. doi:10.1126/science.abb8497.
- "UN health chief announces global 'solidarity trial' to jumpstart search for COVID-19 treatment". UN News. 18 March 2020. Archived from the original on 23 March 2020. Retrieved 23 March 2020.
- "Citing safety concerns, the W.H.O. paused tests of a drug Trump said he had taken". The New York Times. 26 May 2020. Archived from the original on 26 May 2020.
-
 This article incorporates text from this source, which is in the public domain: Hydroxychloroquine does not benefit adults hospitalized with COVID-19. National Institutes of Health (NIH). 9 November 2020. Retrieved 9 November 2020.
This article incorporates text from this source, which is in the public domain: Hydroxychloroquine does not benefit adults hospitalized with COVID-19. National Institutes of Health (NIH). 9 November 2020. Retrieved 9 November 2020.
-
 This article incorporates text from this source, which is in the public domain: Coronavirus (COVID-19) Update: FDA Warns of Newly Discovered Potential Drug Interaction That May Reduce Effectiveness of a COVID-19 Treatment Authorized for Emergency Use. U.S. Food and Drug Administration (FDA). 15 June 2020. Retrieved 15 June 2020.
This article incorporates text from this source, which is in the public domain: Coronavirus (COVID-19) Update: FDA Warns of Newly Discovered Potential Drug Interaction That May Reduce Effectiveness of a COVID-19 Treatment Authorized for Emergency Use. U.S. Food and Drug Administration (FDA). 15 June 2020. Retrieved 15 June 2020.
- "France bans use of hydroxychloroquine, drug touted by Trump, in coronavirus patients". CBS News. 27 May 2020.
- Boseley S (16 June 202). "Recovery trial for Covid-19 treatments: what we know so far". The Guardian. Retrieved 21 June 2020.
- WHO welcomes preliminary results about dexamethasone use in treating critically ill COVID-19 patients. World Health Organization (WHO). 16 June 2020. Retrieved 21 June 2020.
- Q&A: Dexamethasone and COVID-19. World Health Organization (WHO). Retrieved 12 July 2020.
- "Corticosteroids". COVID-19 Treatment Guidelines. National Institutes of Health. Retrieved 12 July 2020.
- ^ World Health Organization (2020). Corticosteroids for COVID-19: living guidance, 2 September 2020 (Report). hdl:10665/334125. WHO/2019-nCoV/Corticosteroids/2020.1.
- "WHO updates clinical care guidance with corticosteroid recommendations". World Health Organization (WHO). Retrieved 25 January 2022.
- Sterne JA, Murthy S, Diaz JV, Slutsky AS, Villar J, Angus DC, et al. (The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group) (October 2020). "Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis". JAMA. 324 (13): 1330–1341. doi:10.1001/jama.2020.17023. PMC 7489434. PMID 32876694. S2CID 221467783.
- Prescott HC, Rice TW (October 2020). "Corticosteroids in COVID-19 ARDS: Evidence and Hope During the Pandemic". JAMA. 324 (13): 1292–1295. doi:10.1001/jama.2020.16747. PMID 32876693. S2CID 221468015.
- ^ EMA endorses use of dexamethasone in COVID-19 patients on oxygen or mechanical ventilation. European Medicines Agency (EMA). 18 September 2020. Retrieved 21 September 2020. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- Dexamethasone in hospitalised patients with COVID-19 (PDF) (Report). European Medicines Agency. 17 September 2020.
- ^
 This article incorporates text from this source, which is in the public domain: Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibody for Treatment of COVID-19. U.S. Food and Drug Administration (FDA). 9 November 2020. Retrieved 9 November 2020.
This article incorporates text from this source, which is in the public domain: Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibody for Treatment of COVID-19. U.S. Food and Drug Administration (FDA). 9 November 2020. Retrieved 9 November 2020.
-
 This article incorporates text from this source, which is in the public domain: FDA Authorizes Monoclonal Antibodies for Treatment of COVID-19. U.S. Food and Drug Administration (FDA). 10 February 2021. Retrieved 9 February 2021.
This article incorporates text from this source, which is in the public domain: FDA Authorizes Monoclonal Antibodies for Treatment of COVID-19. U.S. Food and Drug Administration (FDA). 10 February 2021. Retrieved 9 February 2021.
-
 This article incorporates text from this source, which is in the public domain: Coronavirus (COVID-19) Update: FDA Revokes Emergency Use Authorization for Monoclonal Antibody Bamlanivimab. U.S. Food and Drug Administration (FDA). 16 April 2021. Retrieved 16 April 2021.
This article incorporates text from this source, which is in the public domain: Coronavirus (COVID-19) Update: FDA Revokes Emergency Use Authorization for Monoclonal Antibody Bamlanivimab. U.S. Food and Drug Administration (FDA). 16 April 2021. Retrieved 16 April 2021.
- Li X, Geng M, Peng Y, Meng L, Lu S (April 2020). "Molecular immune pathogenesis and diagnosis of COVID-19". Journal of Pharmaceutical Analysis. 10 (2): 102–108. doi:10.1016/j.jpha.2020.03.001. PMC 7104082. PMID 32282863.
- Zhao Z, Wei Y, Tao C (January 2021). "An enlightening role for cytokine storm in coronavirus infection". Clinical Immunology. 222: 108615. doi:10.1016/j.clim.2020.108615. PMC 7583583. PMID 33203513.
- Liu R, Miller J (3 March 2020). "China approves use of Roche drug in battle against coronavirus complications". Reuters. Archived from the original on 12 March 2020. Retrieved 14 March 2020.
- Xu X, Han M, Li T, Sun W, Wang D, Fu B, et al. (May 2020). "Effective treatment of severe COVID-19 patients with tocilizumab". Proceedings of the National Academy of Sciences of the United States of America. 117 (20): 10970–10975. Bibcode:2020PNAS..11710970X. doi:10.1073/pnas.2005615117. PMC 7245089. PMID 32350134.
- Ovadia D, Agenzia Z. "COVID-19 – Italy launches an independent trial on tocilizumab". Univadis from Medscape. Aptus Health. Retrieved 22 April 2020.
- "Tocilizumab in COVID-19 Pneumonia (TOCIVID-19) (TOCIVID-19)". clinicaltrials.gov. Retrieved 22 April 2020.
- Various sources:
- "How doctors can potentially significantly reduce the number of deaths from Covid-19". Vox. 12 March 2020. Archived from the original on 19 March 2020. Retrieved 14 March 2020.
- Ruan Q, Yang K, Wang W, Jiang L, Song J (May 2020). "Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China". Intensive Care Medicine. 46 (5): 846–848. doi:10.1007/s00134-020-05991-x. PMC 7080116. PMID 32125452.
- Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ (March 2020). "COVID-19: consider cytokine storm syndromes and immunosuppression". Lancet. 395 (10229): 1033–1034. doi:10.1016/S0140-6736(20)30628-0. PMC 7270045. PMID 32192578.
- Slater H (26 March 2020). "FDA Approves Phase III Clinical Trial of Tocilizumab for COVID-19 Pneumonia". cancernetwork.com. Cancer Network. Retrieved 22 April 2020.
- Locke FL, Neelapu SS, Bartlett NL, Lekakis LJ, Jacobson CA, Braunschweig I, et al. (2017). "Preliminary Results of Prophylactic Tocilizumab after Axicabtageneciloleucel (axi-cel; KTE-C19) Treatment for Patients with Refractory, Aggressive Non-Hodgkin Lymphoma (NHL)". Blood. 130 (Supplement 1): 1547. doi:10.1182/blood.V130.Suppl_1.1547.1547. S2CID 155698207.
- Sterner RM, Sakemura R, Cox MJ, Yang N, Khadka RH, Forsman CL, et al. (February 2019). "GM-CSF inhibition reduces cytokine release syndrome and neuroinflammation but enhances CAR T cell function in xenografts". Blood. 133 (7): 697–709. doi:10.1182/blood-2018-10-881722. PMC 6376281. PMID 30463995.
- ^ Casadevall A, Pirofski LA (April 2020). "The convalescent sera option for containing COVID-19". The Journal of Clinical Investigation. 130 (4): 1545–1548. doi:10.1172/JCI138003. PMC 7108922. PMID 32167489.
- ^ Iannizzi C, Chai KL, Piechotta V, Valk SJ, Kimber C, Monsef I, et al. (10 May 2023). "Convalescent plasma for people with COVID-19: a living systematic review". The Cochrane Database of Systematic Reviews. 2023 (5): CD013600. doi:10.1002/14651858.CD013600.pub6. ISSN 1469-493X. PMC 10171886. PMID 37162745.
- ^ Ho M (April 2020). "Perspectives on the development of neutralizing antibodies against SARS-CoV-2". Antibody Therapeutics. 3 (2): 109–114. doi:10.1093/abt/tbaa009. PMC 7291920. PMID 32566896.
- Yang L, Liu W, Yu X, Wu M, Reichert JM, Ho M (July 2020). "COVID-19 antibody therapeutics tracker: a global online database of antibody therapeutics for the prevention and treatment of COVID-19". Antibody Therapeutics. 3 (3): 205–212. doi:10.1093/abt/tbaa020. PMC 7454247. PMID 33215063.
- Maccaro A, Piaggio D, Pagliara S, Pecchia L (June 2021). "The role of ethics in science: a systematic literature review from the first wave of COVID-19". Health and Technology. 11 (5): 1063–1071. doi:10.1007/s12553-021-00570-6. ISSN 2190-7188. PMC 8175060. PMID 34104626.
- McGuire AL, Aulisio MP, Davis FD, Erwin C, Harter TD, Jagsi R, et al. (July 2020). "Ethical Challenges Arising in the COVID-19 Pandemic: An Overview from the Association of Bioethics Program Directors (ABPD) Task Force". The American Journal of Bioethics. 20 (7): 15–27. doi:10.1080/15265161.2020.1764138. PMID 32511078. S2CID 219552665.
- Hotez PJ, Batista C, Amor YB, Ergonul O, Figueroa JP, Gilbert S, et al. (2021). "Global public health security and justice for vaccines and therapeutics in the COVID-19 pandemic". eClinicalMedicine. 39: 101053. doi:10.1016/j.eclinm.2021.101053. PMC 8330385. PMID 34368661.
- Sparke M, Levy O (15 August 2022). "Competing Responses to Global Inequalities in Access to COVID Vaccines: Vaccine Diplomacy and Vaccine Charity Versus Vaccine Liberty". Clinical Infectious Diseases. 75 (Supplement_1): S86 – S92. doi:10.1093/cid/ciac361. ISSN 1058-4838. PMC 9376271. PMID 35535787.
- Wenham C, Smith J, Morgan R (March 2020). "COVID-19: the gendered impacts of the outbreak". Lancet. 395 (10227): 846–848. doi:10.1016/S0140-6736(20)30526-2. PMC 7124625. PMID 32151325.
- Tolchin B, Hull SC, Kraschel K (October 2020). "Triage and justice in an unjust pandemic: ethical allocation of scarce medical resources in the setting of racial and socioeconomic disparities". Journal of Medical Ethics. 47 (3): 200–202. doi:10.1136/medethics-2020-106457. PMID 33067315. S2CID 223558059.
- Sabatello M, Burke TB, McDonald KE, Appelbaum PS (October 2020). "Disability, Ethics, and Health Care in the COVID-19 Pandemic". American Journal of Public Health. 110 (10): 1523–1527. doi:10.2105/AJPH.2020.305837. PMC 7483109. PMID 32816541.
- Chin T, Kahn R, Li R, Chen JT, Krieger N, Buckee CO, et al. (September 2020). "US-county level variation in intersecting individual, household and community characteristics relevant to COVID-19 and planning an equitable response: a cross-sectional analysis". BMJ Open. 10 (9): e039886. doi:10.1136/bmjopen-2020-039886. PMC 7467554. PMID 32873684.
- Elgar FJ, Stefaniak A, Wohl MJ (October 2020). "The trouble with trust: Time-series analysis of social capital, income inequality, and COVID-19 deaths in 84 countries". Social Science & Medicine. 263: 113365. doi:10.1016/j.socscimed.2020.113365. PMC 7492158. PMID 32981770.
- Abu El Kheir-Mataria W, Khadr Z, El Fawal H, Chun S (21 March 2024). "COVID-19 vaccine intercountry distribution inequality and its underlying factors: a combined concentration index analysis and multiple linear regression analysis". Frontiers in Public Health. 12. doi:10.3389/fpubh.2024.1348088. ISSN 2296-2565. PMC 10993910. PMID 38577285.
- Mortiboy M, Zitta JP, Carrico S, Stevens E, Smith A, Morris C, et al. (2024). "Combating COVID-19 Vaccine Inequity During the Early Stages of the COVID-19 Pandemic". Journal of Racial and Ethnic Health Disparities. 11 (2): 621–630. doi:10.1007/s40615-023-01546-0. ISSN 2197-3792. PMC 10019425. PMID 36929491.
Further reading
- "Progress report on the coronavirus pandemic". Nature. 584 (7821): 325. 1 August 2020. doi:10.1038/D41586-020-02414-1. ISSN 1476-4687. PMID 32814893. Wikidata Q98568681.
- COVID-19 infection prevention and control measures for primary care, including general practitioner practices, dental clinics and pharmacy settings: first update. European Centre for Disease Prevention and Control (ECDC) (Report). October 2020.
- Erola Pairo-Castineira, Sara Clohisey, Lucija Klarić, Andrew Bretherick, Konrad Rawlik, Dorota Pasko, et al. (11 December 2020). "Genetic mechanisms of critical illness in Covid-19". Nature. doi:10.1038/S41586-020-03065-Y. ISSN 1476-4687. PMID 33307546. Wikidata Q104287299.
{{cite journal}}: CS1 maint: numeric names: authors list (link) Scholia Q104287299.
External links
[REDACTED] Scholia has a profile for COVID-19 (Q84263196).Health agencies
- Coronavirus disease (COVID‑19) Facts by the World Health Organization (WHO)
- Coronavirus (COVID‑19) by the UK National Health Service (NHS)
- Coronavirus 2019 (COVID-19) by the US Centers for Disease Control and Prevention (CDC)
Directories
- Coronavirus Resource Center at the Center for Inquiry
- COVID‑19 Information on FireMountain.net Archived 13 January 2022 at the Wayback Machine
- COVID‑19 Resource Directory on OpenMD
Medical journals
- BMJ's Coronavirus (covid‑19) Hub by the BMJ
- Coronavirus (Covid‑19) by The New England Journal of Medicine
- Coronavirus (COVID‑19) Research Highlights by Springer Nature
- Coronavirus Disease 2019 (COVID‑19) by JAMA
- COVID‑19 Resource Centre by The Lancet
- Covid‑19: Novel Coronavirus Archived 24 September 2020 at the Wayback Machine by Wiley Publishing
- Novel Coronavirus Information Center by Elsevier
Treatment guidelines
- "Bouncing Back From COVID-19: Your Guide to Restoring Movement" (PDF). Johns Hopkins Medicine.
- "Coronavirus Disease 2019 (COVID-19) Treatment Guidelines" (PDF). National Institutes of Health.
- "Guidelines on the Treatment and Management of Patients with COVID-19". Infectious Diseases Society of America.
- "JHMI Clinical Recommendations for Available Pharmacologic Therapies for COVID-19" (PDF). Johns Hopkins Medicine.
- NHS England and NHS Improvement. National Guidance for post-COVID syndrome assessment clinics (Report).
- World Health Organization (2022). Therapeutics and COVID-19: living guideline, 14 January 2022 (Report). hdl:10665/351006. WHO/2019-nCoV/therapeutics/2022.1.
- [REDACTED] Media from Commons
- [REDACTED] News from Wikinews
- [REDACTED] Quotations from Wikiquote
- [REDACTED] Resources from Wikiversity
- [REDACTED] Data from Wikidata
| Classification | D |
|---|
| Severe acute respiratory syndrome (SARS) | |
|---|---|
| General topics | |
| People | |
| In popular culture | |
| Related articles | |
| Diseases of the respiratory system | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Upper RT (including URTIs, common cold) |
| ||||||||||||||||||||||
| Lower RT/ lung disease (including LRTIs) |
| ||||||||||||||||||||||
| Pleural cavity/ mediastinum |
| ||||||||||||||||||||||
| Other/general | |||||||||||||||||||||||
| Infectious diseases – viral systemic diseases | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Oncovirus | |||||||||
| Immune disorders | |||||||||
| Central nervous system |
| ||||||||
| Cardiovascular | |||||||||
| Respiratory system/ acute viral nasopharyngitis/ viral pneumonia |
| ||||||||
| Human digestive system |
| ||||||||
| Urogenital | |||||||||
