This is an old revision of this page, as edited by CheMoBot (talk | contribs) at 05:44, 1 September 2011 (Updating {{drugbox}} (no changed fields - added verified revid - updated 'ChEMBL_Ref', 'ChEBI_Ref', 'ChEBI_Ref') per Chem/Drugbox validation (report errors or bugs)). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 05:44, 1 September 2011 by CheMoBot (talk | contribs) (Updating {{drugbox}} (no changed fields - added verified revid - updated 'ChEMBL_Ref', 'ChEBI_Ref', 'ChEBI_Ref') per Chem/Drugbox validation (report errors or bugs))(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff) Pharmaceutical compound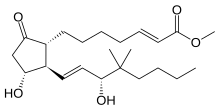 | |
| Clinical data | |
|---|---|
| Other names | methyl (E)-7--5-oxo-cyclopentyl]hept-2-enoate |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Pessary |
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.058.869 |
| Chemical and physical data | |
| Formula | C23H38O5 |
| Molar mass | 394.545 g/mol g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Gemeprost (16, 16-dimethyl-trans-delta2 PGE1 methyl ester) is an analogue of prostaglandin E1.
Clinical use
It is used as a treatment for obstetric bleeding.
It is used with RU486 to terminate pregnancy up to 24 weeks gestation.
Side effects
Vaginal bleeding, cramps, nausea, vomiting, loose stools or diarrhea, headache, muscle weakness; dizziness; flushing; chills; backache; dyspnoea; chest pain; palpitations and mild pyrexia. Rare: Uterine rupture, severe hypotension, coronary spasms with subsequent myocardial infarctions.
References
- Bartley J, Brown A, Elton R, Baird DT (2001). "Double-blind randomized trial of mifepristone in combination with vaginal gemeprost or misoprostol for induction of abortion up to 63 days gestation". Human reproduction (Oxford, England). 16 (10): 2098–102. doi:10.1093/humrep/16.10.2098. PMID 11574498. Retrieved 2008-10-29.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link)
| Uterotonics/labor inducers/oxytocics (G02A) | |||||
|---|---|---|---|---|---|
| Cervical ripening |
| ||||
| Contraction induction | |||||
| |||||
| Eicosanoids | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Precursor | |||||||||||||||
| Prostanoids |
| ||||||||||||||
| Leukotrienes (LT) |
| ||||||||||||||
| Eoxins (EX) |
| ||||||||||||||
| Nonclassic |
| ||||||||||||||
| By function | |||||||||||||||
This drug article relating to the genito-urinary system is a stub. You can help Misplaced Pages by expanding it. |