 | |
| Identifiers | |
|---|---|
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
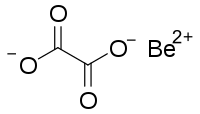
| Chemical formula | C 2BeO 4 |
| Molar mass | 97.03 |
| Appearance | Transparent crystals |
| Boiling point | 365.1 °C (689.2 °F; 638.2 K) |
| Solubility in water | Soluble |
| Hazards | |
| Flash point | 188.8 °C (371.8 °F; 461.9 K) |
| Related compounds | |
| Related compounds | Calcium oxalate Sodium oxalate Magnesium oxalate Strontium oxalate Barium oxalate Iron(II) oxalate Iron(III) oxalate Lithium oxalate Praseodymium oxalate |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Beryllium oxalate is an inorganic compound, a salt of beryllium metal and oxalic acid with the chemical formula C
2BeO
4. It forms colorless crystals, dissolves in water, and also forms crystalline hydrates. The compound is used to prepare ultra-pure beryllium oxide by thermal decomposition.
Synthesis
The action of oxalic acid on beryllium hydroxide:
Chemical properties
Crystalline hydrates lose water when heated:
References
- "BERYLLIUM OXALATE". chemicalbook.com. Retrieved 15 June 2021.
- "beryllium,oxalate". chemsrc.com. Retrieved 15 June 2021.
- Novoselova, Aleksandra Vasilʹevna; Bat︠s︡anova, Li︠u︡dmila Rafailovna (1969). Analytical Chemistry of Beryllium. Ann Arbor-Humphrey Science Publishers. p. 25. Retrieved 15 June 2021.
- Dollimore, David; Konieczay, Julie L. (1998-09-07). "The thermal decomposition of beryllium oxalate and related materials". Thermochimica Acta. 318 (1–2): 155–163. doi:10.1016/S0040-6031(98)00340-2. Retrieved 15 June 2021.
- Walsh, Kenneth A. (2009-01-01). Beryllium Chemistry and Processing. ASM International. p. 125. ISBN 978-0-87170-721-5. Retrieved 15 June 2021.
- Moore, Raymond E. (1960). Purification of Beryllium Compounds: A Literature Survey. Oak Ridge National Laboratory. p. 6. Retrieved 15 June 2021.
| Beryllium compounds | |
|---|---|
| Beryllium(I) | |
| Beryllium(II) |
|
| Compounds of the oxalate ion | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

